Abstract
Context
Sickle cell anemia (SCA) is a chronic illness causing progressive deterioration in quality of life. Brain dysfunction may be the most important and least studied problem affecting individuals with this disease.Objective
To measure neurocognitive dysfunction in neurologically asymptomatic adults with SCA vs healthy control individuals.Design, setting, and participants
Cross-sectional study comparing neuropsychological function and neuroimaging findings in neurologically asymptomatic adults with SCA and controls from 12 SCA centers, conducted between December 2004 and May 2008. Participants were patients with SCA (hemoglobin [Hb] SS and hemoglobin level < or = 10 mg/dL) aged 19 to 55 years and of African descent (n = 149) or community controls (Hb AA and normal hemoglobin level) (n = 47). Participants were stratified on age, sex, and education.Main outcome measures
The primary outcome measure was nonverbal function assessed by the Wechsler Adult Intelligence Scale, third edition (WAIS-III) Performance IQ Index. Secondary exploratory outcomes included performance on neurocognitive tests of executive function, memory, attention, and language and magnetic resonance imaging measurement of total intracranial and hippocampal volume, cortical gray and white matter, and lacunae.Results
The mean WAIS-III Performance IQ score of patients with SCA was significantly lower than that of controls (adjusted mean, 86.69 for patients with SCA vs 95.19 for controls [mean difference, -5.50; 95% confidence interval {CI}, -9.55 to -1.44]; P = .008), with 33% performing more than 1 SD (<85) below the population mean. Among secondary measures, differences were observed in adjusted mean values for global cognitive function (full-scale IQ) (90.47 for patients with SCA vs 95.66 for controls [mean difference, -5.19; 95% CI, -9.24 to -1.13]; P = .01), working memory (90.75 vs 95.25 [mean difference, -4.50; 95% CI, -8.55 to -0.45]; P = .03), processing speed (86.50 vs 97.95 [mean difference, -11.46; 95% CI, -15.51 to -7.40]; P < .001), and measures of executive function. Anemia was associated with poorer neurocognitive function in older patients. No differences in total gray matter or hippocampal volume were observed. Lacunae were more frequent in patients with SCA but not independently related to neurocognitive function.Conclusion
Compared with healthy controls, adults with SCA had poorer cognitive performance, which was associated with anemia and age.Free full text

Neuropsychological Dysfunction and Neuroimaging Abnormalities in Neurologically Intact Adults With Sickle Cell Anemia
Abstract
Context
Sickle cell anemia (SCA) is a chronic illness causing progressive deterioration in quality of life. Brain dysfunction may be the most important and least studied problem affecting individuals with this disease.
Objective
To measure neurocognitive dysfunction in neurologically asymptomatic adults with SCA vs healthy control individuals.
Design, Setting, and Participants
Cross-sectional study comparing neuropsychological function and neuroimaging findings in neurologically asymptomatic adults with SCA and controls from 12 SCA centers, conducted between December 2004 and May 2008. Participants were patients with SCA (hemoglobin [Hb] SS and hemoglobin level ≤10 mg/dL) aged 19 to 55 years and of African descent (n=149) or community controls (Hb AA and normal hemoglobin level) (n=47). Participants were stratified on age, sex, and education.
Main Outcome Measures
The primary outcome measure was nonverbal function assessed by the Wechsler Adult Intelligence Scale, third edition (WAIS-III) Performance IQ Index. Secondary exploratory outcomes included performance on neurocognitive tests of executive function, memory, attention, and language and magnetic resonance imaging measurement of total intracranial and hippocampal volume, cortical gray and white matter, and lacunae.
Results
The mean WAIS-III Performance IQ score of patients with SCA was significantly lower than that of controls (adjusted mean, 86.69 for patients with SCA vs 95.19 for controls [mean difference, −5.50; 95% confidence interval {CI}, −9.55 to −1.44]; P =.008), with 33% performing more than 1 SD (<85) below the population mean. Among secondary measures, differences were observed in adjusted mean values for global cognitive function (full-scale IQ) (90.47 for patients with SCA vs 95.66 for controls [mean difference, −5.19; 95% CI, −9.24 to −1.13]; P =.01), working memory (90.75 vs 95.25 [mean difference, −4.50; 95% CI, −8.55 to −0.45]; P =.03), processing speed (86.50 vs 97.95 [mean difference, −11.46; 95% CI, −15.51 to −7.40]; P <.001), and measures of executive function. Anemia was associated with poorer neurocognitive function in older patients. No differences in total gray matter or hippocampal volume were observed. Lacunae were more frequent in patients with SCA but not independently related to neurocognitive function.
Conclusion
Compared with healthy controls, adults with SCA had poorer cognitive performance, which was associated with anemia and age.
Sickle cell anemia (SCA) WAS once associated with high pediatric mortality. While the average life span for patients with SCA now exceeds 50 years,1,2 SCA has become a chronic illness associated with progressive deterioration in quality of life.2,3 Neurocognitive dysfunction may be the most important and least studied problem affecting this aging population.4-8
The Cooperative Study of Sickle Cell Disease found that 24% of individuals with SCA experienced a clinical stroke by age 45 years.4 Prospective pediatric imaging and neurocognitive studies have identified the serious problem of unrecognized brain injury in children with SCA.6,9,10 Global neurocognitive impairment was observed in patients with overt strokes, but neurologically intact children also had impaired neurocognitive function that increased with age. Declining IQ scores, learning difficulties, and impairment of executive function were common in children with normal findings on imaging studies.9,11-16 To our knowledge, controlled studies of neurocognitive function in adult patients have not been reported, and routine screening after childhood is not performed.
Several risk factors for ischemic brain dysfunction in SCA increase the likelihood of neurocognitive impairment with age, including hypoxia and chronic anemia, and these have been associated with disturbances in cerebral oxygenation and perfusion4-8,17 in the general population. If neurocognitive dysfunction is found to be common in adult patients with SCA, it may contribute to deterioration of their quality of life.3
We hypothesized that neurologically asymptomatic adult patients with SCA and chronic anemia (hemoglobin level ≤10 g/dL) would score lower on the Performance IQ (PIQ) Index of the Wechsler Adult Intelligence Scale, third edition (WAIS-III) compared with community controls. This measure of nonverbal function was chosen as the primary outcome because of the strong association between nonverbal abilities and central nervous system dysfunction in children with SCA. Secondary exploratory hypotheses included (1) neurologically intact adults with SCA would have lower scores on other measures of neurocognitive function; (2) impairment on these measures of neurocognitive function would be associated with abnormal magnetic resonance imaging (MRI) results; and (3) volumetric MRI would detect brain dysfunction in patients with abnormal neurocognitive test results but normal qualitative MRI results.
METHODS
Participants
This cross-sectional study was designed to compare neuropsychological function in neurologically asymptomatic adult patients with hemoglobin (Hb) SS and Sβ0thalassemia (Sβ0) with healthy control individuals from their communities. Patients aged 19 to 55 years with hemoglobin electrophoresis confirmation of their diagnosis and hemoglobin levels of 10 g/dL or less were eligible.
Patients routinely seen in the clinic setting were enrolled from 12 US medical centers participating in the Comprehensive Sickle Cell Centers program between December 2004 and May 2008. Because approximately 95% of the patients with SCA cared for at these centers are of African descent, to ensure sample homogeneity all of the individuals enrolled in the study were African American (self-reported). Enrollment was stratified using age (19-29, 30-39, 40-49, and 50-55 years), sex, and education (≤8 years, 9-11 years, high school completion, >12 years). A 3:1 stratified enrollment ratio of patients to controls was used so that estimates for patients would have a high degree of confidence while enrolling a sufficient number of controls for 80% power in a patient-control comparison.
Patients with any history of neurologic injury, stroke, or abnormal neurologic examination or imaging findings were excluded. Patients with serious cognitive impairment, depression, or both were excluded by screening with the Mini-Mental State Examination18 and Profile of Mood States.19 Patients with a chronic disorder (eg, diabetes, chronic lung disease, hypertension, liver or renal disease), recent acute illness, or medication use that could affect neurocognitive function were excluded. Patients with a history of recent transfusions and Hb A level greater than 15% were not eligible. All patients completed neurocognitive testing and neuroimaging at steady state.
Controls of African American descent with Hb AA on electrophoresis and a normal hemoglobin level (>12 g/dL for women, >13.5 g/dL for men) were recruited from the same communities with the same exclusionary criteria. Recruitment of controls was facilitated through mailing of institutional review board–approved information flyers. Additionally, controls were recruited from local churches, shopping centers, colleges, hospital staffs, and community events.
Participant Disposition
Written informed consent was provided by 214 patients and 72 controls. Fifty-four patients and 20 controls did not meet eligibility requirements after screening. Of the 160 patients and 52 controls enrolled, 11 patients and 5 controls were discontinued prior to completing the neuropsychological battery or MRI. A total of 149 patients and 47 controls completed neuropsychological batteries, MRIs, or both, resulting in participation rates of 93% and 90%, respectively. The neurocognitive battery was completed by 146 patients and 47 controls, while 141 patients and 44 controls completed the MRIs. Eighty-two patients and 22 controls had MRIs of sufficient quality for complete volumetric analysis. Reasons for volumetric MRI failure were related to coordinating a multicenter MRI study and not to participant-related reasons.
Participating Centers and Data Collection
This study was conducted using Good Clinical Practice guidelines.20 Institutional review boards at each site approved the study. Each institution had a principal investigator, neuropsychologist, radiologist, and coordinator assigned to the study.
Neuropsychological Protocol
Participating neuropsychologists were trained to administer and score the neurocognitive protocol. All data were reviewed, rescored, and validated by the coordinating center neuropsychologist. The assessment protocol measured global cognitive function, executive function, academic achievement, memory, attention, motor skills, and processing speed. Tests, functions, and score characteristics for neurocognitive measures are detailed in Table 1. The scores for the analyses were adjusted for age at the time of testing.
Table 1
Neurocognitive Measures, Functional Area Assessed, and Score Characteristics
| Measure | Functional Area Assessed | Score Characteristics | ||
|---|---|---|---|---|
| Mean (SD) | Range | Clinical Significance | ||
| Primary outcome (WAIS-III Performance IQ Index) | Index of nonverbal function; includes 5 subtests of visual and motor ability | 100 (115)a | 50-150b | ≥70 to <85 indicate below average; <70, impaired |
| Secondary outcomes | ||||
 WAIS-III WAIS-III | ||||
  Verbal IQ Index Verbal IQ Index | Index of verbal function; includes 6 subtests of language, abstract reasoning | 100 (115)a | 50-150b | ≥70 to <85 indicate below average; <70, impaired |
  Full-scale IQ Index Full-scale IQ Index | Global cognitive index composed of performance IQ and verbal IQ; age normed | 100 (115)a | 50-150b | ≥70 to <85 indicate below average; <70, impaired |
  Processing Speed Index Processing Speed Index | Index composite of 2 measures of visual scanning speed with motor response | 100 (115)a | 50-150b | ≥70 to <85 indicate below average; <70, impaired |
  Working Memory Index Working Memory Index | Index composite of 3 measures of auditory short-term memory | 100 (115)a | 50-150b | ≥70 to <85 indicate below average; <70, impaired |
  Verbal Comprehension Index Verbal Comprehension Index | Index composite of 3 measures of word knowledge, general information, reasoning | 100 (115)a | 50-150b | ≥70 to <85 indicate below average; <70, impaired |
  Perceptual Organization Index Perceptual Organization Index | Index composite of 3 measures of visual-motor perception and organization of motor responses | 100 (115)a | 50-150b | ≥70 to <85 indicate below average; <70, impaired |
 WMS-III WMS-III | Comprehensive set of 9 subtests assessing short- and long-term memory, auditory and visual memory | 100 (115)a | 50-150b | ≥70 to <85 indicate below average; <70, impaired |
 WCST-CV4 WCST-CV4 | Cognitive flexibility and ability to shift “set” | 50 (10)c | 10-90b | ≥37 to <42 indicate below average; <36, impaired |
 D-KEFS D-KEFS | Integrated battery of 5 tests of planning ability, verbal fluency, concentration, deductive reasoning | 10 (3)d | 1-19 | ≥3.0 to <7.0 indicate below average; <3.0, impaired |
 TEA TEA | 8 subtests of visual and auditory attention | 10 (3)d | 1-19 | ≥3.0 to <7.0 indicate below average; <3.0, impaired |
 CVLT-II CVLT-II | Word learning over multiple trials; both recall and recognition measures | 0 (1) | −4.0 to 4.0b | ≤−1.5 indicates below average |
Abbreviations: CVLT-II, California Verbal Learning Test, second edition; D-KEFS, Delis-Kaplan Executive Function System; TEA, Tests of Everyday Attention; WAIS-III, Wechsler Adult Intelligence Scale, third edition; WCST-CV4, Wisconsin Card Sorting Test, computer version 4; WMS-III, Wechsler Memory Scale, third edition.
Evaluations were completed across 2 sessions lasting approximately 6 to 7 hours. Participants were debriefed at study conclusion by the neuropsychologist and local investigators. This included outlining cognitive strengths and weaknesses. Recommendations and consultations were provided to address any concerns related to mental health, memory, motor impairment, problem solving, planning, and organization.
MRI Evaluation
The University of California, San Francisco, Neuroimaging Center was the MRI reading center. A standardized protocol, software, and 1.5-T newer-generation scanners with volumetric T1-weighted MRI acquisition capability were used.
Phantom scans were provided by each site for assessment of qualification and reliability. Central readings were performed by 2 qualified independent neuroradiologists masked to the participant’s medical history. Descriptive analysis was standardized. Lacunae were defined as regions of at least 5 mm in diameter that were hyperintense on the T2-weighted images as well as the proton-density weighted images, with corresponding hypointensity on T1-weighted images.
Volumetric measurements included total intracranial volume, hippocampal volume, cortical gray matter, white matter, and cortical and subcortical lacunae. Intracranial volume was determined by creating a binary mask of the T2-weighted image. Hippocampal volumes were obtained using Surgical Navigation Technologies, a brain-mapping method based on a high-dimensional fluid transformation algorithm.21 The Expectation Maximizing Segmentation algorithm22 was used to segment all MRIs into gray matter, white matter, and cerebrospinal fluid. Output was confirmed with a segmentation review combined with the corresponding lobe parcellation results. An atlas-based method was used to identify the lobes and subcortical nuclei, 23 with the MRI of a healthy 36-year-old man as the reference. An entropy-driven B-spline free-form deformation algorithm24 was used to register individual scans to the reference atlas. All automated marked MRIs were visually inspected to ensure accuracy. The lobar and subcortical markings were then combined with the Expectation Maximizing Segmentation tissue masks to generate measurements of gray matter, white matter, and cerebrospinal fluid by lobe.
Statistical Analysis
Based on a priori sample-size calculations, a sample of 120 patients and 36 controls would have 80% power, with a 2-sided α of .05, to detect an 8-point difference (>0.5 SD and clinically significant) on the WAIS-III PIQ Index. The primary hypothesis (WAIS-III PIQ score) was tested at the .05 α level; no adjustments for multiple comparisons on the secondary analyses were made, because these P values were considered exploratory.
Demographic and baseline measures were descriptively compared for imbalance using the Fisher exact test for categorical variables and Wilcoxon 2-sample nonparametric test for continuous variables. No missing data were imputed, but the effects of possible biases attributable to missing data were studied using sensitivity analyses.
To compare patients and controls, mixed linear models were fit to the data for each test. Each mixed model had age, sex, education, group, subtest, and the group × subtest interaction as fixed effects and random intercepts at the participant level to account for the within-participant correlations between an individual’s set of subtests within each test. Using the mixed models, 2-sided significance tests were completed and 2-sided 95% confidence intervals (CIs) were constructed for differences in each subtest. Categories of the WAIS-III measures were designated by the number of standard deviations away from the mean and the percentage of patients and controls in each category reported compared with expected norms. The WAIS-III norms are based on a representative sample of the US adult population and include the range of ethnic/racial and socioeconomic categories that exists in the general population.
General linear models of volumetric MRI measures were fit, adjusting for total intracranial volume, age, and group. A group × age interaction was used to estimate age-dependent patient minus control differences. χ2 Tests were used to assess the statistical significance of patient-control differences in clinical MRI event prevalence.
The differences between the groups were statistically significant for WAIS-III PIQ score and the presence of lacunae. An additional general linear model was fit to the subset of data that included patients with lacunae, patients without lacunae, and controls without lacunae. To explore possible confounding of age on these results, age was added to the model and the 2 sets of model results were compared. To explore the relationship between PIQ score and hemoglobin level among the patients, 4 strata of age (<25, 25 to 30, 30 to 40, and ≥40 years) were created based approximately on quartiles of the data. Also, 4 strata of hemoglobin level (<7.6, 7.6 to <8.3, 8.3 to <9.0, and ≥9.0 g/dL) were created. Within each stratum combination, the sample size, mean PIQ score, and standard deviation were tabulated. Statistical analyses were generated using SAS version 9.1 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Analyses were performed to identify differences between participants with and without volumetric MRI results, as well as those with and without results for neuropsychological batteries or clinical MRIs. No significant differences were observed in the demographic or disease variables, WAIS-III measures, or MRI measures of atrophy, lacunae, or white matter lesion incidence. Even though a large proportion of the MRIs were volumetric MRI failures, the failures did not appear to impose a bias on the volumetric MRI comparisons between patients and controls.
Mean patient age was 31.6 (SD, 8.95) years vs 33.1 (SD, 10.06) years for controls (P =.45); 63% of patients were women, and 95% had Hb SS. Compared with controls, patients had lower mean hemoglobin values (8.3 [SD, 0.98] g/dL vs 13.8 [SD, 1.13] g/dL) and higher values than controls for Hb F, platelets, white blood cells, and lactate dehydrogenase (Table 2).
Table 2
Demographic and Baseline Measures Among Patients With Sickle Cell Anemia and Healthy Controls
| Variable | No. (%) | P Valuea | |
|---|---|---|---|
| Patients (n = 160) | Controls (n = 52) | ||
| Age, y | |||
 <25 <25 | 39 (27) | 15 (32) | .85 |
 25-30 25-30 | 44 (30) | 8 (17) | |
 >30 >30 | 63 (43) | 24 (51) | |
| Sex | |||
 Men Men | 54 (37) | 24 (51) | .09 |
 Women Women | 92 (63) | 23 (49) | |
| Diagnosisb | |||
 Hb SS Hb SS | 139 (95) | 0 | NA |
 Sβ0 Sβ0 | 7 (5) | 0 | |
 Hb AA Hb AA | 0 | 47 (100) | |
| Education level, y | |||
 ≤12 ≤12 | 52 (36) | 15 (32) | .62 |
 >12 >12 | 93 (64) | 32 (68) | |
| Medical history | |||
 Asthma Asthma | 12 (8) | 4 (9) | .95 |
 Avascular necrosis Avascular necrosis | 43 (29) | 0 (0) | <.001 |
 Headaches Headaches | 31 (21) | 5 (11) | .11 |
| Clinical values | |||
 Hemoglobin Hemoglobin | (n = 146) | (n = 47) | |
  Mean (SD), g/dL Mean (SD), g/dL | 8.2 (1.00) | 13.8 (1.15) | <.001 |
 Platelets Platelets | (n = 145) | (n = 46) | |
  Mean (SD), ×103/μL Mean (SD), ×103/μL | 395.9 (130.93) | 262.2 (55.48) | <.001 |
 WBC count WBC count | (n = 146) | (n = 47) | |
  Mean (SD), ×103/μL Mean (SD), ×103/μL | 10.3 (3.28) | 6.2 (2.08) | <.001 |
 Hemoglobin F Hemoglobin F | (n = 131) | (n = 34) | |
  Mean (SD), % of total Hb variants Mean (SD), % of total Hb variants | 10.5 (8.21) | 0.6 (1.11) | <.001 |
 LDH LDH | (n = 92) | (n = 37) | |
  Mean (SD), U/Lc Mean (SD), U/Lc | 574.8 (412.99) | 290.0 (151.55) | <.001 |
 ALT ALT | (n = 146) | (n = 47) | |
  Mean (SD), U/L Mean (SD), U/L | 33.5 (33.84) | 31.2 (17.77) | .81 |
 AST AST | (n = 145) | (n = 47) | |
  Mean (SD), U/L Mean (SD), U/L | 43.9 (20.85) | 27.3 (10.95) | <.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; Hb, hemoglobin; LDH, lactate dehydrogenase; NA, not applicable; Sβ0, Sβ0 thalassemia; WBC, white blood cell.
SI conversion factors: To convert LDH, ALT, and AST values to μkat/L, multiply by 0.0167.
Neurocognitive Findings
Comparison of the primary neurocognitive outcome (WAIS-III PIQ score) between adult patients with SCA and controls is summarized in the Figure. After adjusting for age, sex, and education level, the patients had statistically significant lower mean scores than controls (adjusted mean, 89.69 for patients vs 95.19 for controls [mean difference, −5.50; 95% CI, −9.55 to −1.44]; P = .008). The WAIS-III PIQ score was more than 1 SD below the normative mean for 33% of patients and 15% of controls, compared with an expected 16% from the national norms (Table 3).
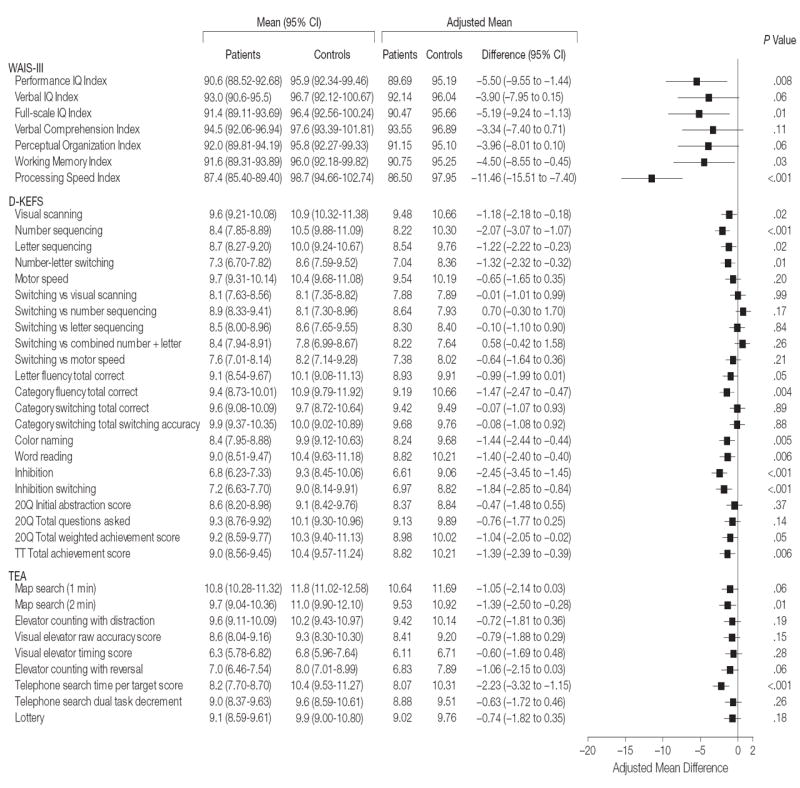
Mean difference indicates mean patient minus control differences. Wechsler Adult Intelligence Scale, third edition (WAIS-III) measures are index scores; Delis-Kaplan Executive Function System (D-KEFS) and Tests of Everyday Attention (TEA) measures are scaled scores. No attempt was made to normalize the mean differences or the 95% confidence intervals (CIs) to a common scale, so no attempt should be made to compare precision across tests without keeping in mind the natural scale of the raw data. Size of data markers indicates the weight of each measure in the study. For patients, sample size for all measures was n=146 except for D-KEFS number-letter switching and TEA map search (1 min), map search (2 min), telephone search time, and lottery (n=145); TEA elevator counting with distraction, visual elevator raw accuracy, and telephone search dual-task decrement (n=144); and TEA visual elevator timing and elevator counting with reversal (n=140). For controls, sample size for all measures was n=47 except for D-KEFS inhibition switching and TEA elevator counting with reversal and telephone search dual-task decrement (n=46); D-KEFS 20-question (20Q) initial abstraction, 20Q total questions, and 20Q total weighted achievement (n=45); and TEA map search (2 min) (n=44).
Table 3
Proportion of Patients With Sickle Cell Anemia (n = 146) and Healthy Controls (n = 47) in Wechsler Adult Intelligence Scale III Categoriesa
| Category | No. (%) | P Valueb | |||
|---|---|---|---|---|---|
| ≤69 | 70-84 | 85-99 | ≥100 | ||
| Expected %c | 2 | 14 | 34 | 50 | |
| Performance IQ Index | |||||
 Patients Patients | 4 (3) | 44 (30) | 67 (46) | 31 (21) | .03 |
 Controls Controls | 2 (4) | 5 (11) | 24 (51) | 16 (34) | |
| Verbal IQ Index | |||||
 Patients Patients | 5 (3) | 40 (27) | 55 (38) | 46 (32) | .16 |
 Controls Controls | 1 (2) | 7 (15) | 22 (47) | 17 (36) | |
| Full-scale IQ Index | |||||
 Patients Patients | 6 (4) | 43 (29) | 59 (40) | 38 (26) | .06 |
 Controls Controls | 1 (2) | 9 (19) | 19 (40) | 18 (38) | |
| Perceptual Organization Index | |||||
 Patients Patients | 8 (5) | 34 (23) | 69 (47) | 35 (24) | .03 |
 Controls Controls | 1 (2) | 5 (11) | 25 (53) | 16 (34) | |
| Working Memory Index | |||||
 Patients Patients | 5 (3) | 47 (32) | 61 (42) | 33 (23) | .01 |
 Controls Controls | 0 | 7 (15) | 25 (53) | 15 (32) | |
| Verbal Comprehension Index | |||||
 Patients Patients | 6 (4) | 32 (22) | 51 (35) | 57 (39) | .48 |
 Controls Controls | 1 (2) | 9 (19) | 17 (36) | 20 (43) | |
| Processing Speed Index | |||||
 Patients Patients | 8 (5) | 53 (36) | 63 (43) | 22 (15) | <.001 |
 Controls Controls | 0 | 5 (11) | 22 (47) | 20 (43) | |
Additional differences between patients and controls were noted on mean scores for secondary measures of processing speed (adjusted mean, 86.50 for patients vs 97.95 for controls [mean difference, −11.46; 95% CI, −15.51 to −7.40]; P <.001), working memory (90.75 vs 95.25 [mean , −4.50]; 95% CI, −8.55 to −0.45]; P =.03), global cognitive function (full-scale IQ) (90.47 vs 95.66 [mean difference, −5.19; 95% CI, −9.24 to −1.13]; P =.01), and the majority of measures of executive function (Delis-Kaplan Executive Function System), after adjusting for age, sex, and education (Figure).
Difficulties with selective attention in patients were illustrated by lower mean scores for visual scanning and attention on the Tests of Everyday Attention (map search [2 minutes] adjusted mean difference, −1.39; 95% CI, −2.50 to −0.28; P =.01) and telephone search (adjusted mean difference, −2.23; 95% CI, −3.32 to −1.15; P <.001) (Figure). The differences in means between patients and controls were not statistically significant for other TEA subtests or scales. Differences between patients and controls were not statistically significant for any subtests from the Wechsler Memory Scale, third edition (memory), Wisconsin Card Sorting Test, computer version 4 (cognitive flexibility), or California Verbal Learning Test, second edition (verbal learning, recall, and recognition) (Figure).
MRI Findings
A cortical infarct was detected in 1 patient and 1 control. Lacunae were seen in 13% of patients and 2% of controls (difference, 11%; 95% CI, 4.1% to 18.4%; P =.05) and were mainly in the frontal lobe, parietal lobe, and basal ganglia. Atrophy was seen in 23% of patients and 16% of controls (difference, 7%; 95% CI, −5.4% to 20.4%; P =.40), and white matter lesions occurred in 15% of patients and 7% of controls (difference, 8%; 95% CI, −1.4% to 17.6%; P =.21).
The difference between patients and controls in mean total intracranial volume was statistically significant (1152.6 mL vs 1218.5 mL [difference, −65.9 mL; 95% CI, −125.2 to −6.5]; P =.03). This difference represents 5.7% of the mean total intracranial volume among patients. Exploratory analysis revealed that total intracranial volume decreased by 2.6 mL per year on average among patients while remaining constant among controls (95% CI, −4.8 to −0.5). No statistically significant difference in total cortical gray matter volume (difference, −1.58 mL; 95% CI, −7.11 to 10.26; P =.72) or total hippocampal volume (difference, −0.16 mL; 95% CI, −0.16 to 0.49; P =.33) was observed between patients and controls when controlling for total intracranial volume, age, and group. Globally, there was a statistically significant reduction in total cortical gray matter volume as age increased (−1.02 mL/y; 95% CI, −1.37 to −0.67; P <.001); however, group differences in the reduction were not statistically significant (−0.85 mL/y among controls and −1.07 mL/y among patients [difference, 0.22 mL/y; 95% CI, −1.04 to 0.60]; P =.59). A reduction in total hippocampal volume with age was statistically significant in patients but not controls (−0.015 mL/y; 95% CI, −0.03 to −0.005; P =.04).
Correlates of WAIS-III PIQ Score
Performance IQ score was not correlated with sex, white blood cell count, platelet count, or levels of Hb F, lactate dehydrogenase, or hemoglobin. Further investigation suggested an age-dependent relationship between PIQ score and hemoglobin level. As hemoglobin level decreased, the difference in mean PIQ score between patients younger than 25 years and those 40 years or older tended to increase (eTable, available at http://www.jama.com). A similar pattern was not observed for verbal IQ, full-scale IQ, Perceptual Organizational Index, and Verbal Comprehension Index scores, although patients with hemoglobin levels less than 7.6 g/dL consistently had large reductions in mean score after the fourth decade. Neither the Working Memory Index nor the Processing Speed Index demonstrated a relationship with hemoglobin levels.
Patients with lacunae had the lowest mean WAIS-III PIQ scores compared with patients without lacunae or controls without lacunae. Mean PIQ scores in patients with lacunae were 85.1 compared with 91.1 for patients without lacunae (difference, 6.0; 95% CI, 0.1 to 12.0; P =.05). After adjusting for age, however, this difference was not statistically significant. Additional analyses showed that total intracranial volume and total cortical gray matter volume were not significantly correlated with PIQ score, and the correlation with total hippocampal volume of 0.21 (95% CI, −0.01 to 0.41) was also not statistically significant.
COMMENT
To our knowledge, this is the first comprehensive assessment of neurocognitive function in neurologically intact adults with SCA. The major findings are that (1) adults with SCA showed poorer performance on neurocognitive tests when compared with community controls; (2) anemia is associated with the age-related decline in cognitive performance; and (3) MRI findings do not explain the performance differences in the subset of patients with neuroimaging studies, despite the presence of more lacunae infarcts in patients than in controls.
Because silent infarction and impaired performance on neurocognitive tests was previously demonstrated in neurologically intact pediatric patients, we studied a similar population of adults with SCA who had no previous history of neurologic events or of abnormal findings on imaging or neurologic examination. The study sample excluded patients with chronic disease and complications of SCA resulting in end-organ failure that are known to be associated with neurocognitive dysfunction. While the study sample is not representative of the entire SCA population, it represents an important subgroup of neurologically intact individuals without evidence of other organ dysfunction.
The study demonstrates important differences in neurocognitive performance on the WAIS-III verbal IQ, PIQ, and full-scale IQ indexes, as well as on tests of memory, language, learning, attention, retrieval, and overall executive functioning. Executive functioning includes cognitive ability, volition, planning, and effective execution of plans.26,27 While the mean scores of the patients with SCA are at the low end of the average range, 33% of this group had PIQ scores in the below-average range, suggesting possible challenges in skills of daily life such as employment, financial management, medication adherence, utilization of community resources, and social functioning.27,28 The majority of adult patients with SCA have other sickle cell–related complications and risk factors4,29,30 associated with neurocognitive decline in the general population,29 such as chronic lung disease, renal failure, stroke, intracranial hemorrhage, chronic liver disease, mental health disorders, and systemic hypertension. Therefore, these results may underestimate the level of cognitive difficulties experienced in the larger community of adults with SCA.
Hemoglobin level, age, and education were associated with lower neurocognitive performance. We found no association between PIQ scores and lung injury, disease severity, Hb F level, white blood cell count, platelet count, or lactate dehydrogenase level.
Anemia is a potentially important correlate for poorer neurocognitive performance in this population, as indicated by the effect of anemia on age-related neurocognitive decline. Previous studies have consistently found reduced hemoglobin levels to be a risk factor for neurocognitive dysfunction in individuals with SCA as well as the general population.11-14 Hemoglobin is likely a surrogate marker for reduced oxygen delivery to the brain. Increased cerebral blood flow is associated with lower cognitive function and is caused by anemia and hypoxia.13,31 Patients with SCA appear to have a decreased cerebral vascular reserve and may have a state of chronic cerebral ischemia resulting in neurocognitive dysfunction. This is supported by studies that find lower cerebral oxygen saturation in patients with SCA than in controls and that also indicate that performance associated with anemia and oxygen desaturation may be reversible. 32-34 Both transfusion and administration of hydroxyurea may improve cerebral blood flow, oxygenation, and neurocognitive function in patients with SCA.15,32,35 In studies of healthy controls and other chronic diseases, anemia is predictive of poorer neurocognitive performance, and increasing hemoglobin levels improves test results. 33,34 These observations suggest that a possible cause of cognitive difficulties in SCA is diffuse, possibly reversible, hypoxic dysfunction.
Compared with controls, patients with SCA had an increased number of lacunae, reduced total intracranial volume, and reduced total hippocampal volume with age. Patients with SCA both with and without lacunae had a significant reduction in PIQ scores compared with controls without lacunae. When adjusted for age, however, the effect of lacunae on cognitive function in SCA was no longer statistically significant. Other MRI measures did not significantly correlate with PIQ scores. Anemia appears to be a more important correlate with neurocognitive performance; however, a larger sample size is necessary to establish a lack of relationship between MRI findings and neurocognitive function. Furthermore, other studies in SCA have found that anemia is a stronger predictor of neurocognitive function than silent MRI findings.12,14
The quantitative MRI results of this study demonstrate preliminary evidence that total intracranial and hippocampal volumes decrease with age in patients with SCA but not controls. The lack of correlation of total intracranial volume and hippocampal volume with age in the control group may indicate that volume loss is accelerated in young patients with SCA, but the narrow age range in patients and controls may limit the detection of some volume loss in older controls. The neurocognitive impairment may be caused by cerebral blood flow velocity and hypoxia.11,12,36 Because MRI does not accurately predict this velocity, neurocognitive injury from anemia may be better predicted by functional flow studies using transcranial Doppler ultrasonography.13,15,35,37
Study Limitations
By design, the patient group was limited to a healthy group of adult patients with SCA and was not representative of the population at large. A more heterogeneous sample is needed for generalization to the population. Because this was a cross-sectional study, follow-up of patients was not included. The age range of patients as well as controls was not distributed widely enough to include older patients, thereby limiting some conclusions. Including a control group with chronic anemia would strengthen the study. Because thalassemia and other hemolytic anemias appear to pose a high risk of central nervous system injury secondary to a vasculopathy or prothrombotic state, individuals with such disorders were not recruited as controls.5 A homogeneous group of patients with nonhemolytic anemias would be the ideal control but difficult to obtain.
Analysis of secondary outcome variables was exploratory in nature, and P values were not corrected for multiple comparisons. Their interpretation should occur in the context of patterns observed across a variety of neurocognitive test results. The reduced number of individuals with a volumetric MRI result could have affected the estimate of the relationship between MRI result findings and the neurocognitive measures. Also, the study did not include functional or perfusion studies. This limits interpretation of the secondary analyses and underscores the need for further investigation with longitudinal designs.
Summary and Recommendations
Adult patients with SCA who are neurologically asymptomatic are still at risk for neurocognitive performance deficits, because their anemia may be inducing neurocognitive impairment secondary to cerebral hypoxemia undetectable by standard neuroimaging studies. Several practical steps can be taken. First, early identification of patients with difficulties on specific measures of neurocognitive function may allow these patients to enroll in and benefit from cognitive rehabilitation programs.27 Additionally, longitudinal studies are necessary to understand and evaluate disease progression. These studies can be linked to biological components to improve understanding of neurocognitive function in SCA.
Overall, the results of this study suggest that neurocognitive performance is not adequately explained by findings on standard neuroimaging studies and support the need for intervention studies evaluating transfusion therapy, neuroprotective agents, hydroxyurea, and oxygenation to determine whether such treatments will improve cognition.
Acknowledgments
Funding/Support: This study was supported by the National Heart, Lung, and Blood Institute Award No. U54HL070587.
Role of the Sponsor: The National Heart, Lung, and Blood Institute had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Appendix
Neuropsychological Dysfunction and Neuroimaging Adult Sickle Cell Anemia Study Group
Compensation was not received and the investigators had no conflicts of interest. National Institutes of Health: Greg Evans, PhD, was the National Heart, Lung, and Blood Institute project officer for this effort. Investigative Team: F. Daniel Armstrong, PhD, Barry Eggleston, MS, Jeffrey I. Gold, PhD, Karen Kesler, PhD, Susan Lieff, PhD, Lynne Neumayr, MD, Elliott Vichinsky, MD, and Michael W. Weiner, MD. Statistical and Data Coordinating Center: Rho Federal Systems of Rho Inc with Karen Kesler, PhD, Susan Lieff, PhD, Barry Eggleston, MS, Emily Kunka, Marsha McMurray, Allison Muma, MHA, Cathie Snyder, and Jamie Spencer. Central Neuropsychology Site: Childrens Hospital Los Angeles/ University of Southern California with Jeffrey I. Gold, PhD, and Nicole Mahrer. Central Neuroimaging Site: The Veterans Health Research Institute (NCIRE) with Michael Weiner, MD, Randall Rule, PhD, Diana Truran, and Jeffrey Kasten.
Participating Sites
This study was a collaboration of the following institutions, investigators, neuropsychologists, radiologists, and study coordinators. Site principal investigators are indicated by asterisks. Boston Medical Center, Boston, Massachusetts (Lillian McMahon, MD,* Glenn Barest, MD, Peter Mosbach, PhD, Asif Qureshi); Children’s Hospital & Research Center Oakland, Oakland, California (Elliott Vichinsky, MD,* Audrey Bethke, PhD, Kenneth Martin, MD, Lynne Neumayr, MD,* Susan Paulukonis); Cincinnati Children’s Hospital Medical Center and University Hospital Cincinnati, Cincinnati, Ohio (Clinton Joiner, MD,* Karen Kalinyak, MD,* Monique Lumpkin, MSW, Lori Crosby, PsyD, Donna Diedenhofer, BA, Annette R. Lavender, RN, MSN, APRN-BC, Tamara Nordheim, BSN, RN, Marsha Nortz Gragert, PhD, Zahida Yasin, MD); Duke University Medical Center, Durham, North Carolina (Laura M. De Castro, MD,* Mary R. Abrams, MPH, Christle Cameron, Miriam Feliu, PhD, Jude C Jonassaint, RN, Christopher Lascola, MD, PhD, Marilyn J. Telen, MD); Medical College of Georgia, Augusta (Abdullah Kutlar, MD,* Gregory Lee, PhD, Kavita Natarajan, MD, Leigh Wells, MSN, Adrienne Wilson, MS); Memorial Cancer Institute, Hollywood, Florida (Lanetta Jordan, MD, MPH,* Bonnie Aberson, PsyD, Bruce Braffman, MD, Atif Hussein, MD, Peter A. Gold, PsyD, Cheri G. Surloff, PhD, Mallika Venkataramani); University of Miami, Miami, Florida (F. Daniel Armstrong, PhD,* Rita Bhatia, MD, Thomas Harrington, MD, Elizabeth Willen, PhD); University of North Carolina, Chapel Hill (Eugene P. Orringer, MD,* Kenneth I. Ataga, MD, Sutapa Ford, PhD, Susan K. Jones, RN, J. Keith Smith, MD, PhD); University of Southern California, Los Angeles (Cage Johnson, MD,* Pat Corley, RN, Carol McCleary, PhD, Chi-Shing Zee, MD); University of Texas Medical Branch, Galveston ( Joel David Bessman, MD,* Phyllis Crawford, RN, Lisa Hernandez Garcia, Stephen S. Ladner, MD, Alison Rutledge, PhD, Vicki M. Soukup, MD); University of Texas Southwestern, Dallas (Cynthia J. Rutherford, MD,* Munro Cullum, PhD, Bonnie L. Davis, RN, Laurie Rilling, PhD).
Footnotes
Author Contributions: Dr Vichinsky had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Vichinsky, Neumayr, Gold, Rule, Armstrong.Acquisition of data: Vichinsky, Weiner, Rule, Truran, Kasten, Eggleston, Kesler, McMahon, Orringer, Harrington, Kalinyak, De Castro, Kutlar, Rutherford, Johnson, Bessman, Jordan, Armstrong.
Analysis and interpretation of data: Vichinsky, Neumayr, Gold, Rule, Truran, Kasten, Eggleston, Kesler, McMahon, De Castro, Bessman, Jordan, Armstrong.
Drafting of the manuscript: Vichinsky, Neumayr, Gold, Rule, Eggleston, Kesler, McMahon, Jordan, Armstrong.
Critical revision of the manuscript for important intellectual content: Vichinsky, Neumayr, Gold, Weiner, Rule, Truran, Kasten, Eggleston, Kesler, McMahon, Orringer, Harrington, Kalinyak, De Castro, Kutlar, Rutherford, Johnson, Bessman, Jordan, Armstrong.
Statistical analysis: Eggleston, Kesler.
Obtained funding: Vichinsky, Neumayr, Gold, Rutherford, Bessman, Armstrong.
Administrative, technical, or material support: Vichinsky, Neumayr, Weiner, Rule, Truran, Kasten, Kesler, McMahon, Harrington, Rutherford, Johnson, Bessman, Armstrong.
Study supervision: Vichinsky, Neumayr, Gold, Rule, Truran, McMahon, De Castro, Armstrong.
Financial Disclosures: None reported.
Online-Only Material: The eTable is available at http://www.jama.com.
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jama.2010.562
Read article for free, from open access legal sources, via Unpaywall:
https://cdr.lib.unc.edu/downloads/1c18dp85h
Free to read at jama.ama-assn.org
http://jama.ama-assn.org/cgi/content/abstract/303/18/1823
Subscription required at jama.ama-assn.org
http://jama.ama-assn.org/cgi/reprint/303/18/1823.pdf
Subscription required at jama.ama-assn.org
http://jama.ama-assn.org/cgi/content/full/303/18/1823
Citations & impact
Impact metrics
Article citations
The Association Between Sickle Cell Anemia and Cognitive Dysfunction: A Systematic Review.
Cureus, 16(9):e69104, 10 Sep 2024
Cited by: 0 articles | PMID: 39391457 | PMCID: PMC11466366
Review Free full text in Europe PMC
Determinants of cognitive dysfunction in adults with sickle cell-related stroke or suspected neurological morbidity.
Blood Adv, 8(15):3993-4002, 01 Aug 2024
Cited by: 1 article | PMID: 38815229 | PMCID: PMC11339041
Cognitive outcomes of children and adults with sickle cell anaemia: A contemporary cohort.
Br J Haematol, 205(3):1238-1241, 09 Jul 2024
Cited by: 0 articles | PMID: 38981601
Neurocognitive outcome in children with sickle cell disease after myeloimmunoablative conditioning and haploidentical hematopoietic stem cell transplantation: a non-randomized clinical trial.
Front Neurol, 15:1263373, 22 May 2024
Cited by: 0 articles | PMID: 38841694
End Organ Affection in Sickle Cell Disease.
Cells, 13(11):934, 29 May 2024
Cited by: 0 articles | PMID: 38891066 | PMCID: PMC11174153
Review Free full text in Europe PMC
Go to all (162) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neuroimaging abnormalities in adults with sickle cell anemia: associations with cognition.
Neurology, 82(10):835-841, 12 Feb 2014
Cited by: 43 articles | PMID: 24523480 | PMCID: PMC3959758
Anemia predicts lower white matter volume and cognitive performance in sickle and non-sickle cell anemia syndrome.
Am J Hematol, 94(10):1055-1065, 23 Jul 2019
Cited by: 26 articles | PMID: 31259431 | PMCID: PMC6857783
Effect of age, cerebral infarcts, vasculopathy and haemoglobin on cognitive function, in Tanzanian children with sickle cell anaemia.
Eur J Paediatr Neurol, 37:105-113, 14 Jan 2022
Cited by: 7 articles | PMID: 35182942
Neurocognitive sequelae of pediatric sickle cell disease: a review of the literature.
Child Neuropsychol, 13(2):120-131, 01 Mar 2007
Cited by: 75 articles | PMID: 17364569
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (5)
Grant ID: U54 HL070587-07
Grant ID: U54HL070587
Grant ID: U54 HL070583
Grant ID: U54 HL070583-010002
Grant ID: U54 HL070587





