Abstract
Background
During pathology of the nervous system, increased extracellular ATP acts both as a cytotoxic factor and pro-inflammatory mediator through P2X(7) receptors. In animal models of amyotrophic lateral sclerosis (ALS), astrocytes expressing superoxide dismutase 1 (SOD1G93A) mutations display a neuroinflammatory phenotype and contribute to disease progression and motor neuron death. Here we studied the role of extracellular ATP acting through P2X(7) receptors as an initiator of a neurotoxic phenotype that leads to astrocyte-mediated motor neuron death in non-transgenic and SOD1G93A astrocytes.Methods
We evaluated motor neuron survival after co-culture with SOD1G93A or non-transgenic astrocytes pretreated with agents known to modulate ATP release or P2X(7) receptor. We also characterized astrocyte proliferation and extracellular ATP degradation.Results
Repeated stimulation by ATP or the P2X(7)-selective agonist BzATP caused astrocytes to become neurotoxic, inducing death of motor neurons. Involvement of P2X(7) receptor was further confirmed by Brilliant blue G inhibition of ATP and BzATP effects. In SOD1G93A astrocyte cultures, pharmacological inhibition of P2X(7) receptor or increased extracellular ATP degradation with the enzyme apyrase was sufficient to completely abolish their toxicity towards motor neurons. SOD1G93A astrocytes also displayed increased ATP-dependent proliferation and a basal increase in extracellular ATP degradation.Conclusions
Here we found that P2X(7) receptor activation in spinal cord astrocytes initiated a neurotoxic phenotype that leads to motor neuron death. Remarkably, the neurotoxic phenotype of SOD1G93A astrocytes depended upon basal activation the P2X(7) receptor. Thus, pharmacological inhibition of P2X(7) receptor might reduce neuroinflammation in ALS through astrocytes.Free full text

Extracellular ATP and the P2X7 receptor in astrocyte-mediated motor neuron death: implications for amyotrophic lateral sclerosis
Abstract
Background
During pathology of the nervous system, increased extracellular ATP acts both as a cytotoxic factor and pro-inflammatory mediator through P2X7 receptors. In animal models of amyotrophic lateral sclerosis (ALS), astrocytes expressing superoxide dismutase 1 (SOD1G93A) mutations display a neuroinflammatory phenotype and contribute to disease progression and motor neuron death. Here we studied the role of extracellular ATP acting through P2X7 receptors as an initiator of a neurotoxic phenotype that leads to astrocyte-mediated motor neuron death in non-transgenic and SOD1G93A astrocytes.
Methods
We evaluated motor neuron survival after co-culture with SOD1G93A or non-transgenic astrocytes pretreated with agents known to modulate ATP release or P2X7 receptor. We also characterized astrocyte proliferation and extracellular ATP degradation.
Results
Repeated stimulation by ATP or the P2X7-selective agonist BzATP caused astrocytes to become neurotoxic, inducing death of motor neurons. Involvement of P2X7 receptor was further confirmed by Brilliant blue G inhibition of ATP and BzATP effects. In SOD1G93A astrocyte cultures, pharmacological inhibition of P2X7 receptor or increased extracellular ATP degradation with the enzyme apyrase was sufficient to completely abolish their toxicity towards motor neurons. SOD1G93A astrocytes also displayed increased ATP-dependent proliferation and a basal increase in extracellular ATP degradation.
Conclusions
Here we found that P2X7 receptor activation in spinal cord astrocytes initiated a neurotoxic phenotype that leads to motor neuron death. Remarkably, the neurotoxic phenotype of SOD1G93A astrocytes depended upon basal activation the P2X7 receptor. Thus, pharmacological inhibition of P2X7 receptor might reduce neuroinflammation in ALS through astrocytes.
Background
Amyotrophic lateral sclerosis (ALS) is characterized by the progressive degeneration of motor neurons in the spinal cord, brainstem and motor cortex, leading to respiratory failure and death of affected patients within a few years of diagnosis [1]. The discovery of mutations in the gene encoding the antioxidant enzyme Cu/Zn superoxide dismutase-1 (SOD1) in a subset of patients with familial ALS has led to the development of transgenic animal models expressing different SOD1 mutations [2]. These animal models recapitulate the human disease, exhibiting aberrant oxidative chemistry [3,4], neuroinflammation [5], endoplasmic reticulum stress [6], glutamate excitotoxicity [7], mitochondrial dysfunction [8] and protein misfolding and aggregation [9]. However, the mechanisms behind motor neuron death are unknown.
Accumulating evidence indicates that non-neuronal cells contribute to motor neuron dysfunction and death in ALS by the maintenance of a chronic inflammatory response [10-12]. Activated microglia accumulate in the spinal cord, producing inflammatory mediators and reactive oxygen and nitrogen species [11]. Astrocytes, the most abundant cells in the adult nervous system, also become reactive and display inflammatory features [12,13]. Remarkably, astrocytes carrying SOD1 mutations release soluble factors that selectively induce the death of motor neurons [14-18]. Astrocytes carrying the SOD1G93A mutation display mitochondrial dysfunction, increased nitric oxide and superoxide production and altered cytokine liberation profile [14,17,19-22]. Thus, SOD1 mutation causes astrocytes to display a neurotoxic phenotype dependent on autocrine/paracrine pro-inflammatory signaling and increased oxidative and nitrative stress [14,19,23].
In the central nervous system, extracellular adenosine-5'-triphosphate (ATP) has physiological roles in neurotransmission, glial communication, neurite outgrowth and proliferation [24]. Extracellular ATP levels markedly increase in the nervous system in response to ischemia, trauma and inflammatory insults [25-28]. In these cases, ATP is a potent immunomodulator regulating the activation, migration, phagocytosis and release of pro-inflammatory factors in immune and glial cells.
Extracellular ATP effects are mediated by metabotropic (P2Y) and ionotropic (P2X) receptors, both widely expressed in the nervous system [24]. The P2X7 receptor (P2X7r) is a ligand-gated cation channel that elicits a robust increase in intracellular calcium [29]. Of all P2 receptors, P2X7r has the highest EC50 (>100 μM) for ATP. The high extracellular concentrations of ATP needed to activate P2X7r are most likely to arise under pathological conditions. In the normal rodent brain, P2X7r expression in astrocytes is generally low, but quickly upregulated in response to brain injury or pro-inflammatory stimulation in cell culture conditions [30-32]. In astrocytes, P2X7r activation can potentiate pro-inflammatory signaling, as it enhances IL-1β-induced activation of NF-κB and AP-1, leading to increased production of nitric oxide as well as increased production of the chemokines MCP-1 and IL-8 [33,34].
Inhibition of P2X7r and other P2X receptors is neuroprotective in animal models of experimental autoimmune encephalomyelitis and Alzheimer's and Huntington's disease [35-37]. In addition, P2X7r mediates motor neuron death after traumatic spinal cord injury, and systemic inhibition in vivo protects motor neurons and promotes functional recovery [25,38]. In ALS patients as well as SOD1G93A animals, increased immunoreactivity for P2X7r has been found in spinal cord microglia [39,40]. Furthermore, SOD1G93A microglia in culture display an increased sensitivity to ATP, and P2X7r activation drives a pro-inflammatory activation that leads to decreased survival of neuronal cell lines [41].
Despite the recognized detrimental role of extracellular ATP and P2X7r signaling during nervous system pathology, little is known about its effects on astrocytes or its possible role in ALS. We investigated whether ATP acting through P2X7r could trigger a neurotoxic transformation of astrocytes leading to motor neuron death. We also explored whether ATP signaling in SOD1G93A astrocytes is involved in the maintenance of their neurotoxic phenotype towards motor neurons.
Methods
Chemicals and reagents
Cell culture media and reagents, 5-bromo-2-deoxyuridine (BrdU), primary antibody against BrdU and secondary antibodies were purchased from Invitrogen (Life Technologies). The Malachite Green Phosphate Assay kit was purchased from Cayman Chemical. All other reagents were from Sigma.
Animals
Procedures using laboratory animals were in accordance with the international guidelines for the use of live animals and were approved by the Institutional Animal Care Organization of the School of Medicine, Universidad de la República (Uruguay) and by the Oregon State University IACUC.
Primary astrocyte cultures
Astrocytes were prepared from spinal cords of 1 day old rat pups as previously described [42]. Astrocytes were plated at a density of 2 × 104 cells/cm2 and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, HEPES (3.6 g/L), penicillin (100 IU/mL) and streptomycin (100 μg/mL). Monolayers were >98% pure as determined by GFAP immunoreactivity and devoid of OX42-positive microglial cells. Transgenic SOD1G93A and non-transgenic astrocytes were prepared in parallel using littermate pups previously genotyped by PCR.
Primary motor neuron cultures
Motor neurons were prepared from embryonic day 15 rat spinal cords as previously described [42,43]. Briefly, the dorsal horns of spinal cords were dissected and incubated in 0.05% trypsin for 15 minutes at 37°C, followed by mechanical dissociation. Motor neurons were then purified by centrifugation on an Optiprep cushion, followed by isolation of p75NTR expressing motor neurons by immunoaffinity selection with the IgG 192 monoclonal antibody. For co-culture experiments, astrocyte monolayers were washed twice with phosphate buffered saline (PBS) after experimental treatments and then non-transgenic motor neurons were plated on top at a density of 350 cells/cm2. Co-cultures were maintained for 48 hours in L15 medium supplemented with 0.63 mg/ml sodium bicarbonate, 5 μg/ml insulin, 0.1 mg/ml conalbumin, 0.1 mM putrescine, 30 nM sodium selenite, 20 nM progesterone, 20 mM glucose, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2% horse serum [42,43]. Pure motor neuron cultures were cultured for 48 hours on a polyornithine-laminin substrate in Neurobasal media supplemented with 2% horse serum, 25 μM L-glutamate, 25 μM 2-mercaptoethanol, 500 μM L-glutamine, and 2% B-27 supplement [42,43]. Survival was maintained by the addition of GDNF (1 ng/ml).
Astrocyte treatments
All astrocyte treatments were performed in DMEM 2% FBS for 48 hours unless otherwise stated. Stock solutions were prepared as 100× and added directly to the well after media change. Inhibitors were added 1 hour prior to subsequent treatment. As noted in Figure Figure1A,1A, to determine the time-dependency of ATP exposure, media was replenished every 48 hours and 100 μM ATP was added. Thus, astrocytes treated for one day received a single ATP addition while astrocytes treated for three and five days correspondingly received 2 and 3 ATP additions.
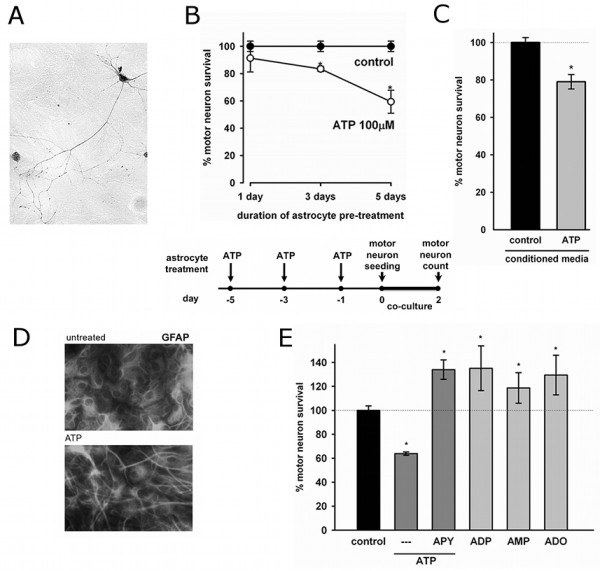
ATP induced a neurotoxic phenotype in non-transgenic astrocytes. (A) Motor neuron stained for p75NTR cultured on the top of an astrocyte monolayer (B) Motor neuron survival in coculture with astrocytes pretreated with ATP (100 μM, top graph) as described in the diagram (bottom). Astrocytes treated for 5, 3 or 1 day(s) received 3, 2 or 1 ATP addition(s) correspondingly. (C) Survival of motor neurons in pure cultures exposed to conditioned media from control or ATP-pretreated astrocytes (100 μM, 5 days, 3 additions). (D) GFAP immunofluorescence of control and ATP-treated astrocytes (100 μM, 5 days, 3 additions) (E) Motor neuron survival in co-culture with astrocytes pretreated with ATP and apyrase (5 U/ml), ADP, AMP or Adenosine (ADO, 0.1 μM, 5 days) on motor neuron survival. Data are expressed as percentage of control, mean ± SEM from at least three independent experiments. *p < 0.05, significantly different from untreated control.
Production of conditioned media and treatment of pure motor neuron cultures
To produce conditioned media, astrocytes were treated with ATP 3 times during the course of 5 days. Twenty-four hours after the last treatment, monolayers were washed 3 times with PBS and then incubated for 48 hours with Neurobasal media supplemented with 2% horse serum. Conditioned media was centrifuged to remove debris, aliquoted and stored at -80°C until use. Pure motor neuron cultures were exposed to astrocyte conditioned media 3 hours after plating by replacing 50% of their complete media with conditioned media. GDNF was then added to a final concentration of 1 ng/ml.
Motor neuron survival assessment
Motor neuron survival was assessed after 48 hours by counting all cells displaying intact neurites longer than 4 cells in diameter [42]. Counts were performed over an area of 0.9 cm2 in 24-well plates. In pure cultures, motor neurons were counted under phase contrast. In co-cultures cells were fixed, immunostained for p75NTR (Figure (Figure1A)1A) and counted [42]. In primary motor neuron cultures, the range of motor neuron death is generally limited to a subpopulation of 40 to 50% [44].
GFAP immunofluorescence
Astrocytes grown on coverslips were fixed with ice-cold 4% paraformaldehyde in PBS for 15 minutes. Cultures were permeabilized with 0.1% Triton X-100 in PBS for 15 min and blocked for 1 hour with 10% goat serum, 2% bovine serum albumin, and 0.1% Triton X-100 in PBS. Anti-GFAP monoclonal antibody diluted in blocking solution (1:400) was incubated overnight at 4°C. After washing, cultures were incubated for 1 hour at room temperature with Alexa Fluor 488-conjugated goat anti-mouse antibody (1:500). Nuclei were stained with DAPI (1 μg/mL).
Assessment of astrocyte proliferation
Confluent astrocyte monolayers were treated with apyrase for 48 hours in DMEM 2% FBS. At the end of the first 24 hours, BrdU (10 μg/mL) was added. BrdU immunofluorescence was performed as described for GFAP with the addition of a DNA denaturalization step with 1 M hydrochloric acid (30 min at room temperature) after permeabilization. Percentage of BrdU nuclei was calculated as the number of coincident BrdU and DAPI stained nuclei over the total number of DAPI-stained nuclei.
Determination of ATP degradation by phosphate measurement
To determine extracellular ATP hydrolysis, extracellular phosphate production was measured with the Malachite Green Phosphate Assay kit. After the treatment, astrocyte cultures in 24 well plates were washed 3 times with a phosphate free buffer (2 mM CaCl2, 120 mM NaCl, 5 mM KCl, 10 mM glucose, 20 mM Hepes, pH 7.4) and incubated in 500 μl with 3 mM ATP as described [45]. After 10 minutes, an aliquot of each well was removed and phosphate was immediately measured following the manufacturer's instructions.
Statistics
Each experiment was repeated at least three times and data are reported as mean ± SEM. Statistical analysis was performed by one-way analysis of variance, followed by a Student-Newman-Keuls test. Differences were declared statistically significant if p < 0.05. Statistics were performed using SigmaStat (Jandel Scientific, San Rafael, CA, USA).
Results
ATP caused non-transgenic astrocytes to induce motor neuron death
Exposure to extracellular ATP caused a neurotoxic activation of spinal cord astrocytes, which lead to death of co-cultured motor neurons in a time dependent-manner. Because ATP is quickly hydrolyzed in the extracellular media and to mimic pathological conditions with persistent ATP stimuli, we treated astrocytes repeatedly as shown in diagram in Figure Figure1B.1B. Before plating motor neurons on top, astrocyte monolayers were thoroughly washed to remove any traces of the treatment. After 2 days of co-culture, motor neuron survival was assessed. Astrocytes exposed to a single addition of ATP 24 hours before co-culture showed no significant toxicity to motor neurons (Figure (Figure1B).1B). However, astrocytes exposed to two additions of ATP (3 and 1 days before co-culture) decreased motor neuron survival by 27 ± 17%, and astrocytes treated with three ATP additions (5, 3 and 1 days before co-culture) decreased motor neuron survival by 36 ± 1.4% (Figure (Figure1B).1B). In addition, conditioned media from these astrocytes applied to purified motor neuron cultures plated on a laminin substrate induced a 20% decrease in survival (Figure (Figure1C),1C), suggesting ATP leads to the release of a diffusible factor from astrocytes able to induce motor neuron death. Immunocytochemical analysis of these astrocytes evidenced morphological changes associated with activation, displaying long and thin processes with intense GFAP immunoreactivity as compared to the typical polygonal shape of resting astrocytes (Figure (Figure1D1D).
To confirm that the effects seen on astrocytes were caused by ATP and not its degradation products ADP, AMP or adenosine (ADO), we treated astrocytes with ATP in combination with the enzyme apyrase (5 U/mL), which rapidly degrades ATP to AMP and phosphate. In this condition, the death of co-cultured motor neurons was completely prevented. Moreover, motor neuron survival increased above controls to 134 ± 8% (Figure (Figure1E).1E). Pretreatment of astrocytes directly with the products of ATP degradation ADP, AMP or adenosine (ADO) (0.1 μM added 3 times over five days as was done with ATP) caused an equivalent increase in astrocytic trophic support to motor neurons (Figure (Figure1E1E).
P2X7r activation causes astrocytes to promote motor neuron death
To investigate the role of P2X7r as an initiator of astrocyte-mediated motor neuron death, we used the preferential P2X7r agonist 2',3'-O-(4-benzoylbenzoyl)ATP (BzATP). Figure Figure2A2A shows that a 48-hour treatment of astrocytes with BzATP (10 μM) resulted in death of 30 ± 3% of co-cultured motor neurons. The effects of ATP and BzATP were prevented by the P2X7r antagonist BBG (1 μM), suggesting that P2X7r activation was required to induce the astrocyte neurotoxic phenotype (Figure (Figure2A2A).
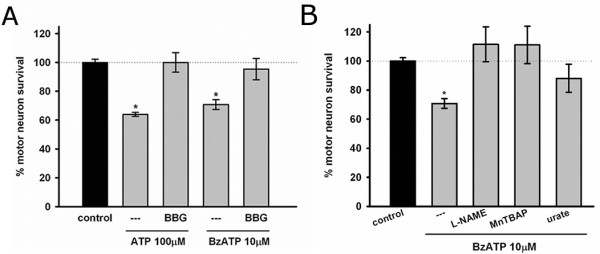
P2X7r activation triggered astrocyte-mediated neurotoxicity by inducing oxidative stress. (A) Motor neuron survival in co-culture with astrocytes pre-treated with ATP (100 μM, 5 days) or BzATP (10 μM, 48 hours) and the P2X7r inhibitor BBG (1 μM). (B) Motor neuron survival in co-culture with astrocytes pre-treated with NAME (1 mM), MnTBAP (0.1 mM) or urate (0.2 mM) and BzATP before co-culture. Data are expressed as the percentage of control, mean ± SEM from at least three independent experiments. *p < 0.05, significantly different from untreated control.
We then investigated whether the P2X7r-induced phenotypic change in astrocytes could be prevented by agents known to modulate oxidative and nitrative stress. BzATP-treated astrocytes were no longer toxic to motor neurons when the astrocytes were treated with the nitric oxide synthase inhibitor L-NAME (nitro-L-arginine methyl ester, 1 mM), the superoxide scavenger MnTBAP (Manganese (III) tetrakis (4-benzoic acid) porphyrin, 100 μM) and urate (200 μM) (Figure (Figure2B).2B). Urate efficiently scavenges peroxynitrite-derived free radicals and thereby inhibits tyrosine nitration of proteins [46,47].
Inhibition of ATP signaling in SOD1G93A astrocytes prevents astrocyte-mediated motor neuron death and cell proliferation
Consistent with previous reports [14], spinal cord astrocytes from SOD1G93A rats induced death of 37 ± 8% of co-cultured motor neurons. Remarkably, pre-incubation of SOD1G93A astrocytes with apyrase to degrade endogenous extracellular ATP for 48 hours before co-culture completely prevented motor neuron death (Figure (Figure3A).3A). Pretreatment with the P2X7r inhibitor BBG also restored motor neuron survival to non-transgenic levels (Figure (Figure3A).3A). This suggests that P2X7r could be basally activated in SOD1G93A astrocytes in an autocrine/paracrine manner, resulting in neurotoxicity to motor neurons.
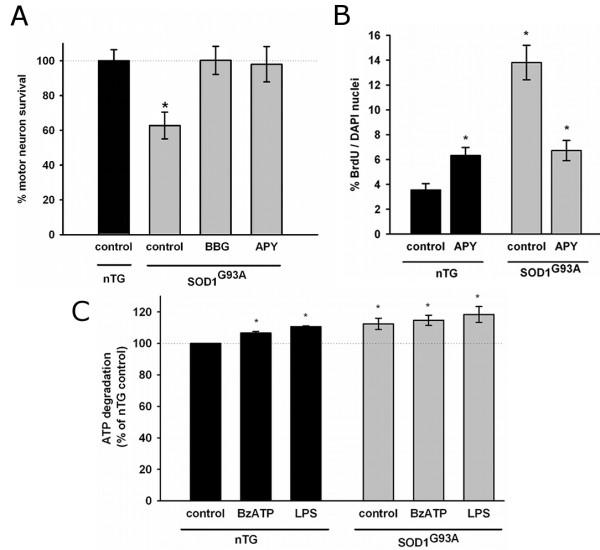
SOD1G93A astrocytes exhibit ATP-dependent neurotoxicity, proliferation, and increased ATP degradation. (A) Motor neuron survival in co-culture with SOD1G93A astrocytes pre-treated for 48 hours with the P2X7r inhibitor BBG (1 μM) or the ATP-hydrolyzing enzyme apyrase (5 U/ml) (B) Effect of apyrase treatment on SOD1G93A astrocyte proliferation in culture. (C) Degradation of exogenously added ATP by SOD1G93A or non-transgenic astrocytes astrocytes. Data are expressed as percentage of non-transgenic control, mean ± SEM from at least three independent experiments. Data are expressed as percentage of non-transgenic control, mean ± SEM from at least three independent experiments. *p < 0.05, significantly different from non-transgenic control.
Because purinergic signaling plays a key role in modulating astrocyte proliferation in pathological conditions [48,49], we assessed whether increased ATP signaling was involved in the proliferation of SOD1G93A astrocytes. Cultured SOD1G93A astrocytes showed a 4- to 5-fold increased proliferation rate as compared with non-transgenic astrocytes (Figure (Figure3B).3B). Proliferation in SOD1G93A astrocytes was decreased in half by apyrase to the same level as apyrase-treated non-transgenic astrocytes (Figure (Figure3B).3B). The small increase in proliferation of non-transgenic astrocytes caused by apyrase could be caused by generation of adenosine, which has been shown to stimulate proliferation of astrocytes [49,50].
The increase in ATP signaling observed in SOD1G93A astrocytes did not result from decreased extracellular degradation. On the contrary, ATP hydrolysis was 11% greater in SOD1G93A astrocytes (Figure (Figure3C).3C). Similarly, stimulation with LPS or BzATP induced a comparable increase in ATP degradation in non-transgenic astrocytes. In SOD1G93A astrocytes, these agents did not induce further ATP degradation.
Discussion
Extracellular ATP has become increasingly recognized to have a major role in neurodegenerative processes, but its role in astrocyte-mediated neuronal death has not been explored. Here, we found that spinal cord astrocytes assume a neurotoxic phenotype in response to extracellular ATP, leading to the induction of motor neuron death in co-cultures. Furthermore, evidence indicates that endogenous ATP stimulates SOD1G93A astrocytes in basal conditions and contributes to the maintenance of their neurotoxic phenotype.
Non-transgenic astrocytes required multiple stimuli with ATP over several days to induce the neurotoxic phenotype, while a single stimulus with the P2X7r-selective agonist BzATP was sufficient to activate astrocytes to induce the same extent of motor neuron death. BzATP is most potent as an agonist for P2X7r, but it is also a weaker agonist of P2X1r and P2X3r [51-53]. The involvement of P2X7r was further implicated in the activation of astrocyte neurotoxicity by the antagonist BBG, as it completely inhibited the action of ATP and BzATP. BBG is a selective antagonist for both P2X7r and P2X5r. [51-53]. Thus, P2X7r appears to be the most likely receptor responsible for inducing the neurotoxic phenotype in astrocytes.
We have previously shown that oxidative stress induced by superoxide and nitric oxide forming peroxynitrite in non-transgenic astrocytes leads to a neurotoxic phenotype [19,42]. Here we found that oxidative stress induced by BzATP stimulation mediated the transition of non-transgenic astrocytes to a neurotoxic phenotype, as NOS inhibitors as well as superoxide and peroxynitrite scavengers prevented their neurotoxicity towards motor neurons. In a similar way, Skaper et al showed that P2X7r activation in microglia stimulated peroxynitrite production and led to death of co-cultured neurons [54]. Thus, amplification of oxidative stress by P2X7r signaling in microglia and astrocytes could lead to the generation of an adverse environment for vulnerable neurons during neurodegenerative processes.
Because SOD1G93A astrocytes in culture display a neurotoxic phenotype that is maintained by chronic oxidative stress and autocrine pro-inflammatory signaling [14,17,19,20,22], we investigated whether they also presented alterations in extracellular ATP signaling. Indeed, our results indicate that SOD1G93A astrocytes display basally augmented extracellular ATP signaling as evidenced by an ATP-dependent neurotoxic phenotype, increased ATP-dependent proliferation, and increased extracellular ATP metabolism. Thus, ATP emerges as an extracellular factor that could chronically maintain the SOD1G93A astrocyte aberrant phenotype in an autocrine/paracrine manner.
We found that SOD1G93A astrocytes degraded ATP faster than non-transgenic astrocytes, ruling out that their basal alteration in ATP signaling could be caused by a decrease in its extracellular degradation, thereby allowing ATP to accumulate near receptors. An increase in ATP degradation could also be induced in non-transgenic astrocytes exposed to BzATP or LPS. We have previously shown that LPS induces a neurotoxic phenotype in astrocytes, leading to motor neuron death [42]. Increased ATP degradation and/or ectonucleotidase upregulation has been previously described in neural tissue after cortical stab wound and acute ischemia [55,56]. This phenomenon might reflect a cellular attempt to prevent over-activation of purinergic receptors during increases in extracellular ATP, thus promoting the return of extracellular ATP signaling to homeostasis.
Degradation of ATP by ectonucleotidases cannot only terminate deleterious ATP signaling, but also initiates ADP and adenosine signaling through P2Y and P1 receptors. To our surprise, in non-transgenic astrocytes, ATP degraded with apyrase, ADP, AMP, or adenosine led to ~35% more motor neuron attachment and survival compared to untreated controls. Because survival is determined 48 hours after plating of the motor neurons freshly isolated from spinal cords, any treatment that increases attachment of motor neurons will result in an increase of motor neuron survival above the untreated control. These results illustrate how the astrocyte phenotype can be modulated from toxic to highly trophic by changing the balance between ATP, ADP and adenosine signaling through P2X, P2Y or adenosine receptors.
In animal models of ALS, proliferative activated astrocytes interact with microglia to accelerate disease progression [57]. Remarkably, we found that modulating ATP signaling in SOD1G93A astrocytes with apyrase or BBG blocked their neurotoxic phenotype, completely preventing astrocyte-mediated death of motor neurons. A role for ATP and P2X7r in the SOD1G93A model was recently proposed by D'Ambrosi et al [41], who showed that SOD1G93A microglia are sensitized to BzATP activation. A combination of aberrant ATP signaling in astrocytes and microglia could generate a positive feedback loop driving a sustained inflammatory response in the spinal cord. The results presented here and the findings in SOD1G93A microglia [41] suggest that P2X7r inhibition in ALS could slow disease progression by decreasing astrocyte and microglial activation.
Taken together, the present work supports the idea that extracellular ATP acting through P2X7r causes astrocytes to develop a neurotoxic phenotype. In SOD1G93A astrocytes evidence suggests that P2X7r is basally activated and contribute to their toxicity towards motor neurons. Thus, modulation of astrocyte P2X7r during disease could lead to decreased oxidative stress and inflammatory signaling and in turn the switch to a more trophic phenotype towards neurons. A better understanding of ATP and P2X7r signaling in astrocytes could contribute to the development of novel protective therapies in ALS and other neurodegenerative diseases where astrocytes are involved.
Authors' contributions
MG, PC, LB participated in the design of the study. MG, HP and PC prepared astrocyte and motor neuron cultures and co-cultures. MG collected the co-culture data and carried out all other experiments. All authors reviewed the data and contributed to the preparation of the manuscript. All authors have read and approved the final manuscript.
Acknowledgements
We wish to thank the Cell Biology Unit at Institut Pasteur Montevideo for providing cell culture and microscopy facilities, Verónica Abudara and Mauricio Garré for providing reagents, Mark Levy for critical reading of this manuscript and Laura Martínez Palma, Raquel Castellanos, Pablo Díaz-Amarilla and Andrés de Leon for excellent technical help and support. This work was financially supported in part by funding from the National Institute of Health grants NS058628, AT002034 and ES00240 and by Comision Sectorial de Investigacion Cientifica (CSIC, Principal investigator Patricia Cassina).
References
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. 10.1056/NEJM200105313442207. [Abstract] [CrossRef] [Google Scholar]
- Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. [Abstract] [Google Scholar]
- Beckman JS, Estevez AG, Crow JP, Barbeito L. Superoxide dismutase and the death of motoneurons in ALS. Trends Neurosci. 2001;24:S15–20. 10.1016/S0166-2236(00)01981-0. [Abstract] [CrossRef] [Google Scholar]
- Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. [Europe PMC free article] [Abstract] [Google Scholar]
- Papadimitriou D, Le Verche V, Jacquier A, Ikiz B, Przedborski S, Re DB. Inflammation in ALS and SMA: Sorting out the good from the evil. Neurobiol Dis. 2009. [Europe PMC free article] [Abstract]
- Kikuchi H, Almer G, Yamashita S, Guegan C, Nagai M, Xu Z, Sosunov AA, McKhann GM, Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc Natl Acad Sci USA. 2006;103:6025–6030. 10.1073/pnas.0509227103. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch BL, Coyle JT. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28:18–25. 10.1002/ana.410280106. [Abstract] [CrossRef] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. 10.1016/0896-6273(95)90259-7. [Abstract] [CrossRef] [Google Scholar]
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. 10.1126/science.281.5384.1851. [Abstract] [CrossRef] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–72. 10.1083/jcb.200908164. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004;1035:104–116. 10.1196/annals.1332.007. [Abstract] [CrossRef] [Google Scholar]
- Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev. 2004;47:263–274. 10.1016/j.brainresrev.2004.05.003. [Abstract] [CrossRef] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. 10.1146/annurev.neuro.27.070203.144244. [Abstract] [CrossRef] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Beckman JS, Barbeito L. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem. 2006;97:687–696. 10.1111/j.1471-4159.2006.03742.x. [Abstract] [CrossRef] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. 10.1016/j.stem.2008.09.017. [Abstract] [CrossRef] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. 10.1038/nn1885. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. 10.1016/j.stem.2008.10.001. [Abstract] [CrossRef] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. 10.1038/nn1876. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de Leon A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28:4115–4122. 10.1523/JNEUROSCI.5308-07.2008. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cassina P, Pehar M, Vargas MR, Castellanos R, Barbeito AG, Estevez AG, Thompson JA, Beckman JS, Barbeito L. Astrocyte activation by fibroblast growth factor-1 and motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2005;93:38–46. 10.1111/j.1471-4159.2004.02984.x. [Abstract] [CrossRef] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Martinez-Palma L, Thompson JA, Beckman JS, Barbeito L. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J Biol Chem. 2005;280:25571–25579. 10.1074/jbc.M501920200. [Abstract] [CrossRef] [Google Scholar]
- Hensley K, Abdel-Moaty H, Hunter J, Mhatre M, Mou S, Nguyen K, Potapova T, Pye QN, Qi M, Rice H, Stewart C, Stroukoff K, West M. Primary glia expressing the G93A-SOD1 mutation present a neuroinflammatory phenotype and provide a cellular system for studies of glial inflammation. J Neuroinflammation. 2006;3:2. 10.1186/1742-2094-3-2. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pehar M, Vargas MR, Robinson KM, Cassina P, England P, Beckman JS, Alzari PM, Barbeito L. Peroxynitrite transforms nerve growth factor into an apoptotic factor for motor neurons. Free Radic Biol Med. 2006;41:1632–1644. 10.1016/j.freeradbiomed.2006.08.010. [Abstract] [CrossRef] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. 10.1038/nrd2605. [Abstract] [CrossRef] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. 10.1038/nm1082. [Abstract] [CrossRef] [Google Scholar]
- Phillis JW, O'Regan MH, Perkins LM. Adenosine 5'-triphosphate release from the normoxic and hypoxic in vivo rat cerebral cortex. Neurosci Lett. 1993;151:94–96. 10.1016/0304-3940(93)90054-O. [Abstract] [CrossRef] [Google Scholar]
- Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47:442–448. 10.1016/j.neuint.2005.05.014. [Abstract] [CrossRef] [Google Scholar]
- Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. 10.1073/pnas.0709684105. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. [Abstract] [Google Scholar]
- Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. [Abstract] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. 10.1523/JNEUROSCI.3404-07.2007. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49:245–258. 10.1002/glia.20110. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- John GR, Simpson JE, Woodroofe MN, Lee SC, Brosnan CF. Extracellular nucleotides differentially regulate interleukin-1beta signaling in primary human astrocytes: implications for inflammatory gene expression. J Neurosci. 2001;21:4134–4142. [Europe PMC free article] [Abstract] [Google Scholar]
- Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci. 2001;21:7135–7142. [Europe PMC free article] [Abstract] [Google Scholar]
- Ryu JK, McLarnon JG. Block of purinergic P2X(7) receptor is neuroprotective in an animal model of Alzheimer's disease. Neuroreport. 2008;19:1715–1719. 10.1097/WNR.0b013e3283179333. [Abstract] [CrossRef] [Google Scholar]
- Matute C, Torre I, Perez-Cerda F, Perez-Samartin A, Alberdi E, Etxebarria E, Arranz AM, Ravid R, Rodriguez-Antiguedad A, Sanchez-Gomez M, Domercq M. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci. 2007;27:9525–9533. 10.1523/JNEUROSCI.0579-07.2007. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Diaz-Hernandez M, Diez-Zaera M, Sanchez-Nogueiro J, Gomez-Villafuertes R, Canals JM, Alberch J, Miras-Portugal MT, Lucas JJ. Altered P2X7-receptor level and function in mouse models of Huntington's disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23:1893–1906. 10.1096/fj.08-122275. [Abstract] [CrossRef] [Google Scholar]
- Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA. 2009;106:12489–12493. 10.1073/pnas.0902531106. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. 10.1186/1471-2377-6-12. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Casanovas A, Hernandez S, Tarabal O, Rossello J, Esquerda JE. Strong P2X4 purinergic receptor-like immunoreactivity is selectively associated with degenerating neurons in transgenic rodent models of amyotrophic lateral sclerosis. J Comp Neurol. 2008;506:75–92. 10.1002/cne.21527. [Abstract] [CrossRef] [Google Scholar]
- D'Ambrosi N, Finocchi P, Apolloni S, Cozzolino M, Ferri A, Padovano V, Pietrini G, Carri MT, Volonte C. The proinflammatory action of microglial P2 receptors is enhanced in SOD1 models for amyotrophic lateral sclerosis. J Immunol. 2009;183:4648–4656. 10.4049/jimmunol.0901212. [Abstract] [CrossRef] [Google Scholar]
- Cassina P, Peluffo H, Pehar M, Martinez-Palma L, Ressia A, Beckman JS, Estevez AG, Barbeito L. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J Neurosci Res. 2002;67:21–29. 10.1002/jnr.10107. [Abstract] [CrossRef] [Google Scholar]
- Henderson CE, Bloch-Gallego E, Camu W. Purification and culture of embryonic motor neurons. Oxford: IRL Press; 1995. [Google Scholar]
- Estevez AG, Sahawneh MA, Lange PS, Bae N, Egea M, Ratan RR. Arginase 1 regulation of nitric oxide production is key to survival of trophic factor-deprived motor neurons. J Neurosci. 2006;26:8512–8516. 10.1523/JNEUROSCI.0728-06.2006. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wink MR, Braganhol E, Tamajusuku AS, Casali EA, Karl J, Barreto-Chaves ML, Sarkis JJ, Battastini AM. Extracellular adenine nucleotides metabolism in astrocyte cultures from different brain regions. Neurochem Int. 2003;43:621–628. 10.1016/S0197-0186(03)00094-9. [Abstract] [CrossRef] [Google Scholar]
- Teng RJ, Ye YZ, Parks DA, Beckman JS. Urate produced during hypoxia protects heart proteins from peroxynitrite-mediated protein nitration. Free Radic Biol Med. 2002;33:1243–1249. 10.1016/S0891-5849(02)01020-1. [Abstract] [CrossRef] [Google Scholar]
- Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999;372:285–294. 10.1006/abbi.1999.1491. [Abstract] [CrossRef] [Google Scholar]
- Neary JT, Kang Y. Signaling from P2 nucleotide receptors to protein kinase cascades induced by CNS injury: implications for reactive gliosis and neurodegeneration. Mol Neurobiol. 2005;31:95–103. 10.1385/MN:31:1-3:095. [Abstract] [CrossRef] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Kim JK, Gysbers JW, DeForge SP, Smith RW, Hughes DW. Adenosine and its nucleotides stimulate proliferation of chick astrocytes and human astrocytoma cells. Neurosci Res. 1992;13:1–17. 10.1016/0168-0102(92)90030-G. [Abstract] [CrossRef] [Google Scholar]
- Ciccarelli R, Di Iorio P, D'Alimonte I, Giuliani P, Florio T, Caciagli F, Middlemiss PJ, Rathbone MP. Cultured astrocyte proliferation induced by extracellular guanosine involves endogenous adenosine and is raised by the co-presence of microglia. Glia. 2000;29:202–211. 10.1002/(SICI)1098-1136(20000201)29:3<202::AID-GLIA2>3.0.CO;2-C. [Abstract] [CrossRef] [Google Scholar]
- Cotrina ML, Nedergaard M. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signal. 2009;5:223–232. 10.1007/s11302-009-9138-2. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Surprenant A. Functional properties of native and cloned P2X receptors. Ciba Found Symp. 1996;198:208–219. discussion 219-222. [Abstract] [Google Scholar]
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. 10.1016/S0014-2999(99)00350-7. [Abstract] [CrossRef] [Google Scholar]
- Skaper SD, Facci L, Culbert AA, Evans NA, Chessell I, Davis JB, Richardson JC. P2X(7) receptors on microglial cells mediate injury to cortical neurons in vitro. Glia. 2006;54:234–242. 10.1002/glia.20379. [Abstract] [CrossRef] [Google Scholar]
- Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci. 1998;18:4891–4900. [Europe PMC free article] [Abstract] [Google Scholar]
- Nedeljkovic N, Bjelobaba I, Lavrnja I, Stojkov D, Pekovic S, Rakic L, Stojiljkovic M. Early temporal changes in ecto-nucleotidase activity after cortical stab injury in rat. Neurochem Res. 2008;33:873–879. 10.1007/s11064-007-9529-0. [Abstract] [CrossRef] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. 10.1038/nn2047. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Articles from Journal of Neuroinflammation are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/1742-2094-7-33
Read article for free, from open access legal sources, via Unpaywall:
https://jneuroinflammation.biomedcentral.com/track/pdf/10.1186/1742-2094-7-33
HAL Open Archive
http://hal-pasteur.archives-ouvertes.fr/pasteur-00684666
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1186/1742-2094-7-33
Article citations
A Pro-Inflammatory Stimulus versus Extensive Passaging of DITNC1 Astrocyte Cultures as Models to Study Astrogliosis.
Int J Mol Sci, 25(17):9454, 30 Aug 2024
Cited by: 0 articles | PMID: 39273404 | PMCID: PMC11394751
Unlocking the therapeutic potential of P2X7 receptor: a comprehensive review of its role in neurodegenerative disorders.
Front Pharmacol, 15:1450704, 30 Jul 2024
Cited by: 1 article | PMID: 39139642 | PMCID: PMC11319138
Review Free full text in Europe PMC
Molecular hallmarks of ageing in amyotrophic lateral sclerosis.
Cell Mol Life Sci, 81(1):111, 02 Mar 2024
Cited by: 1 article | PMID: 38430277 | PMCID: PMC10908642
Review Free full text in Europe PMC
Role and therapeutic targets of P2X7 receptors in neurodegenerative diseases.
Front Immunol, 15:1345625, 02 Feb 2024
Cited by: 2 articles | PMID: 38370420 | PMCID: PMC10869479
Review Free full text in Europe PMC
The Key Role of Astrocytes in Amyotrophic Lateral Sclerosis and Their Commitment to Glutamate Excitotoxicity.
Int J Mol Sci, 24(20):15430, 21 Oct 2023
Cited by: 4 articles | PMID: 37895110 | PMCID: PMC10607805
Review Free full text in Europe PMC
Go to all (95) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis.
Brain, 134(pt 9):2627-2641, 01 Sep 2011
Cited by: 132 articles | PMID: 21908873 | PMCID: PMC3170534
Motor neuron dysfunction in a mouse model of ALS: gender-dependent effect of P2X7 antagonism.
Toxicology, 311(1-2):69-77, 11 Apr 2013
Cited by: 43 articles | PMID: 23583883
The proinflammatory action of microglial P2 receptors is enhanced in SOD1 models for amyotrophic lateral sclerosis.
J Immunol, 183(7):4648-4656, 04 Sep 2009
Cited by: 78 articles | PMID: 19734218
Complexity of astrocyte-motor neuron interactions in amyotrophic lateral sclerosis.
Neurodegener Dis, 2(3-4):139-146, 01 Jan 2005
Cited by: 48 articles | PMID: 16909019
Review
Funding
Funders who supported this work.
NCCIH NIH HHS (1)
Grant ID: AT002034
NIEHS NIH HHS (1)
Grant ID: ES00240
NINDS NIH HHS (1)
Grant ID: NS058628

 1,4
1,4



