Abstract
Free full text

Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression
Abstract
Regulatory T cells (Tregs) are thought to play a major role in pregnancy by inhibiting the maternal immune system and preventing fetal rejection. In decidual tissues, NK cells (dNK) reside in close contact with particular myelomonocytic CD14+ (dCD14+) cells. Here we show that the interaction between dNK and dCD14+ cells results in induction of Tregs. The interaction is mediated by soluble factors as shown by transwell experiments, and the prominent role of IFN-γ is revealed by the effect of a neutralizing monoclonal antibody. Following interaction with dNK cells, dCD14+ cells express indoleamine 2,3-dioxygenase (IDO), which, in turn, induces Tregs. Notably, unlike peripheral blood NK (pNK) cells, dNK cells are resistant to inhibition by the IDO metabolite L-kynurenine. “Conditioned” dCD14+ cells also may induce Tregs through transforming growth factor-β (TGF-β) production or CTLA-4–mediated interactions, as indicated by the effect of specific neutralizing Abs. Remarkably, only the interaction between dNK and dCD14+ cells results in Treg induction, whereas other coculture combinations involving either NK or CD14+ cells isolated from peripheral blood are ineffective. Our study provides interesting clues to understanding how the crosstalk between decidual NK and CD14+ cells may initiate a process that leads to Treg induction and immunosuppression. Along this line, it is conceivable that an impaired function of these cells may result in pregnancy failure.
In successful human pregnancy the balance between active immunity and tolerance at the site of contact between mother and child, i.e., the decidua, is of critical importance. Thus, whereas effective immunity must be maintained to protect the mother from harmful pathogens, no immune response should occur against fetal antigens. Notably, because the fetus represents a semiallograft, the process of pregnancy must also include mechanisms capable of preventing allograft rejection (1).
A particular T cell subset, represented by regulatory T cells (Tregs), proved to be expanded during early pregnancy (2). Tregs are thought to play a central role in tempering immunity and in maintaining immune homeostasis (3). Different Treg subpopulations have been described, including naturally occurring Tregs that arise from the thymus (4, 5) and adaptive Tregs that develop in the periphery (6–8). The development of Tregs requires the transcription factor Forkhead box P3 (FOXP3), which currently represents their most specific marker (9). The secretion of inhibitory cytokines and the contact-dependent inhibition are among the identified mechanisms of Treg-mediated suppression (10, 11). Interestingly, several recent observations have suggested that they may exert a protective role when the maternal tissues come into contact with fetal antigens expressed by invading placental trophoblast cells (8).
A possible mediator involved in the establishment of maternal–fetal tolerance is represented by indoleamine 2,3-dioxygenase (IDO) (12). IDO is generally absent or inactive in cells involved in the immune response, but can be induced in macrophages and in some dendritic cells (DC) by cytokines, primarily IFN-γ (13), or by the CTLA-4–dependent pathway (14). IDO has been shown to promote peripheral immune tolerance by inhibiting T cell activation and proliferation through tryptophan catabolism (15–17). Notably, the initial evidence that IDO can mediate immunosuppression in vivo was obtained in studies focused on the maternal–fetal interface. Thus, inhibition of IDO activity by the competitive inhibitor 1-methyl-tryptophan (1MT) resulted in fetus rejection (12). Although several evidences suggest that both the presence of Tregs and the induction of IDO activity could significantly contribute to tolerance toward the fetus, limited information is presently available on how Tregs and IDO activity may be induced and regulated in decidual tissues. A possible candidate for this role is represented by decidual myelomonocytic CD14+ (dCD14+) cells because these cells may actively interact with T cells and, upon appropriate stimulation, produce IDO (18, 19). Previous studies indicated that these cells expressed CD206 (20), CD209 (considered a typical M2 marker) (21), and low levels of the costimulatory molecules CD80 and CD86, whereas they were negative for CD1a. Notably, in the presence of GM-CSF and IL4, dCD14+ cells did not differentiate into DC (21). Regulatory function could also be exerted by decidual NK (dNK) cells. During the first trimester of pregnancy these cells constitute the large majority of the lymphoid cells present in the decidua (1, 22). Recent studies revealed that, at this stage of gestation, dNK cells play an important role in regulating trophoblastic growth, differentiation, and invasion (23, 24). Remarkably, histochemical analysis revealed that dNK cells can reside in close association with dCD14+ cells (25), a condition that may result in functional crosstalk between the two cell types (22, 26). Thus, upon interaction with dCD14+ cells, dNK cells may undergo activation and induction of their functional program.
In the present study, we analyze the result of the interaction between dNK and dCD14+ cells. We show that the crosstalk between these two cell types results in selective expansion of Tregs.
Results
CD4+CD25highFOXP3+ T Cells Are Generated in Culture as a Result of the Crosstalk Between Myelomonocytic dCD14+ Cells and dNK Cells.
In these experiments, we investigated whether the interaction between CD14+ cells and NK cells isolated from decidual tissues had any effect on T cell responses. Freshly isolated dCD14+ cells (see phenotype in Fig. S1) were cultured with Carboxyfluorescein succinimidyl ester (CFSE)-labeled allogeneic peripheral blood T lymphocytes in the presence or in the absence of dNK or peripheral blood NK (pNK) cells. T cell proliferation occurred in response to the allogeneic stimulus. The phenotype of proliferating T cells was assessed by cytofluorimetric analysis for CFSE staining intensity and surface expression of CD4 and CD8β. As shown in Fig. 1A (at day 7 of culture), the percentage of proliferating T cells was similar in the different coculture conditions. In contrast, the CD4+/CD8+ cell ratio was markedly different. Thus, in cocultures of T cells with dCD14+ cells alone or in combination with pNK cells, the percentages of proliferating CD4+ T cells were similar to or even lower than that of proliferating CD8+ T cells. In contrast, in culture combinations containing dCD14+ cells and dNK cells, CD4+ greatly outnumbered CD8+ T cells. On the other hand, neither dNK nor pNK cells alone could induce T cell proliferation. These experiments were repeated using dCD14+ cells isolated from 15 different donors. In all instances, the CD4+/CD8+ proliferating T cell ratio was significantly higher in T/dCD14+/dNK cell cocultures as compared with T/dCD14+/pNK or T/dCD14+ cell cocultures (Fig. 1B).
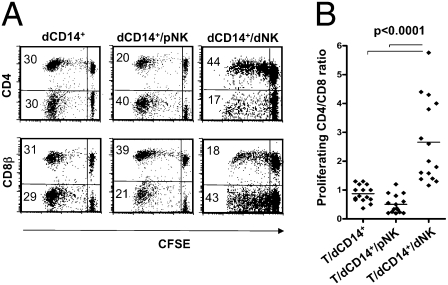
Effect of dCD14+ cells cocultured with dNK or pNK cells on T cell proliferation. (A) CD4 and CD8β surface expression in CFSE-labeled T cells cocultured (for 7 d) with dCD14+, dCD14+/pNK, or dCD14+/dNK cells. Analysis was performed by gating on the lymphocyte fraction according to the FSC and SSC parameters. CFSE-negative lymphocytes (i.e., NK cells) were excluded from the analysis. Percentages of proliferating cells are indicated. A shows a representative experiment. (B) Similar results were obtained by analyzing dCD14+ and dNK cells derived from 15 different donors: a statistical analysis of the CD4+/CD8+ proliferating T-cell ratio under different coculture conditions is shown.
Given the high proportion of proliferating CD4+ T cells detected in T/dCD14+/dNK cell cocultures, we investigated whether these increases reflected the preferential expansion of given T cell subsets, namely Tregs. Tregs are thought to play a relevant regulatory role during pregnancy. Human CD4+ Tregs are characterized by the expression of the CD25highFOXP3+ phenotype (7). They also express glucocorticoid-induced TNF receptor (GITR) (7), CD39 (27), CTLA-4 (14), low levels of IL-7Rα (CD127) (7), and CD49d (28). The Treg phenotype was assessed in proliferating CD4+ cells by using specific monoclonal antibodies (mAbs). Fig. 2A shows the results of a representative experiment (of six performed). T cell populations that had been cocultured either with dCD14+ cells alone or in combination with pNK cells contained low percentages of CD4+CD25high cells. In contrast, large proportions of CD4+CD25high cells were detected in T cells cocultured with dCD14+ and dNK cells. In addition, staining with anti-FOXP3 mAb was mostly confined to proliferating T cells cocultured with dCD14+ and dNK cells, whereas only low percentages of FOXP3+ cells were present under the other coculture conditions. These results (obtained in 10 independent experiments) were statistically significant (Fig. 2B). CD4+ T cells derived from cocultures with dCD14+ alone or with dCD14+/pNK cells expressed high levels of CD127 and CD49d. On the other hand, CD4+ T cells obtained from dCD14+/dNK cell cocultures expressed high levels of GITR, CD39, and CTLA-4 and did not express or expressed low levels of CD127 and CD49d (Fig. 2C).
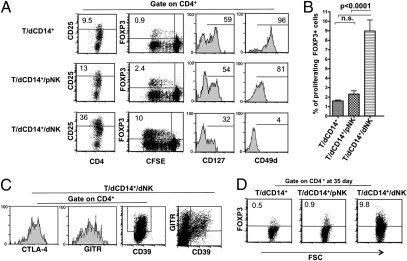
dCD14+/dNK cell interaction induces Tregs. (A) T cells cocultured with the indicated cell combinations were analyzed (at day 7) for the surface expression of CD25, CD127, and CD49d and for the intracellular expression of FOXP3. Two- or three-color immunofluorescence analyses were performed to evaluate cell proliferation and expression of the indicated markers. A shows a representative experiment of six performed. (B) The expression of FOXP3 on proliferating T cells was evaluated in 10 different experiments (i.e., by using pNK, dNK, and dCD14+ cells derived from 10 different donors). Bars indicate the percentage mean (±SEM) of proliferating FOXP3+ T cells assessed in the indicated coculture conditions (n.s., not significant). (C) T cells cocultured with dCD14+/dNK cells were analyzed (at day 7) for the expression of CD39, GITR, and CTLA-4. Two- or three-color immunofluorescence analyses were performed to evaluate the expression of the indicated markers. C shows a representative experiment of three performed. (D) T cells were cocultured as indicated for 35 d and analyzed for the intracellular expression of FOXP3. D shows a representative experiment of three performed.
In T cells cultured with dCD14+/dNK cells, FOXP3 expression was maintained after a long culture interval (35 d) (Fig. 2D). These data are in line with previous studies (28) showing that Tregs maintain FOXP3 expression over time.
CD4+FOXP3+ T Cells Induced in dCD14+/dNK Cell Cocultures Exhibit Suppressive Capability.
To verify whether CD4+FOXP3+ T cells generated in vitro upon coculture with dCD14+ and dNK cells could indeed exert a regulatory function, they were tested for their capability of suppressing T cell proliferation induced by anti-CD3 mAb or Mixed Lymphocyte Reaction (MLR) (Materials and Methods). Purified allogeneic T cells labeled with CFSE were used as responders. As shown in Fig. 3, CD4+ T cells isolated from dCD14+/dNK cell cocultures strongly inhibited proliferation of CFSE-labeled T cells stimulated with anti-CD3 mAb. A similar inhibition was detected when CD4+ T cells were added to T cells responding in MLR (Fig. S2). In contrast, CD4+ T cells isolated from dCD14+/pNK cell cocultures had no inhibitory effect.
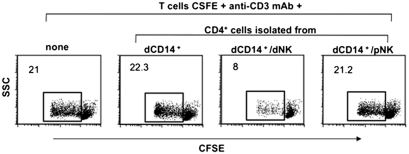
CD4+ T cells isolated from dCD14+/dNK cell cocultures exhibit suppressive capacity on T cell proliferation. CD4+ T cells isolated from the indicated coculture combinations were added to anti-CD3 mAb-stimulated allogeneic CFSE-labeled T cells (1:2 inhibitor:responder ratio) to assess their ability to inhibit T-cell proliferation. The percentages of proliferating cells are indicated. Data are representative of three independent experiments. To exclude dead cells, the experiments were performed in the presence of 7AAD.
Role of IFN-γ and IDO in the Crosstalk Between dNK Cells and dCD14+ Cells and in the Induction of Tregs.
To assess whether the dCD14+/dNK cell crosstalk could involve soluble factors, coculture experiments were performed in transwell cultures (Fig. 4A). As shown in Fig. 4B, dCD14+ cells that had been cultured in transwell with a combination of dNK and dCD14+ cells were able to induce T cells with inhibitory activity on anti-CD3 mAb-induced T cell proliferation. Other transwell coculture combinations (such as dCD14+ cells cultured in transwell with pNK and dCD14+ or with dNK cells alone) had no effect. These data indicate that the ability of dCD14+ cells to induce Tregs is promoted by soluble mediators specifically released upon interaction between dNK cells and dCD14+ cells. Previous studies showed that IDO (which can be induced by IFN-γ) could play a relevant role in the maintenance of pregnancy (12, 13). To explore the possible role of IDO and IFN-γ in our experimental setting, cocultures were set up using the IDO inhibitor 1MT or an anti-IFN-γ–neutralizing mAb. In these experiments, CFSE-labeled T cells were cultured for 7 d with dCD14+/dNK cells in the absence or in the presence of 1MT or anti-IFN-γ mAb and analyzed for cell proliferation and expression of CD4, CD25, and FOXP3. Figure 4C shows a representative experiment (of five performed). The proportions of proliferating T cells were similar in the various coculture combinations. However, there was a substantial difference in the percentages of cells expressing CD4. Thus, in the presence of 1MT or anti-IFN-γ mAb, the CD4+/CD4− ratio was decreased compared with that detected in the absence of the inhibitors. In addition, in the presence of the inhibitors, CD4+ T cells expressed low percentages of CD25highFOXP3+ cells (Fig. 4D). That IFN-γ could be directly responsible for conditioning dCD14+ cells was suggested by experiments in which these cells were cultured for 3 d with IFN-γ. Such IFN-γ–conditioned dCD14+ cells could induce FOXP3+CD25high Tregs with a strong inhibitory activity on T cell proliferation (Fig. S3). To obtain further evidence for the role of IFN-γ and IDO in conditioning dCD14+ cells and in Treg induction, IDO mRNA expression was analyzed in dCD14+ cells that were either freshly isolated or exposed overnight to IFN-γ. As shown in Fig. 5A, no IDO mRNA could be detected in fresh dCD14+ cells. However, after incubation with IFN-γ at different concentrations (1–100 IU/mL), dCD14+ cells expressed high IDO mRNA levels. The fact that freshly isolated dCD14+ did not contain IDO mRNA despite their close proximity with dNK cells could reflect a rapid reversibility of IDO induction. Notably, isolation and purification of dCD14+ cells required several steps. Figure 5B shows that IDO mRNA was induced in dCD14+ cells upon transwell coculture with dCD14+/dNK cells. However, it was completely lost after dCD14+ cells were cultured alone for 24 h.

IFN-γ and IDO production during dNK/dCD14+ cell interaction and involvement in Treg induction. The role of factors produced during dCD14+/dNK crosstalk in the induction of Tregs was analyzed in the transwell culture system depicted in A. (B) T cells, isolated from cultures in which dCD14+TW cells were conditioned by dNK + dCD14+ or by pNK + dCD14+ cells, were added to anti-CD3 mAb–stimulated allogeneic CFSE-labeled T cells. Their inhibitory capability was analyzed in a 5-d culture assay. T cells were added at a 1:2 (inhibitor:responder) ratio. The percentage of proliferating CFSE-labeled T cells is indicated. Data are representative of three independent experiments. (C) T cells were cocultured for 7 d with dCD14+ and dNK cells in the absence or in the presence of 1MT or anti-IFN-γ mAb. T cells were analyzed for surface expression of CD4. Percentages of proliferating cells are indicated. (D) Cells cultured as in C were analyzed by two- or three-color immunofluorescence by gating on CD4+ cells for the expression of CD25 and FOXP3. Similar results were obtained in five different experiments. To exclude dead cells, the experiments were performed in the presence of 7AAD.
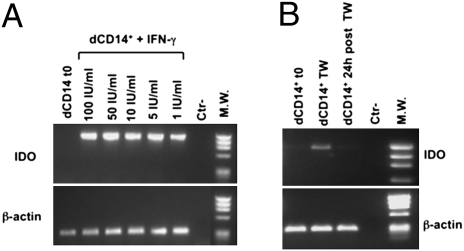
IDO mRNA induction in dCD14+ cells following treatment with IFN-γ or transwell coculture with dCD14+/dNK cells. (A) IDO mRNA expression was analyzed in freshly isolated dCD14+ cells or in dCD14+ cells incubated for 14 h with the indicated IFN-γ concentrations. (B) Freshly isolated dCD14+ cells that had been conditioned in transwell cocultures with dCD14+/dNK cells (Fig. 4A) or that were further cultured alone for 24 h were analyzed for IDO mRNA expression. RT–PCR was performed with primers specific for IDO and for β-actin as positive control. PCR products were run on a 0.8% agarose gel and visualized by ethidium bromide staining. Similar results were obtained in five independent experiments.
IDO-Induced Tryptophan Catabolite L-Kynurenine Does Not Affect dNK Cell Phenotype and Function.
Recent studies have shown that IDO, by generating tryptophan catabolites (in particular L-kynurenine), can inhibit NK cell function, including cytokine production (29, 30). Because IDO expressed by dCD14+ cells could interfere with the production of IFN-γ by dNK cells, freshly isolated dNK cells were cultured either in the absence or in the presence of L-kynurenine for 2 d and then analyzed for IFN-γ production. As shown in Fig. 6A, L-kynurenine did not affect the ability of dNK cells to release IFN-γ in response to mAb-mediated cross-linking of the activating NK receptor NKp46. In agreement with these results, no down-regulation of NKp46, NKp30, and NKG2D activating receptors occurred in dNK cells cultured with L-kynurenine (Fig. 6B). In contrast, a marked modulation was observed in CD56dimCD16+ pNK cells, i.e., in the largely predominant peripheral blood NK subset. Interestingly, CD56brightCD16− pNK cells displayed only a partial modulation of NKp46 but not of NKG2D (Fig. 6B) (29, 31). Our results indicate that at variance with pNK, dNK cells may be resistant to the inhibitory effect of IDO.
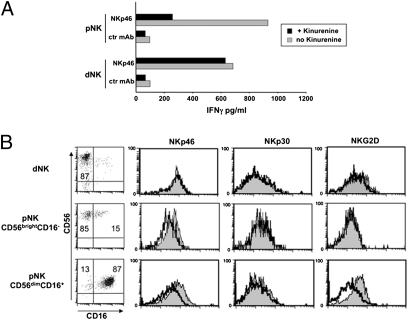
Effect of L-kynurenine on dNK and pNK cell function and phenotype and involvement of TGF-β in the induction of Tregs. (A) Freshly isolated NK cells were cultured for 2 d in the presence of IL-2 either alone or in combination with L-kynurenine and then stimulated with anti-NKp46 mAb (or with anti-CD56 mAb as negative control). Supernatants were assessed for their IFN-γ content by ELISA. (B) Treated (black line) or untreated (shaded profile) dNK and pNK cell subsets (CD56brightCD16− and CD56dimCD16+) were analyzed for the surface expression of NKp46, NKp30, and NKG2D receptors.
TGF-β and CTLA-4 Participate in the dCD14+ Cell-Mediated Induction of Tregs
Previous studies indicated that tolerogenic DC (32, 33) isolated from tumors (18, 34) or certain normal tissues (e.g., gut) (19) induce Tregs via the production of TGF-β (10). Thus, we analyzed the inhibitory activity of conditioned dCD14+ cells on allogeneic T cell proliferation in the presence or in the absence of an anti-TGF-β neutralizing Ab. As shown in Fig. 7, cultures in the presence of anti-TGF-β Ab contained low percentages of CD25highFOXP3+ T cells. In addition, these T cells could not mediate substantial inhibition of T cell proliferation in MLR (Fig. S3). These data indicate that TGF-β, together with IDO, may play a substantial role in the induction of Tregs by dCD14+ cells interacting with dNK cells.
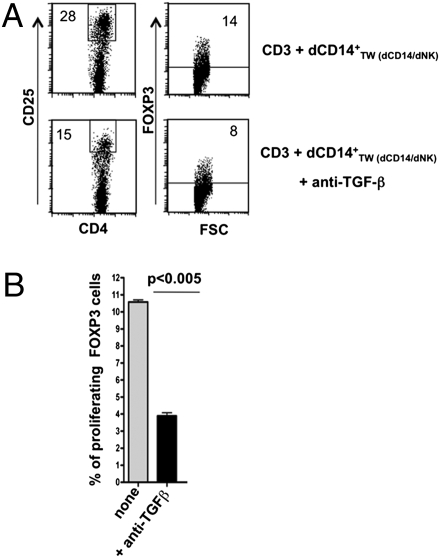
Involvement of TGF-β in the induction of Tregs. (A) T cells that had been cocultured with conditioned dCD14+ TW(dCD14/dNK) in the absence or in the presence of anti-TGF-β mAb were analyzed at day 7 for the expression of CD25 and FOXP3. A shows a representative experiment of three experiments performed. (B) Statistical analysis of three different experiments. Bars indicate the percentage mean (±SEM) of proliferating FOXP3+ T cells assessed in the indicated coculture conditions.
We further explored additional molecular mechanisms that might be involved in the induction of IDO in dCD14+ cells. In this context, previous studies indicated that CTLA-4, expressed on Tregs, induced IDO expression in DC (14, 35). Therefore, we analyzed the expression of CTLA-4 on Tregs generated in our experimental setting (Fig. 2C) and the effect of a neutralizing anti-CTLA-4 mAb on Treg-mediated suppression. As shown in Fig. S4A, a CTLA-4 blockade resulted in a partial restoration of T cell proliferation. Because PDL1 and PDL2 (expressed on DC) are involved in the functional interaction between DC and Tregs (35), we further analyzed whether these molecules were expressed in dCD14+ cells. As shown in Fig. S1, dCD14+ expressed PDL1, but not PDL2. However, as illustrated in Fig. S4B, addition of anti-PDL1 neutralizing mAb did not restore T cell proliferation.
Discussion
In the present study, we provide experimental evidence that a remarkable crosstalk may occur in decidual tissues between two different cell types of innate immunity, which results in the induction of Tregs. Tregs are thought to play a central role in the control of maternal immune effector functions and in preventing fetal rejection (2). In addition, we identified soluble mediators and molecular mechanisms involved in different steps of the process leading to Treg induction. Thus, the interaction between dNK cells and myelomonocytic dCD14+ cells resulted in the production of IFN-γ, which, in turn, promoted IDO expression in dCD14+ cells. Such “conditioned” dCD14+ cells acquired the ability to induce Tregs by a mechanism that involved IDO and TGF-β. Notably, only dNK cells (but not pNK cells) were effective in conditioning dCD14+ cells. This may be explained by the resistance of dNK cells to the antiproliferative and proapoptotic activity of IDO-induced L-kynurenine. In this context, dNK cells substantially differ from pNK cells that are sharply inhibited by L-kynurenine. This effect is primarily related to down-modulation of major activating NK receptors (NKp46 and NKG2D). The resistance of dNK cells to the damping effects of IDO metabolites implies that they can maintain a sustained production of IFN-γ. In turn, IFN-γ induces IDO in dCD14+ cells that promote Treg generation and immunosuppression. In addition, dNK cells also secrete IL-8, VEGF, and SDF-1, thus contributing to tissue remodeling and/or neoangiogenesis (see below) (22, 23). dNK cells, when cocultured with CD14+ cells isolated from peripheral blood, did not induce Tregs. Thus, it appears that both NK and CD14+ cells isolated from decidual tissues display the unique capability of promoting immunosuppression via induction of Tregs as a result of their crosstalk.
Our present data are in line with the concept that decidua-associated NK cells represent a defined NK cell subpopulation with unique phenotypic and functional properties. Indeed, dNK cells express the CD56brightCD16−KIR+ surface phenotype (1, 36), thus differing from both CD56dimCD16+ pNK cells (37, 38) and CD56brightCD16−KIR− NK cells present in different tissues and, in low proportions, in peripheral blood (39, 40). However, it is still unclear whether dNK cells (as well as dCD14+ cells; see below) represent peculiar maturational stages or have been conditioned by the decidual microenvironment. In this context, previous studies indicated that NK cell function may be greatly susceptible to exposure to polarizing cytokines (41–43).
Although dNK cells express normal levels of the major activating NK receptors (26, 44), as well as abundant perforin and granzyme-containing cytoplasmic granules (45), they display a poor ability to kill various targets, including trophoblast cells (23, 46). This had been correlated with their poor ability to form appropriate immunological synapses (47) or with the expression of the inhibitory form of 2B4 (26, 48) or of inhibitory NK receptors specific for HLA-E (CD94/NKG2A), HLA-G (KIR2DL4), or HLA-C (KIR2DL1/2/3) (48–50). Notably, these are the only HLA class I molecules expressed by human trophoblasts (HLA-C expression being limited to the first trimester) (1). It has also been proposed that the combination of maternal KIR genotype and fetal HLA-C, resulting in excessive NK cell inhibition, could be associated with a higher risk of pre-eclampsia due to inappropriate NK cell–trophoblast interactions and inadequate spiral artery formation (39). In this context, dNK cells, by producing a unique pattern of chemokines and proangiogenic factors, are thought to play a central role in inducing trophoblast growth and invasion and an efficient neoangiogenesis (22). Thus, rather than playing a predominant defensive cytolytic function, dNK cells appear to promote/regulate the events leading to building and remodeling of placental tissues (22, 23). Because, as revealed by the present study, dNK cells may play a central role in inducing Tregs upon selective crosstalk with myelomonocytic dCD14+ cells, it is conceivable that an impaired dNK cell function also may contribute to pregnancy failure by interfering with the induction of Tregs.
dCD14+ cells are distinct from conventional CD14+ monocytes/macrophages present in peripheral blood because they express CD206 (20), DC-SIGN (CD209) (21, 25), CD86, CD206, and PDL1 (35). They also differ from DC in the absence of CD1a and CD83. Limited information exists on the functional properties of these cells. In this study, we show that dCD14+ cells do not constitutively express IDO, which can be induced upon their interaction with dNK cells. In agreement with previous reports (16, 51, 52), IDO may be responsible for the induction of Tregs in the decidua. Indeed, the generation of Tregs is inhibited in the presence of 1MT, an IDO inhibitor. In addition, we show that induction of Tregs may be mediated also by TGF-β released by “conditioned” dCD14+ cells. Interestingly, the ability of these cells to produce TGF-β and to mediate immunosuppression is reminiscent of the previously described effect of CD14+ myeloid suppressive cells (33), which were shown to inhibit the immune response to tumor (18, 53), primarily by inducing Tregs. Treg generation was also inhibited by the blockade of CTLA-4. We show that CTLA-4 also is expressed in Tregs generated in our experimental setting and that Tregs are partially inhibited in their suppressive function in the presence of a neutralizing anti-CTLA-4 mAb. On the other hand, neutralizing mAbs specific for PDL1 had no effect. In our experimental setting, Treg generation and proliferation were induced using allogeneic T cells (8). It is notable that during pregnancy the fetus behaves as a semiallograft for maternal T cells, which are thus exposed to an allogeneic stimulus similar to our experimental conditions. In addition, in agreement with previous reports, our study shows that Treg generation is mediated by factors (i.e., IDO and TGF-β) that exert their effect irrespective of HLA barriers.
In conclusion, our present study provides substantial information for a better understanding of the cellular and molecular mechanisms involved in the induction and maintenance of tolerance during physiological pregnancy. Indeed, both decidua-associated CD14+ cells and NK cells appear to play an important and integrated role in the induction of Tregs. In turn, Tregs are thought to prevent the generation of maternal alloreactive T cells that are considered a major cause of immuno-mediated miscarriages (54). In this context, previous studies showed that, in spontaneous abortion during the first trimester, dNK cell populations were reduced, whereas among decidual T cell populations the proportion of CD8+ cells was substantially increased (54, 55). Therefore, our study supports the notion that, in decidual tissues, NK cells may play a central role not only in trophoblast building and remodeling (22, 23), but also in modulating maternal immune responses. Accordingly, it is possible to speculate that defects in NK cell generation and/or function in the decidua may be responsible for fetal losses. Our data might also provide clues to better understand mechanisms of tumor-associated immunosuppression and suggest approaches to inducing tolerance in transplantation.
Materials and Methods
Monoclonal Antibodies and Cytofluorimetric Analysis.
The following mAbs were kindly provided by D. Pende or A. Moretta: c218 (IgG1, anti-CD56), BAB281 (IgG1, anti-NKp46), Z231 (IgG1, anti-NKp44), AZ20 (IgG1, anti-NKp30), and BAT221 (IgG1, anti-NKG2D). Anti-CD56-Cy5, anti-CD4-Cy5, anti-CD8β-Cy5, anti-CD86, anti-CD83, anti-CD80, anti-CD1a, anti-HLA-DR (Immunotech), anti-CD3-PE, anti-CD16-FITC, anti-CD127, anti-CD14, anti-CD49d, anti-CTLA-4, anti-CD39, anti-CD206, anti-CD209 (BD PharMingen), anti-CD25-PE, anti-GITR (Miltenyi Biotech), anti-FOXP3-PE, anti-PDL1, anti-PDL2, anti-CTLA-4 (eBioscience) and 7-amino-actinomycin D (7AAD) (Sigma) were also used. Cell staining and cytofluorimetric analysis were performed as previously described (26). For analysis of cells derived from different cocultures, T or NK cells were gated on the basis of Forward Scatter (FSC) and Side Scatter (SSC) parameters and confirmed by the assessment of CD3 or CD56 expression. To assess proliferation, T cells were labeled with the green fluorescent dye CFSE as described (28).
Isolation and Culture of Cell Populations.
Peripheral blood lymphocytes were isolated and assessed for informative markers by cytofluorimetric analysis as previously described (26). Decidua samples were obtained at 9–12 wk of gestation from singleton pregnancies of mothers requesting termination of the pregnancy for social reasons or who were undergoing evacuation of retained products of conception following spontaneous pregnancy failure. The relevant institutional review boards approved the study, and all patients gave their written informed consent according to the Declaration of Helsinki. Cells were isolated from decidual tissue with GentleMacs (Miltenyi Biotec) and analyzed as described in a previous report (28).
Cell Cultures.
Mixture of pNK or dNK cells and dCD14+ cells were cultured for 7 d with allogeneic peripheral blood T cells in the combinations indicated in the text (T/NK/dCD14+ ratio: 10/5/1). dNK/dCD14+ or pNK/dCD14+ cell cocultures were performed in an allogeneic setting. Cells were seeded in U-bottom 96-well plates (Corning Life Sciences) and cultured in RPMI + 10% FCS without cytokines. When indicated, cocultures were performed in the presence of 1 mM 1MT (Sigma-Aldrich) or saturating amounts of anti-IFN-γ blocking mAb, chicken anti-TGF-β neutralizing Ab (both from R&D System), anti-CTLA-4 neutralizing Ab, or anti-PD1/PDL blocking mAbs (eBioscience). In some experiments, transwell inserts (EuroClone) were used to separate NK + dCD14+ from dCD14+. Transwell cultures were continued for 3 d. The “conditioned” dCD14+ (dCD14+TW) cells were then cultured for 7 d with allogeneic T cells. At the end, T cells were analyzed for informative Treg markers and for their ability to inhibit T-cell proliferation. To analyze the effect of L-kynurenine on NK cells, freshly derived peripheral and decidua NK cells were seeded in U-bottom 96-well plates at 2 × 105 cells/well and cultured in RPMI + 10% FCS. When indicated, 300 U/mL IL-2 (Proleukin; Chiron) and/or 0.5 mM L-kynurenine were added at the onset of the culture. After 3 d, cells were harvested, assessed for viability (by trypan blue staining), and assessed by cytofluorimetric analysis or cytokine production assay.
Functional Assays.
For the suppression assay, CD4+ T cells were isolated from T/dCD14+/NK cell cocultures and cultured again with freshly isolated allogeneic CFSE-labeled T cells (1:2 ratio) stimulated with anti-CD3 mAb in flat-bottom 96-well microtiter plates precoated with goat anti-mouse IgG or in MLR. MLR, using CFSE-labeled T cells, was performed as described above. Controls included CFSE-labeled T cells without suppressor cells. T cell proliferation was analyzed at day 5 (CFSE staining intensity). To exclude dead cells, the experiments were performed in the presence of 7AAD. The IFN-γ production assay by NK cells was performed as previously described (23).
RT–PCR Analysis.
Total RNA was extracted from dCD14+ cells purified as described above, either freshly derived or following overnight incubation with IFN-γ (from 100 to 1 IU/mL) using RNAeasy micro kit (Qiagen). In another set of experiments, total RNA was extracted from freshly isolated dCD14+ cells that had been conditioned in Tw cocultures with dCD14+/dNK cells that were further cultured alone for 24 h. Oligo(dT)-primed cDNA was prepared by standard technique using Transcriptor (Roche). PCR amplifications were performed with the following primers: β-actin up 5′ ACTCCATCATGAAGTGTGACG; β-actin dw 5′ CATACTCCTGCTTGCTGATCC; IDO ORF up 5′ GACTACAAGAATGGCACACG; and IDO ORF dw 5′ AATGTGCTCTTGTTGGGTTAC. Amplifications were performed for 30 cycles (30 s at 95 °C, 30 s at 58 °C, 1 min at 68 °C) using Platinum TAQ (Invitrogen). PCR products (249 bp fragment for β-actin and 1244 bp for IDO) were run on a 0.8% agarose gel and visualized by ethidium bromide staining.
Acknowledgments
This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro; Fondazione Cassa di Risparmio di Genova (CARIGE) (Agreement 2006.1051-53); Ministero dell'Istruzione, dell'Università e della Ricerca-Fondo per gli Investimenti della Ricerca di Base (MIUR-FIRB 2003) Project RBLA039LSF (to L.M.); MIUR-Progetti di Ricerca di Interesse Nazionale (MIUR-PRIN 2007) Project 20077NFBH8_005 (to M.C.M); Ministero del Lavoro, della Salute e delle Politiche Socali (RF2006-Ricerca Oncologica-Project of Integrated Program 2006–08, Agreement Ricerca Oncologica (RO) strategici 3/07) (to L.M.); Agreement Ricerca Oncologica (RO) strategici 8/07 (to M.C.M.); and Ministero della Salute, Progetto Strategico (RFPS-2007-4-633146, to M.C.M.). P.V. is a recipient of a fellowship awarded by Fondazione Italiana per la Ricerca sul Cancro.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/10.1073/pnas.1001749107/-/DCSupplemental.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.1001749107
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2900704?pdf=render
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/reprint/107/26/11918.pdf
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/abstract/107/26/11918
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/full/107/26/11918
Citations & impact
Impact metrics
Article citations
Compromised C3b-VSIG4 axis between decidual NK cells and macrophages contributes to recurrent spontaneous abortion.
J Transl Med, 22(1):1017, 11 Nov 2024
Cited by: 0 articles | PMID: 39529122 | PMCID: PMC11556194
Microbial Gatekeepers of Fertility in the Female Reproductive Microbiome of Cattle.
Int J Mol Sci, 25(20):10923, 10 Oct 2024
Cited by: 0 articles | PMID: 39456706 | PMCID: PMC11507627
Review Free full text in Europe PMC
Mechanism and application of immune interventions in intracerebral haemorrhage.
Expert Rev Mol Med, 26:e22, 08 Oct 2024
Cited by: 0 articles | PMID: 39375846 | PMCID: PMC11488334
Review Free full text in Europe PMC
Beyond CAR-T: The rise of CAR-NK cell therapy in asthma immunotherapy.
J Transl Med, 22(1):736, 05 Aug 2024
Cited by: 0 articles | PMID: 39103889 | PMCID: PMC11302387
Review Free full text in Europe PMC
Beyond Immune Balance: The Pivotal Role of Decidual Regulatory T Cells in Unexplained Recurrent Spontaneous Abortion.
J Inflamm Res, 17:2697-2710, 01 May 2024
Cited by: 1 article | PMID: 38707955 | PMCID: PMC11070170
Review Free full text in Europe PMC
Go to all (144) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Natural killer cells in human pregnancy.
J Reprod Immunol, 97(1):14-19, 01 Mar 2013
Cited by: 34 articles | PMID: 23432867
Review
Stromal cells from human decidua exert a strong inhibitory effect on NK cell function and dendritic cell differentiation.
PLoS One, 9(2):e89006, 20 Feb 2014
Cited by: 46 articles | PMID: 24586479 | PMCID: PMC3930605
Effect of Indoleamine 2,3-Dioxygenase Expressed in HTR-8/SVneo Cells on Decidual NK Cell Cytotoxicity.
Am J Reprod Immunol, 75(5):519-528, 18 Jan 2016
Cited by: 15 articles | PMID: 26782048





