Abstract
Free full text

Effects of Thymoquinone in the Expression of Mucin 4 in Pancreatic Cancer Cells: Implications for the Development of Novel Cancer Therapies
Abstract
Pancreatic cancer is one of the most lethal cancers in the world, as it continues to be resistant to any therapeutic approaches. The high molecular weight glycoprotein mucin 4 (MUC4) is aberrantly expressed in pancreatic cancer and contributes to the regulation of differentiation, proliferation, metastasis, and the chemoresistance of pancreatic cancer cells. The absence of its expression in the normal pancreatic ductal cells makes MUC4 a promising target for novel cancer therapeutics. Natural products have been widely investigated as potential candidates in cancer therapies, and thymoquinone (TQ), extracted from the seeds of Nigella sativa, has shown excellent antineoplastic properties in some systems. In the present study, we evaluated the effect of TQ on pancreatic cancer cells and specifically investigated its effect on MUC4 expression. The MUC4-expressing pancreatic cancer cells FG/COLO357 and CD18/HPAF were incubated with TQ, and in vitro functional assays were done. The results obtained indicate that treatment with TQ downregulated MUC4 expression through the proteasomal pathway and induced apoptosis in pancreatic cancer cells by the activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase pathways. In agreement with previous studies, the decrease in MUC4 expression correlated with an increase in apoptosis, decreased motility, and decreased migration of pancreatic cancer cells. MUC4 transient silencing studies showed that c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase pathways are activated in pancreatic cancer cells, indicating that the activation of these pathways by TQ is directly related to the MUC4 downregulation induced by the drug. Overall, TQ has potential for the development of novel therapies against pancreatic cancer.
Introduction
Pancreatic cancer is a major problem worldwide and the fourth leading cause of cancer-related deaths in the United States. Treatment against this malignancy remains a major challenge in oncology as evidenced by the unchanged overall survival (<5%) of pancreatic cancer patients over the last 25 years (1, 2). Currently, chemotherapy is the only treatment option for patients with metastatic pancreatic cancer, and unfortunately, there are numerous molecular factors involved in the chemotherapeutic resistance of pancreatic cancer tumors (3). Therefore, novel therapies are desperately needed and alternative markers must be evaluated for their potential in improving the prognosis and therapy of pancreatic cancer patients.
Mucins are a family of large, heavily glycosylated proteins that are expressed in various epithelial tissues. Although there is no specific tumor marker for diagnosing pancreatic cancer, mucins have been researched as potential diagnostic candidates (4). It has been long suspected that alterations in mucin expression as well as the expression of aberrant forms of mucins contribute to the development of cancer by influencing growth, differentiation, and immune surveillance (5). In particular, mucin 4 (MUC4), a membrane-bound mucin, which consists of a mucin-type subunit (MUC4α) and a transmembrane growth factor–like subunit (MUC4β), contributes to the regulation of differentiation, proliferation, and metastasis of pancreatic cancer cells (6, 7). It has been reported that MUC4 is aberrantly expressed in precancerous pancreatic intraepithelial neoplasia lesions, and its expression increases with the progression of the disease (8). As it is not expressed in normal pancreatic ductal cells, MUC4 is a promising target for novel anticancer therapies (9). In addition to being a good candidate for targeted therapies against several tumors, we and others have shown that MUC4 is also responsible for the resistance of pancreatic cancer cells to apoptosis induced by chemotherapeutic drugs (i.e., gemcitabine, trastuzumab, and cisplatin; refs. 10–12). Therefore, a logical approach for pancreatic cancer treatment would be to target MUC4 expression in pancreatic cancer cells to overcome their intrinsic resistance to apoptosis.
Among the novel anticancer drugs that are being currently studied, natural products have emerged as promising candidates that have gained considerable attention (13). It has been well documented that the seed extracts of the plant Nigella sativa (black seed), widely used for natural remedies in the Middle East, possess multiple benefits, including antitumorigenic effects (14, 15). One of the extracted compounds that has shown promising antineoplastic properties is thymoquinone (TQ). An important characteristic of TQ is that it induces cytotoxicity and apoptosis of cancer cells, whereas nonneoplastic cells are relatively resistant to the drug (16–19). A comprehensive review of the multiple benefits of TQ provides evidence that, although no clinical studies testing TQ have been established yet, its anticancer properties are well supported on numerous in vitro and in vivo studies (14). Of particular importance is the finding that TQ was shown to be 4- to 5-fold more cytotoxic to cisplatin-resistant osteosarcoma cells (18) and equally sensitive in multidrug-resistant variants of pancreatic adenocarcinoma, uterine sarcoma, and leukemic cell lines (20) when compared with their respective parental controls. Additionally, a particular benefit of TQ is its relative nontoxicity, as it has been reported that the LD50 of TQ in mice and rats is more than 10 and 100 times the effective doses reported for intraperitoneal and oral ingestion, respectively (21).
Although few studies have evaluated the potential of TQ in pancreatic cancer therapy, some of the studies done in pancreatic cancer cells have shown that TQ possesses anti-inflammatory properties (22), reduces proliferation (23, 24), and sensitizes pancreatic cancer cells to conventional chemotherapeutic drugs (25). In the present study, we investigated the effect of TQ in MUC4-expressing pancreatic cancer cells. The pancreatic cancer cell lines FG/COLO357 and CD18/HPAF were incubated with TQ, and functional assays and mechanistic studies were done. The results indicate that TQ induced the apoptosis of pancreatic cancer cells, and specifically, the downregulation of MUC4 expression played a critical role in this process.
Materials and Methods
Drug
TQ (MP Biomedicals) was dissolved in acetone (100 mmol/L) and stored at 4°C protected from light. Subsequent dilutions were prepared in DMEM supplemented with 1% fetal bovine serum (FBS) without antibiotics immediately before adding to cell culture.
Cell culture
The metastatic FG/COLO357 and CD18/HPAF cell lines were purchased from the American Type Culture Collection and cultured in DMEM supplemented with 10% FBS and antibiotics (100 μg/mL penicillin and 100 μg/mL streptomycin). The cells were maintained at 37°C and 5% CO2 in a humidified atmosphere. All the experiments were done on FG/COLO357 cells, whereas data in the CD18/HPAF cell line are included in Supplementary Data.
Antibodies
The MUC4 monoclonal antibody (8G7) used in these studies was developed by our group (26). The antibodies phospho–stress-activated protein kinase/c-Jun NH2-terminal kinase (JNK; Thr183/Tyr185), total JNK, cleaved caspase-9 (Asp330), and caspase-3 were purchased from Cell Signaling. The antibodies Bcl-xL (H-5), Bax, phospho–signal transducer and activator of transcription 1 (STAT1; Ser727), total STAT1, phospho–focal adhesion kinase (FAK; Tyr925), total FAK, total p38, and HER2 were obtained from Santa Cruz Biotechnology. The phospho-Crk/p38 (pY221) antibody was purchased from Epitomics, and β-actin antibody was obtained from Sigma-Aldrich. The secondary antibodies used were the enhanced chemiluminescence anti-mouse and anti-rabbit IgG conjugated to horseradish peroxidase (GE Healthcare). The secondary antibody FITC-conjugated anti-mouse was obtained from Jackson Immunoresearch Labs, Inc.
Cytotoxicity assay
To determine the cytotoxicity of TQ on pancreatic cancer cells, 0.25 × 105 cells were seeded per well on a 96-well plate in DMEM supplemented with 1% FBS and no antibiotics. After overnight incubation, TQ was added at different concentrations (10–100 μmol/L). After 24 hours, the media were replaced with media containing Alamar Blue reagent (Invitrogen), and the fluorescence (λexcitation = 570 nm, λemission = 585 nm) was measured after 4 hours of incubation. Quadruplicate wells were used for each concentration of TQ, and the cytotoxicity was calculated by substituting the corresponding mean fluorescence values in the following equation:
where untreated cells refer to cells that are suspended in media alone (0 μmol/LTQ). The experiment was repeated thrice.
Western blot analysis
For protein analysis, 1 × 106 of pancreatic cancer cells were seeded on each well of a six-well plate in DMEM supplemented with 1% FBS and no antibiotics. After overnight incubation, fresh solutions of TQ (50 and 100 μmol/L) were prepared and added to the respective wells. Cells incubated with the corresponding amount of acetone present in the highest concentrated solution of TQ were used as a negative control (0 μmol/L). After 24 hours of incubation with the drug, protein lysates were isolated with radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors. Samples were stored at −80°C. After several freeze-thaw cycles, the lysates were centrifuged at 16,000 × g for 10 minutes at 4°C. The concentration of the proteins was determined with the micro–bicinchoninic acid protein estimation kit (Bio-Rad). Protein concentrations were adjusted, and corresponding solutions were prepared under reducing conditions with β-mercaptoethanol. To resolve the MUC4 protein, 20 μg of protein lysates were loaded in 2% SDS agarose gels and run for 6 hours at 100 V. For all the other functional proteins, 30 to 60 μg of protein lysates were loaded in 10% SDS-PAGE gels and run at 80 mA for 1 hour. Resolved proteins were transferred onto polyvinylidene difluoride membranes, blocked in 5% fat-free dry milk in PBS, and incubated with the primary antibodies overnight. After washing four times with PBST (PBS and 0.1% Tween), the corresponding secondary antibodies were added. After washing the secondary antibodies with PBST, the proteins were detected by luminol detection reagents (Thermo Scientific) after exposure to X-ray films. The experiment for analyzing all the functional proteins after TQ treatment was repeated a minimum of three times.
Confocal microscopy
For confocal analysis, 0.5 × 106 FG/COLO357 cells were seeded on sterilized round glass coverslips, which were placed on separate wells of a 12-well plate, and incubated overnight. After cells were attached to the coverslips, TQ (50 and 100 μmol/L) was added and cells were incubated for 24 hours. For MUC4 staining, cells were washed with HBSS and fixed with cold methanol at −20°C for 2 minutes. After washing with PBS, cells were blocked with 10% goat serum (Jackson Immunoresearch Labs) in PBST for 30 minutes. Without washing, cells were incubated with anti-MUC4 monoclonal antibody (1:100) for 1 hour at room temperature. Cells were then washed with PBST (thrice for 5 min) and FITC-conjugated anti-mouse antibody was added for 30 minutes. After repeating the washing steps, the glass coverslips were mounted on cover slides with Vectashield mounting medium (Vector Laboratories).
Fluorescent phallotoxins (Invitrogen) were used for staining actin filaments. The instructions of the manufacturer were followed for formaldehyde-fixed cells. After staining was done, glass coverslips were mounted with Vectashield medium. The laser confocal microscope LSM 510 (Carl Zeiss GmbH) was used to image the cells in the respective channels at a magnification of ×63.
Measurement of MUC4 mRNA levels
The transcripts levels of MUC4 in pancreatic cancer cells after treatment with TQ were determined qualitatively by reverse transcription-PCR (RT-PCR) and quantitatively by real-time PCR. As was done for protein lysate isolation, 1 × 106 cells were seeded on each well of a six-well plate in DMEM supplemented with 1% FBS and no antibiotics. After overnight incubation, fresh solutions of TQ (50 μmol/L) were prepared and cells were incubated for 24 hours. RNA was isolated and purified using the RNeasy Mini kit (Qiagen). The cDNA was synthesized using 2 μg RNA, oligo(dT)18 primer, and SuperScript II RNase− reverse transcriptase (Invitrogen). For RT-PCR, amplification was done for 30 cycles at an annealing temperature of 58°C. For MUC4 amplification, the primers used were 5′-CGCGGTGGTGGAGGCGTTCTT-3′ (forward) and 5′-GAAGAATCCTGACAGCCTTCA-3′ (reverse). β-Actin was used as the housekeeping gene. RT-PCR products were resolved on 2% agarose gels stained with ethidium bromide. Photographs were taken under UV light. The experiment was repeated at least twice.
For real-time PCR, 1 μL of cDNA was amplified using the LightCycler 480 SYBR Green I Master Mix (Roche Diagnostics). The instrument used for real-time PCR was the LightCycler 480 (Roche Diagnostics), and the amplification was done in a two-step cyclic process (95°C for 5 min followed by 45 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 10 s). Absolute quantification was based on a calibration curve of MUC4 and β-actin. The sequences of the MUC4-specific primers used were 5′-GTGACCATGGAGGCCAGTG-3′ (forward) and 5′-TCATGCTCAGGTGTCCACAG-3′ (reverse). The levels of MUC4 mRNA were normalized to β-actin mRNA levels. The results are represented as the fold difference of triplicate wells in the MUC4 mRNA level in treated (50 μmol/L TQ) versus untreated (0 μmol/L TQ) cells. The experiment was repeated at least thrice.
Inhibition of proteasomal and global transcription pathways
To determine if MUC4 was downregulated at the transcriptional or translational level, 1 × 106 pancreatic cancer cells were seeded overnight on each well (six-well plate) in DMEM supplemented with 1% FBS and no antibiotics. The next day, 2 hours before adding TQ solutions, the proteasomal inhibitor (PrI) MG132 (330 nmol/L; Assay Designs, Inc.) or the global gene transcription inhibitor (GGTI) garcinol (2 μmol/L; Enzo Life Sciences) was added to the respective wells. Both inhibitors were tested separately. After 2 hours, the media were replaced with fresh media containing the inhibitor and the respective concentration of TQ. The following day, the protein lysates were isolated and MUC4 expression was analyzed. Experimental samples included cells with no inhibitor, inhibitor only, TQ only, and TQ and inhibitor. The experiment was repeated twice.
Wound-healing assay
For wound-healing assays, 3 × 106 of pancreatic cancer cells were seeded in 60-mm Petri dishes in DMEM supplemented with 1% FBS and no antibiotics. After overnight incubation and obtaining confluent (>90%) cultures, a wound was induced with a sterile pipette tip. Images magnified ×4 were obtained before TQ was added. Subsequently, the corresponding concentration of TQ was added (0, 50, and 100 μmol/L). Images of wound areas were obtained after 24 hours, and the motility of the cells was compared among different treatments.
Motility assay
To determine the migration of pancreatic cancer cells as a consequence of TQ treatment, a Transwell assay was done. FG/COLO357 cells (2 × 106) were suspended in 1% FBS DMEM and seeded for 24 hours in polyethylene terephthalate (PET; Becton Dickinson) membranes (pore size, 8 μm; six-well insert) with the respective concentration of TQ (0–100 μmol/L). DMEM supplemented with 10% FBS was added to the bottom of each well of the six-well plate below the PET membrane. After 24 hours of incubation, cells in the top were removed and the cells that migrated through the membrane were stained with Diff-Quick cell staining kit (Dade Behring, Inc.). The number of cells that migrated was quantified in 10 different random fields at the same magnification (×10). The results are represented as the average number of cells in one field. The experiment was repeated thrice.
Detection and quantification of apoptosis and necrosis
The Annexin V-FLUOS staining kit (Roche Diagnostics) was used to determine the number of cells undergoing apoptosis and necrosis after being incubated with 50 μmol/L TQ. Cells (1 × 106) were seeded per well (six-well plate) in DMEM supplemented with 1% FBS and no antibiotics. After overnight incubation, TQ was added on triplicate wells and cells were incubated for 4 and 24 hours. Instructions of the manufacturer were followed for staining cells for flow cytometry and confocal analysis. Cells that were propidium iodide (PI) negative and Annexin V negative were considered healthy, cells that were PI negative and Annexin V positive were considered apoptotic, and cells that were positive to both PI and Annexin V were considered necrotic. Experiment was repeated thrice.
Microarray analysis
Human oligonucleotide array containing probes for 30,000 genes was constructed at the Microarray Core Facility of the University of Nebraska Medical Center. Total RNA was isolated from TQ-treated (50 μmol/L) and untreated (0 μmol/L) pancreatic cancer cells by RNeasy Mini kit. Spotted microarrays were used to determine differential gene expression between these two groups. The samples were competitively hybridized to three arrays. To generate the fluorescently labeled single-stranded cDNA target, total RNA (750 ng) was reverse transcribed to generate cDNA, followed by in vitro transcription to generate aminoallyl RNA using the Amino Allyl Message Amp kit (Ambion). RNA (5 μg) was coupled with either CY5 or CY3 dye as per the manufacturer's suggestion, mixed, and cohybridized (in 20 μL of Ambion hybridization buffer) to microarray slides for 16 hours at 42°C. The slides were washed per the manufacturer's suggestions and scanned using an Axon 4000b scanner to generate .tiff images. The images were extracted using GenePix software, and resultant GPR files were used for downstream analysis. Differentially expressed genes were identified using BRB Array Tools. Several filters and normalization were applied before analysis. Spots were excluded if both the red and the green channels had values <100, and if only one of the red or green channels was <100, then it was increased to the threshold of 100. Median background was subtracted and log2 transformation was applied to all ratios. Normalization was then done to “center” each array using lowess smoother, and genes were excluded if any of the spots were missing or filtered out for any of the samples. Random-variance paired t tests were used to determine which genes are differentially expressed between TQ-treated and untreated FG/COLO357 samples, comparing the log red (TQ-treated FG/COLO357; 50 μmol/L) and green (control FG/COLO357; 0 μmol/L) channel intensities. The random-variance paired t test allows sharing information among genes about variation without assuming that all genes have the same variance, which gives a more accurate estimate of the variability when sample sizes are small. A significance level of 0.001 was selected to help limit the false discovery rate due to multiple comparisons.
Transient knockdown of MUC4
To transiently knock down MUC4 on FG/COLO357 cells, 0.5 × 106 cells were seeded on each well of a six-well plate in complete media. The next day, cells were serum starved for 2 hours. The transfection reagent Lipofectamine (Invitrogen) was used. The instructions of the manufacturer were followed, and MUC4 RNAi and scramble RNAi oligonucleotides (500 pmol; Dharmacon) were added to the respective wells. The MUC4-specific sequence for the RNAi oligonucleotides used was 5′-CAGCGACACUA-GAGGGACA-3′. After 4 hours of adding the transfection solution, 0.5 mL of complete media was added to the cells. The next day, the media were replaced with fresh media and protein lysates were isolated after 24, 48, and 96 hours. The protein lysates were used for Western blot analysis. The experiment was repeated thrice. The samples from the time point where MUC4 was downregulated more significantly were used for further protein analyses (i.e., 48 h).
Results
Effect of TQ on cytotoxicity and MUC4 expression on pancreatic cancer cells
The highly metastatic pancreatic cancer cell line FG/COLO357 was incubated for 24 hours with different doses of TQ. TQ induced the cytotoxicity of FG/COLO357 cells in a dose-dependent (0–100 μmol/L) manner (Fig. 1A). The IC50 of TQ on FG/COLO357 was of 73 μmol/L after 24 hours of treatment. Further, to assess the effect of TQ on MUC4 expression, the protein lysates were analyzed by Western blot and confocal analyses. It was found that TQ downregulated the expression of MUC4 in FG/COLO357 in dose- and time-dependent manners (Fig. 1B and C). Based on these experiments, the dose and time chosen for subsequent analysis were 50 μmol/L TQ and 24 hours. Similar results were obtained in the pancreatic cancer cell line CD18/HPAF (Supplementary Fig. S1).
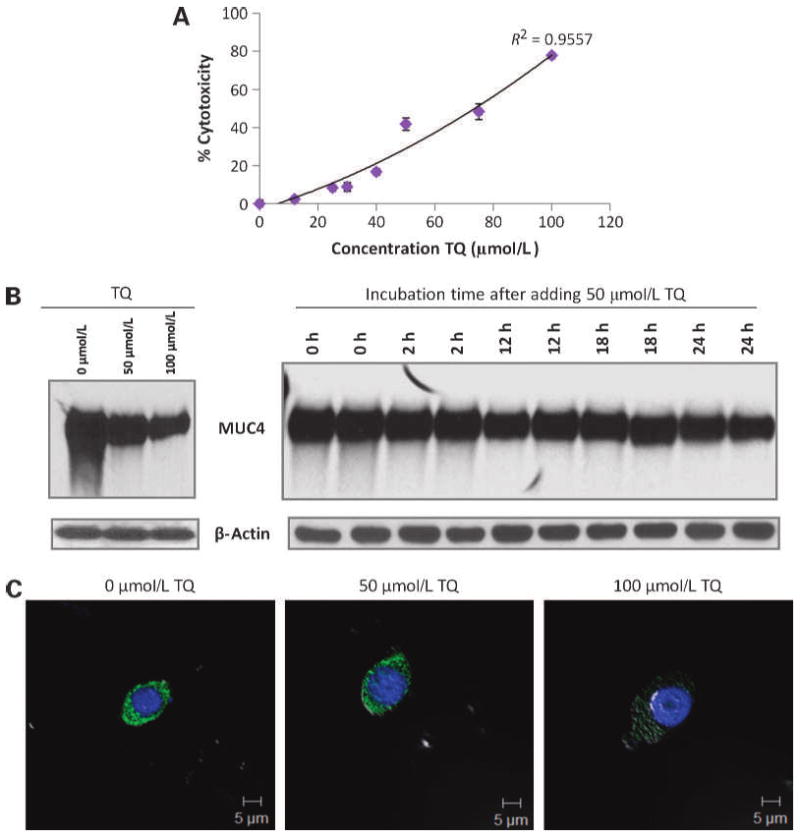
Effect of TQ in cell viability and MUC4 expression on FG/COLO357 cells. A, cytotoxicity of TQ in FG/COLO357 cells after being incubated with the drug for 24 h. Points, mean of quadruplicate values; bars, SE. B, Western blot analysis of MUC4 expression in FG/COLO357 cells after being incubated with different doses of TQ and different time intervals. Protein lysates (20 μg) were resolved on 2% SDS agarose gels. β-Actin was used as the loading control. C, confocal microscopy images of FG/COLO357 cells after being incubated with different concentrations of TQ. Cells were stained with anti-MUC4 monoclonal antibody and FITC-conjugated secondary antibody. The cell nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 5 μm.
TQ downregulates MUC4 expression in pancreatic cancer cells posttranscriptionally
As the expression of MUC4 is regulated by a complex interplay of several signaling pathways, the downregulation of MUC4 expression may require simultaneous targeting of multiple signaling mechanisms. To determine the pathway of MUC4 downregulation of TQ-treated pancreatic cancer cells, the levels of MUC4 transcripts were analyzed qualitatively and quantitatively by RT-PCR and real-time PCR, respectively (Fig. 2A and B). The levels of MUC4 mRNA transcripts on FG/COLO357 after being incubated with TQ did not varied in untreated (0 μmol/L TQ) and TQ-treated (50 μmol/L) cells. These results suggest that TQ decreased MUC4 expression on FG/COLO357 cells posttranscriptionally. Thus, subsequently, the expression of MUC4 was analyzed on cells that were preincubated with a PrI and a GGTI before adding TQ (Fig. 2C and D). The PrI MG132 blocked the effect of TQ on MUC4 downregulation, indicating that MUC4 is targeted to the proteasomal degradation pathway in the presence of TQ (Fig. 2C). To corroborate that MUC4 was indeed not regulated transcriptionally, FG/COLO357 cells were incubated with the GGTI garcinol before adding TQ (Fig. 2D). Garcinol pretreatment had no effect on MUC4 downregulation in FG/COLO357 cells by TQ, supporting PCR data where MUC4 transcript levels did not changed significantly in the presence of TQ.
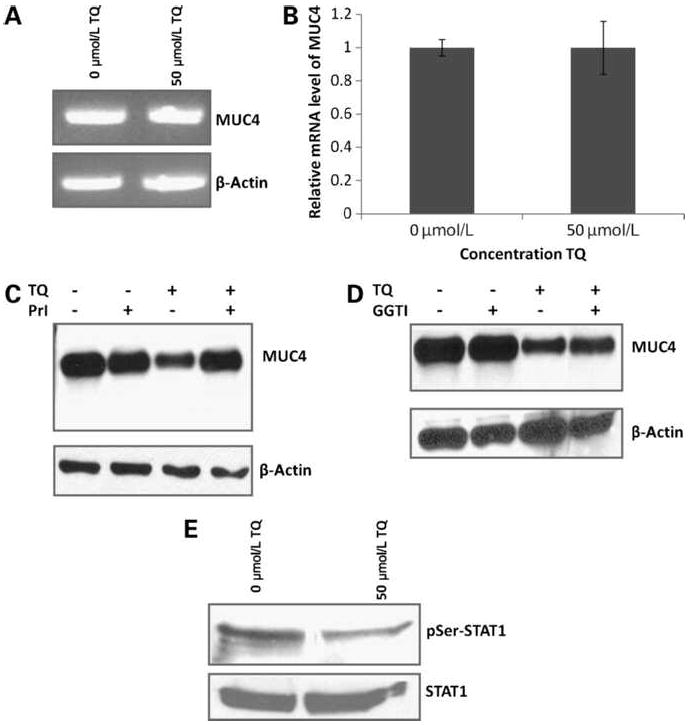
Downregulation of MUC4 expression on FG/COLO357 cells by TQ. A, measurement of MUC4 transcripts of cells incubated with TQ by RT-PCR. The housekeeping gene β-actin was used as a control. B, quantification of MUC4 transcripts of cells incubated with TQ by real-time PCR. Columns, fold difference of the MUC4 mRNA level in untreated cells (0 μmol/L) and TQ-treated cells (50 μmol/L); bars, SE. The housekeeping gene β-actin was used as an internal control. Statistical analysis was done and samples were not significantly different. C, Western blot analysis of MUC4 expression after being incubated with TQ in the presence of the PrI MG132. Experimental samples included media only, PrI only, TQ only, and PrI with TQ. Protein lysates (20 μg) were resolved in 2% SDS agarose gels. β-Actin was used as the loading control. D, Western blot analysis of MUC4 expression after being incubated with TQ in the presence of the GGTI garcinol. Experimental samples included media only, GGTI only, TQ only, and GGTI with TQ. Protein lysates (20 μg) were resolved in 2% SDS agarose gels. β-Actin was used as the loading control. E, Western blot analysis of total STAT1 and its phosphorylated form (pSer-STAT1) of cells incubated with TQ. Protein lysates (40 μg) were resolved in 10% SDS-PAGE gels.
It has been reported that the upregulation of STAT1, rather than its phosphorylation state, plays a crucial role in MUC4 induction (27). Accordingly, the effect of TQ on the expression of STAT1 in FG/COLO357 cells was evaluated (Fig. 2E). After treatment with TQ, the phosphorylation state at Ser727 of STAT1 was decreased, whereas the total STAT1 level remained unchanged. Thus, as the levels of total STAT1 protein in the pancreatic cancer cells remained constant, it is expected that the amount of MUC4 transcripts will remain constant after treatment with TQ, supporting the results obtained with RT-PCR and real-time PCR experiments. In agreement with these results, studies done in the pancreatic cancer cell line CD18/HPAF corroborate that TQ downregulates MUC4 posttranscriptionally by the proteasomal degradation pathway (Supplementary Fig. S2).
Motility of pancreatic cancer cells decreases after treatment with TQ
The migration of epithelial cells is an important step of cancer metastasis. To evaluate TQ as a potential drug in cancer therapy, its effect on the motility and invasiveness of cancer cells needs to be evaluated. The motility of pancreatic cancer cells was analyzed by wound-healing (qualitative) and Transwell assays (quantitative), and the results showed that TQ inhibited the migration of FG/COLO357 cells in a dose-dependent manner (Fig. 3A and B). The wound-healing assay clearly shows that cells in the untreated group (0 μmol/L TQ) migrated across the wound much faster than cells incubated with TQ (Fig. 3A). Similarly, the Transwell assay results indicated that the number of migrating cells decreased significantly (P < 0.005) in the presence of TQ (Fig. 3B). A decreased motility of CD18/HPAF cells in the presence of TQ was also confirmed (Supplementary Fig. S3A).
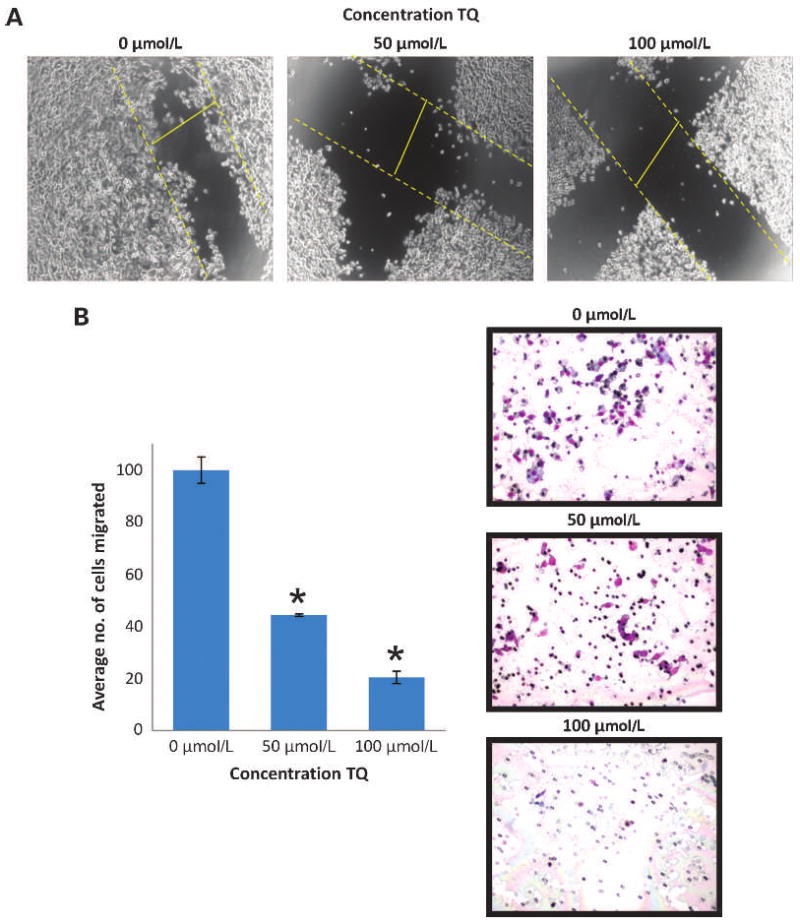
Effect of TQ on the motility and migration of FG/COLO357 cells. A, optical microscopy images (×4) of the wound-healing assay of FG/COLO357 cells after being incubated with different doses of TQ for 24 h. Dashed yellow lines indicate the migration progress of the cells in the 24-h period. B, quantification of FG/COLO357 cells that migrated through the 8-μm pore size PET membrane after being incubated with TQ. Columns, mean number of cells in 10 different random fields (×10); bars, SE. *, P < 0.005 versus control. Representative optical microscope images of the PET membranes are shown.
TQ induces rearrangement of the cytoskeleton of pancreatic cancer cells
As the motility of cells is associated with the arrangement of the cortical actin network in cancer progression (28, 29), the actin filaments on TQ-treated cells in comparison with untreated cells were analyzed by confocal microscopy after phalloidin staining. It was found that TQ-treated cells had reduced cellular projections when compared with untreated cells, suggesting that FG/COLO357 cells have a decreased motility after being incubated with TQ (Fig. 4A).
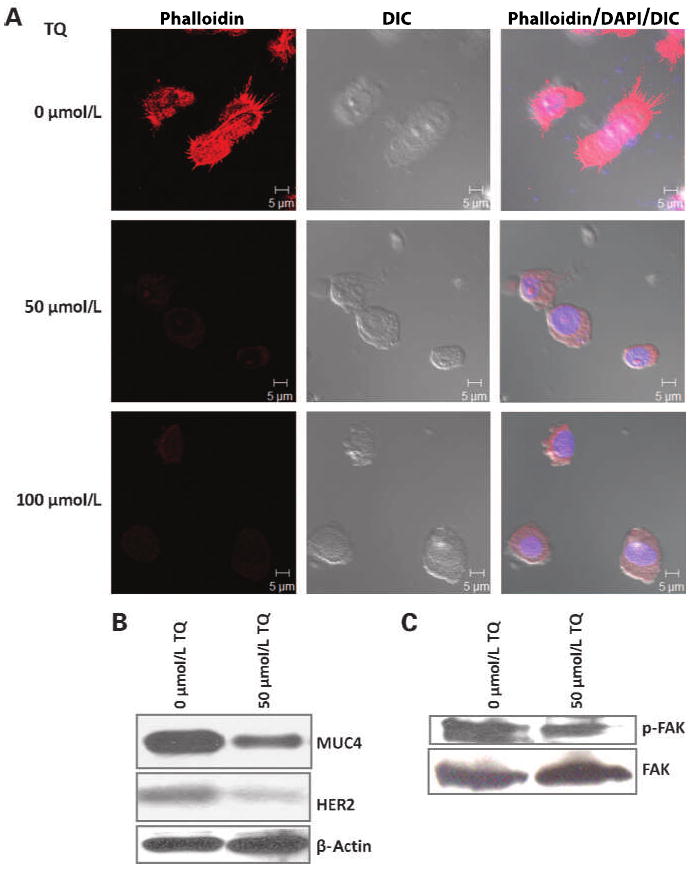
Effect of TQ on the cytoskeleton of FG/COLO357. A, confocal microscopy images of FG/COLO357 cells after being incubated with different concentrations of TQ. Actin filaments in the cells were visualized after staining with fluorescent phallotoxins. The cell nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 5 μm. B, Western blot analysis of MUC4 and HER2 expression in FG/COLO357 cells after treatment with TQ. Protein lysates (20 μg) were resolved in 2% SDS agarose gels. β-Actin was used as the loading control. C, Western blot analysis of FAK and its phosphorylated form in FG/COLO357 cells after TQ treatment. Protein lysates (40 μg) were resolved in 10% SDS-PAGE gels.
It has been reported that MUC4 colocalizes with and stabilizes the protein HER2/ErbB2 on the cell surface (30). Specifically, HER2 expression has been linked to various types of cancer and related with tumor metastasis (30–32). Not surprisingly, after incubating FG/COLO357 cells with TQ, the expression of HER2 was also decreased (Fig. 4B). These results correlated with the decrease in the phosphorylation state of FAK, which is involved in the downstream signaling of HER2 (33), after incubation with TQ (Fig. 4C). Given that FAK controls cell migration and anchorage-dependent differentiation, and is overexpressed in invasive and metastatic tumors (34), its decrease in pancreatic cancer cells after treatment with TQ indicates that the drug has potential in reducing tumor metastasis. Results were validated in the pancreatic cancer cell line CD18/HPAF (Supplementary Fig. S3B and C).
TQ induces apoptosis in pancreatic cancer cells
Resistance to apoptosis is one of the mechanisms responsible for the survival of cancer cells. To determine if pancreatic cancer cells underwent apoptosis or necrosis as a consequence of TQ treatment, these were stained with Annexin V and PI (Fig. 5A and B). The flow cytometry results showed that after 24 hours of incubation of FG/COLO357 cells with 50 μmol/L TQ, the number of apoptotic (47%) and necrotic cells (42%) was balanced, suggesting that TQ may induce cell death by means of different pathways (Fig. 5A). The results obtained with flow cytometry were corroborated by confocal microscopy (Fig. 5B).
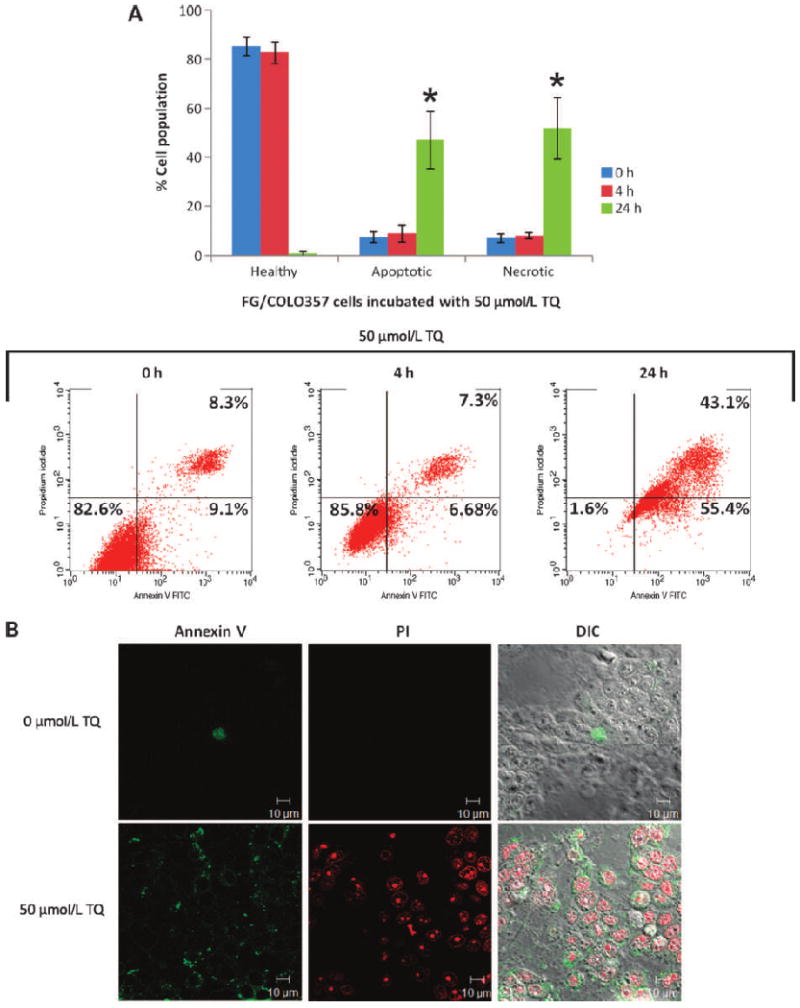
Effect of TQ (50 μmol/L) in the apoptosis and necrosis of FG/COLO357 cells. A, flow cytometry results after staining cells with Annexin V and PI. Triplicate samples were analyzed after 4 and 24 h of incubation, and these were classified as healthy (Annexin V−, PI−), apoptotic (Annexin V+, PI−), and necrotic (Annexin V+, PI+). Columns, mean of triplicate samples at each time point; bars, SE. Representative dot plot data are shown. *, P < 0.05 versus healthy cells (24 h). B, confocal microscopy images of cells incubated with TQ (0 and 50 μmol/L) for 24 h stained with Annexin V and PI. Scale bars, 10 μm.
Genes involved in tumor growth and metastasis of pancreatic cancer cells were downregulated
The genes that were differentially regulated in FG/COLO357 cells following treatment with TQ were determined by microarray analysis (Supplementary Table S1). In general, genes involved in promoting the tumorigenic and metastatic properties of pancreatic cancer cells were downregulated in the TQ-treated cells (50 μmol/L) when compared with untreated cells (0 μmol/L). Some of the genes that were downregulated included TRIM24, S100A4, MMP7, RORA, and MMP13. The level of these genes was downregulated >65% of the original value. On the other hand, the expression of genes involved in inducing apoptosis and growth restriction was increased >4-fold in pancreatic cancer cells incubated with TQ. Several genes that were upregulated in pancreatic cancer cells after TQ treatment were STC2, IL24, TRIB3, ERRFl1, GADD45A, CYP27B1, and RND3. The function of some of the genes (i.e., TRIB3 and GADD45A) after TQ treatment involves the activation of mitogen-activated protein kinase (MAPK) pathways, including JNK and p38 MAPK. The results from the analysis support the functional assays already discussed in this work and led us to analyze the involvement of JNK and MAPK p38 pathways.
TQ downregulates MUC4 through JNK and p38 MAPK pathways
Cancer cells can destabilize JNK and p38 MAPK pathways to facilitate the proliferation, survival, and invasion of tumor cells (35). Functional proteins were analyzed in pancreatic cancer cells after treatment with TQ, and both JNK and p38 MAPK pathways were activated (Fig. 6A), supporting the results from microarray analysis. We also found a downregulation of the cell death suppressor Bcl-xL, and an activation of the apoptotic death accelerator Bax and caspase-9, indicating that TQ activates proapoptotic pathways in FG/COLO357 cells. The levels of caspase-3, however, did not change after treatment with TQ, which implies that apoptosis could be caspase-9 dependent and caspase-3 independent, as has been published elsewhere (36).
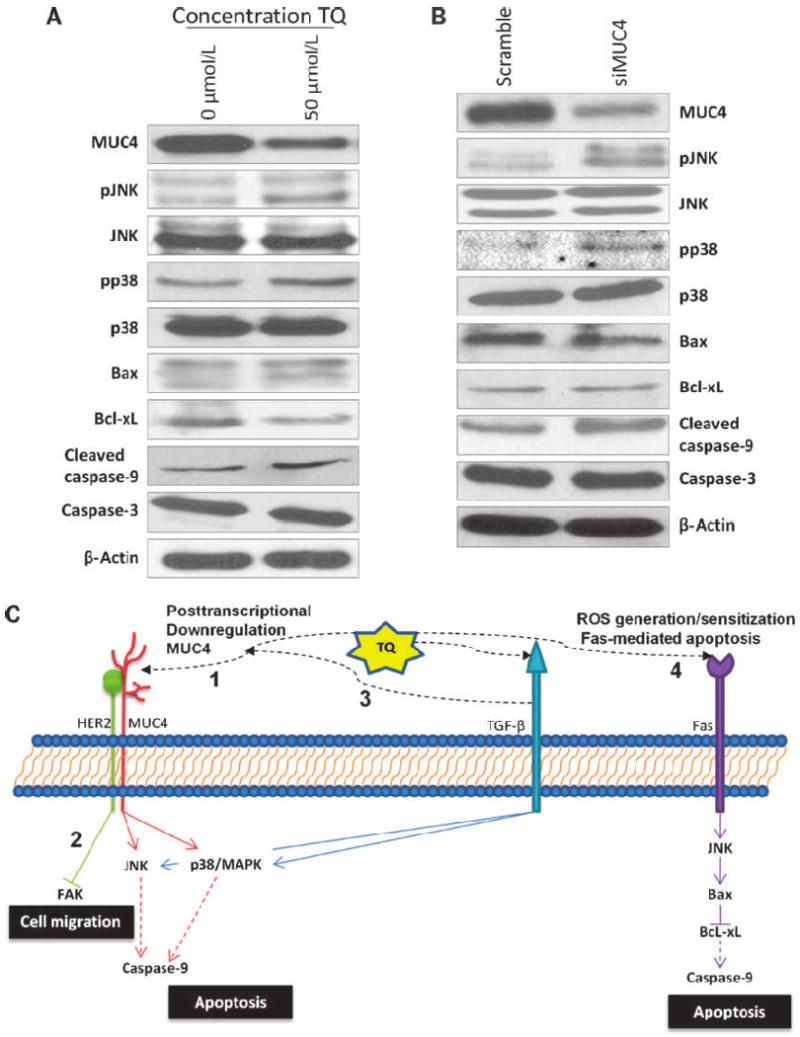
Correlation of MUC4 downregulation and the induction of apoptosis in TQ-treated FG/COLO357 cells. A, Western blot analysis of functional proteins in downstream signaling pathways in cells incubated with TQ. Protein lysates (40–60 μg) were resolved in 10% SDS-PAGE electrophoresis, and the expression of phospho-JNK (pJNK), total JNK, phospho-p38 (pp38), total p38, Bax, Bcl-xL, cleaved caspase-9, caspase-3, and β-actin was analyzed. B, Western blot analysis of functional proteins expressed in MUC4 transiently knocked down FG/COLO357 cells. After doing transient knockdown of MUC4 in FG/COLO357 for 48 h, protein lysates were analyzed and compared with MUC4-expressing cells. C, model of TQ-mediated cytotoxicity in FG/COLO357 cells. TQ induces the apoptosis of cancer cells by different pathways: (1) direct downregulation of MUC4 posttranscriptionally, which leads to apoptosis by the activation of JNK and p38 MAPK pathways; (2) as MUC4 is associated with HER2, the migration of cancer cells is decreased by the inhibition of FAK; (3) TQ might also induce the activation of the TGF-β pathway that can lead to the activation of JNK and p38 MAPK pathways directly or by its induced posttranscriptional downregulation of MUC4; and (4) TQ might sensitize pancreatic cancer cells to Fas-mediated apoptosis through the generation of ROS that activate JNK pathway.
To evaluate the direct association of the apoptotic effects of TQ with MUC4 in pancreatic cancer cells, transient silencing of MUC4 was done on FG/COLO357 cells. The simply transient downregulation of MUC4 on the pancreatic cancer cells was enough to activate the same apoptotic pathways (i.e., JNK and p38 MAPK) that were activated (Fig. 6B). However, the expression of Bax was decreased and the levels of Bcl-xL remained constant in the siMUC4 cells compared with cells transfected with scramble RNA.
Discussion
Pancreatic cancer treatment remains a major challenge in oncology as evidenced by the unchanged overall survival over the last 25 years (2). Numerous encouraging approaches have been developed in targeted therapies against pancreatic cancer, but results in clinical trials are not ideal and the overall survival of pancreatic cancer patients remains the same. Currently, chemotherapy is the only treatment option for patients with metastatic pancreatic cancer, and unfortunately, there are numerous molecular factors involved in the chemotherapeutic resistance of pancreatic cancer tumors (3). Gemcitabine has been the standard chemotherapeutic drug for >10 years and, among all the therapies evaluated, the only therapeutic regimen that improved the overall survival of patients when compared with gemcitabine alone, combined the drug with erlotinib, which targets the epidermal growth factor receptor (37). One of the possible reasons for the resistance to chemotherapy in pancreatic cancer are the alterations in apoptotic signaling pathways (38), which have been directly implicated in gemcitabine resistance (3). Despite all the research that has been done, there are many questions that have not been answered and no major improvement has been documented yet. It is known that novel therapies are desperately needed and alternative markers must be evaluated for their potential in improving the prognosis and therapy for pancreatic cancer patients.
The results of our present study indicate that TQ downregulates MUC4 expression in pancreatic cancer cells. Consequently, TQ reduced the motility of pancreatic cancer cells, as indicated by the reduced migration, minimized cellular projections, decreased HER2 expression, and FAK downregulation. These results agree with previous work done by our group, where it was documented that the overexpression of MUC4 is related to an increased invasiveness, migration, and motility of malignant cells (6–8, 30, 39).
TQ induced apoptosis of pancreatic cancer cells by means of different pathways (Fig. 6C), and the downregulation of MUC4 was directly related to the apoptotic effect of the drug through the activation of JNK and p38 MAPK pathways. The activation of JNK and p38 MAPK pathways is not unexpected as these share upstream regulators, indicating that the activation of both pathways can be activated simultaneously, leading to the activation of a putative tumor suppressor in human cancers (35).
TQ downregulated the expression of MUC4 on pancreatic cancer cells by targeting the protein to the proteasomal degradation pathway. Interestingly, it has been recently published that the transforming growth factor-β (TGF-β) represses MUC4 expression by proteasomal pathway (40). Similarly, other reports indicate that MUC4 expression is inhibited posttranscriptionally by the TGF-β pathway as well (41, 42). As TGF-β induces the activation of JNK and p38 MAPK pathways (43), one possibility is that TQ induces pancreatic cancer cells to secrete TGF-β, which in turn activates the TGF-β pathway, downregulates MUC4, and, consequently, induces apoptosis of pancreatic cancer cells (Fig. 6C). Future studies will aim to determine the role of the TGF-β signaling pathway in TQ-mediated cell signaling.
A second upstream mechanism of TQ-induced pancreatic cancer cytotoxicity is through the generation of reactive oxygen species (ROS). It has been reported that TQ induces apoptosis by the generation of ROS and activating the JNK and extracellular signal-regulated kinase, but not p38 MAPK, pathways in colon cancer cells (19). Similarly, other natural-derived products (i.e., capsaicin) effective against pancreatic cancer cells are also known to induce apoptosis by upregulating ROS production followed by activation of the JNK pathway (44). It is possible that TQ also stimulates the generation of ROS in FG/COLO357 cells, which subsequently activates the JNK pathway and induces apoptosis (Fig. 6C). Nevertheless, further studies need to be done to establish if there is any correlation of ROS generation and MUC4 expression.
A third proposed mechanism of TQ-induced pancreatic cancer cell cytotoxicity is the Fas-mediated apoptosis (Fig. 6C), as other reports had shown that Fas-mediated apoptosis in the pancreatic cancer cell line COLO357 correlated with the activation of JNK and p38 MAPK pathways (45). As evidenced by the results shown in this work, downregulation of MUC4 and apoptosis induced by TQ are not isolated events and TQ is inducing cytotoxicity by a complex interplay between different signaling pathways, where MUC4 plays a major role.
It is important to mention that different cancer cell lines can respond differently to chemotherapeutic drugs. For example, some studies have shown that gemcitabine induced apoptosis in pancreatic cancer cells by activation of p38 MAPK and not the JNK pathway (46), whereas the activation of both pathways was directly related to apoptosis induced by TQ in the studies presented here. Furthermore, a recent published work supports the fact that different cancer cell lines can use various antiapoptotic properties of MUC4 to acquire therapeutic drug resistance (12). Although different cancer cells use different mechanisms for drug metabolism that can lead to cancer cell killing, the fact that MUC4 is involved in conferring aggressive and chemoresistant properties to malignant pancreatic tumors (8), a common denominator in pancreatic cancer therapies must be to target and downregulate this mucin to have a successful therapy.
Finally, an important aspect that need to be clarified is that, although relatively high doses of TQ were used on these experiments, it is well known that TQ is a relatively nontoxic compound as has been shown previously on in vivo experiments, where the LD50 of TQ in mice and rats is much higher than the effective doses of the drug (21). Taking into consideration these results, the concentrations used in the current study were <10% of the LD50 reported.
No study has been published yet that evaluates the effect of TQ in the expression of any mucin. Follow-up studies include the analysis of TQ in the expression of other mucins that are aberrantly expressed in tumors and evaluate its therapeutic and chemosensitizing effect in combination with other natural-derived drugs. Overall, TQ, which has a role in MUC4 downregulation among other antitumorigenic properties, has potential for the development of novel therapies against pancreatic cancer, and further studies must be done to evaluate its therapeutic value in preclinical models.
Acknowledgments
We thank Erik Moore and Kavita Mallya for their invaluable technical support; Janice A. Tayor and James R. Talaska (Confocal Laser Scanning Microscope Core Facility, University of Nebraska Medical Center), Victoria B. Smith and Megan Michalak (University of Nebraska Medical Center Cell Analysis Core Facility), and the Eppley Cancer Center for their support of the core facilities; and Kristi L. Berger for editing the manuscript.
Grant Support
NIH grants CA78590, CA111294, CA133774, and CA131944.
Footnotes
Note: Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.1158/1535-7163.mct-10-0075
Read article for free, from open access legal sources, via Unpaywall:
http://mct.aacrjournals.org/content/9/5/1419.full.pdf
Free after 12 months at intl-mct.aacrjournals.org
http://intl-mct.aacrjournals.org/cgi/reprint/9/5/1419.pdf
Free to read at intl-mct.aacrjournals.org
http://intl-mct.aacrjournals.org/cgi/content/abstract/9/5/1419
Free after 12 months at intl-mct.aacrjournals.org
http://intl-mct.aacrjournals.org/cgi/content/full/9/5/1419
Citations & impact
Impact metrics
Citations of article over time
Article citations
The Emerging Role of Natural Products in Cancer Treatment.
Arch Toxicol, 98(8):2353-2391, 25 May 2024
Cited by: 3 articles | PMID: 38795134
Review
Therapeutic and Phytochemical Properties of Thymoquinone Derived from Nigella sativa.
Curr Drug Res Rev, 16(2):145-156, 01 Jan 2024
Cited by: 1 article | PMID: 37605475
Review
Potential anticancer properties and mechanisms of thymoquinone in colorectal cancer.
Cancer Cell Int, 23(1):320, 12 Dec 2023
Cited by: 0 articles | PMID: 38087345 | PMCID: PMC10717210
Review Free full text in Europe PMC
A Review of the Potential Health Benefits of Nigella sativa on Obesity and Its Associated Complications.
Plants (Basel), 12(18):3210, 08 Sep 2023
Cited by: 1 article | PMID: 37765374 | PMCID: PMC10536791
Review Free full text in Europe PMC
Mucins as contrast agent targets for fluorescence-guided surgery of pancreatic cancer.
Cancer Lett, 561:216150, 29 Mar 2023
Cited by: 2 articles | PMID: 36997106 | PMCID: PMC10150776
Review Free full text in Europe PMC
Go to all (62) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma.
Expert Opin Ther Targets, 21(7):657-669, 29 May 2017
Cited by: 43 articles | PMID: 28460571 | PMCID: PMC5706649
Review Free full text in Europe PMC
Targeting EGF-receptor(s) - STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer.
Oncotarget, 6(7):5164-5181, 01 Mar 2015
Cited by: 36 articles | PMID: 25686822 | PMCID: PMC4467140
Thymoquinone-Induced Tristetraprolin Inhibits Tumor Growth and Metastasis through Destabilization of MUC4 mRNA.
Int J Mol Sci, 20(11):E2614, 28 May 2019
Cited by: 9 articles | PMID: 31141941 | PMCID: PMC6600862
The antagonistic regulation of human MUC4 and ErbB-2 genes by the Ets protein PEA3 in pancreatic cancer cells: implications for the proliferation/differentiation balance in the cells.
Biochem J, 386(pt 1):35-45, 01 Feb 2005
Cited by: 17 articles | PMID: 15461591 | PMCID: PMC1134764
Funding
Funders who supported this work.
NCI NIH HHS (10)
Grant ID: R01 CA131944
Grant ID: CA111294
Grant ID: CA78590
Grant ID: R01 CA078590-13
Grant ID: R01 CA078590-12
Grant ID: U01 CA111294
Grant ID: CA133774
Grant ID: R01 CA078590
Grant ID: CA131944
Grant ID: R01 CA133774




