Abstract
Purpose
AT-101 binds and inhibits the antiapoptotic function of Bcl-2, Bcl-xL, Mcl-1, and Bcl-w and is a potent stimulator of proapoptotic proteins. In this multi-institution phase I/II trial, we evaluated the safety and efficacy of single-agent AT-101, in men with chemotherapy naïve, castrate-resistant prostate cancer (CRPC).Experimental design
Patients with progressive CRPC were to be treated with escalating doses of AT-101 on a continuous daily basis until the maximally tolerated dose was achieved. At the recommended phase 2 dose, an additional 21 patients were planned to assess for preliminary evidence of efficacy.Results
Twenty-three patients were enrolled. The phase I starting dose was 30 mg/day on a continuous basis; however, ongoing trials with AT-101 showed increased gastrointestinal toxicity with this daily schedule when given for repetitive cycles. As a result, the phase II starting dose was chosen to be 30 mg/day for 21 of 28 days. The most frequent observed adverse events (any grade) were diarrhea (43.5%), fatigue (34.8%), nausea (21.7%), anorexia (21.7%), and small intestinal obstruction (21.7%). Due to the high incidence of grade 3 small intestinal obstruction (n = 5; 21.7%), a reduction in dose to 20 mg/day for 21 of 28 days was mandated for all patients. Two patients had a confirmed > or =50% posttherapy prostate-specific antigen decline. No objective responses (Response Evaluation Criteria in Solid Tumors) were observed.Conclusion
AT-101 administered at 20 mg/day for 21 of 28 days was well-tolerated. Evidence of single-agent clinical activity was observed with prostate-specific antigen declines in some patients. Further investigation of AT-101 in prostate cancer is warranted and trials combining AT-101 with androgen deprivation, as well as with docetaxel chemotherapy are ongoing.Free full text

An Open-Label, Multicenter, Phase I/II Study of Single-Agent AT-101 in Men with Castrate-Resistant Prostate Cancer (CRPC)
Abstract
Purpose
AT-101 binds and inhibits the anti-apoptotic function of Bcl-2, Bcl-xL, Mcl-1, and Bcl-w and is a potent stimulator of proapoptotic proteins. In this multi-institution Phase I/II trial, we evaluated the safety and efficacy of single-agent AT-101, in men with chemotherapy naïve, castrate-resistant prostate cancer (CRPC).
Experimental Design
Patients with progressive CRPC were to be treated with escalating doses of AT-101 on a continuous daily basis until the maximally tolerated dose (MTD) was achieved. At the recommended phase 2 dose (RP2D), an additional 21 patients were planned to assess for preliminary evidence of efficacy.
Results
Twenty-three patients were enrolled. The phase I starting dose was 30 mg/day on a continuous basis however, ongoing trials with AT-101 showed increased gastrointestinal (GI) toxicity with this daily schedule when given for repetitive cycles. As a result, the phase II starting dose was chosen to be 30 mg/day for 21 of 28 days. The most frequent observed adverse events (any grade) were diarrhea (43.5%), fatigue (34.8%), nausea (21.7%), anorexia (21.7%), and small intestinal obstruction (21.7%). Due to the high incidence of grade 3 small intestinal obstruction (n = 5; 21.7%), a reduction in dose to 20 mg/day for 21/28 days was mandated for all patients. Two patients had a confirmed ≥ 50% post-therapy PSA decline. No objective responses (RECIST) were observed.
Conclusion
AT-101 administered at 20 mg/day for 21 of 28 day was well tolerated. Evidence of single-agent clinical activity was observed with PSA declines in some patients. Further investigation of AT-101 in prostate cancer is warranted and trials combining AT-101 with androgen deprivation, as well as with docetaxel chemotherapy are ongoing.
INTRODUCTION
Prostate cancer is the most common malignancy among males in the United States with an estimated 186,320 new cases and 28,660 deaths for the year 2008 alone1. While androgen ablation is the standard initial therapy for metastatic prostate cancer, eventually all patients will develop castrate-resistant prostate cancer (CRPC)2. It has been believed for many years that prostate cancer was not responsive to chemotherapy as most trials during that time in patients with metastatic CRPC showed response rates of < 10%3. Although mitoxantrone with prednisone was approved by the Food and Drug Administration (FDA) for the treatment of metastatic CRPC in 1996, it was not widely adopted by clinicians as the studies showed only improvements pain and quality of life parameters, but not survival.4,5.
It has been shown that during the transition to castrate-resistant prostate cancer, certain oncogenes such as bcl-2 can become overexpressed, resulting in chemotherapy resistance6,7. Further, targeting Bcl-2 may be important to enhance sensitivity to chemotherapy-induced apoptotic death. This led to evaluation of taxanes, which were shown to be able to phosphorylate Bcl-2, thereby enhancing chemosensitivity8,9. Docetaxel chemotherapy is one of the more potent inactivators of Bcl-2 preclinically10. Subsequently, docetaxel was found to be superior to mitoxantrone chemotherapy in two randomized Phase 3 trials, leading to its FDA approval for men with metastatic CRPC11,12. Although both trials showed a modest median survival improvement of only 2 – 2.5 months, there was significant improvements in terms of pain and other palliative endpoints. As a result, it is considered standard to use docetaxel in patients with metastatic, symptomatic CRPC. On the other hand, the role of docetaxel chemotherapy in a patient with asymptomatic, advanced CRPC remains undefined. Clearly, newer agents for the management of CRPC are needed, especially in patients with asymptomatic disease when use of chemotherapy is not absolutely indicated.
AT-101 (R-(-)-gossypol acetic acid, Ascenta Therapeutics, Inc.) is a polyphenolic compound derived from the cottonseed plant13. AT-101 inhibits the function of Bcl-2, Bcl-xL, Mcl-1, and Bcl-w by operating as a BH3 mimetic and as a potent stimulator of Noxa and Puma. By blocking the binding of Bcl-2 family members with proapoptotic proteins and upregulating specific proapoptotic factors, AT-101 lowers the threshold for cancer cells to undergo apoptosis14. Preclinically, AT-101 has shown antitumor activity in a variety of tumor models as a single agent and in combination with standard anticancer therapies, including breast15, prostate16, colon17, head and neck18, chronic lymphocytic leukemia19, non-Hodgkin’s lymphoma20 and multiple myeloma21. Here we report the results of a completed Phase I/II study of AT-101 in men with castrate-resistant prostate cancer.
MATERIALS AND METHODS
Patient selection
Patients had to have histologically proven adenocarcinoma of the prostate. A serum testosterone level ≤ 50 ng/mL was required and patients were required to be maintained on androgen-deprivation therapy with a leuteinizing hormone-releasing (LHRH) agonist or antagonist, unless they had a prior bilateral orchiectomy. Patients were considered eligible if they had progressive CRPC documented by two consecutive PSA increases over a reference value. All PSA values used for eligibility determination must be taken at least 1 week apart, with the last value being ≥ 5 ng/mL. Progressive disease after 4 weeks of flutamide, megestrol, or ketoconazole (6 weeks for bicalutamide or nilutamide) withdrawal was required to exclude an antiandrogen withdrawal response. Radiotherapy and/or samarium must have been completed at least 4 weeks (12 weeks for prior strontium) prior to registration. Bisphosphonate use was allowed in patients with known bone metastasis if initiated prior to registration; however, bisphosphonate therapy was not allowed to be initiated during study treatment. Other inclusion criteria included: Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, adequate hematologic function (absolute neutrophil count ≥ 1,500/μl, hemoglobin ≥ 9 gm/dl, platelet count ≥ 100,000/μ1), liver function (albumin ≥ 2.5 gm/dL, total bilirubin ≥ 1.5 × upper limit of normal, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 1.5 × upper limit of normal), renal function (serum creatinine ≤ 2.0 mg/dL), and a life-expectancy of ≥ 3 months. No prior cytotoxic chemotherapy was permitted. No concurrent systemic steroids or concurrent therapy for prostate cancer (e.g. immunologic, biologic, investigational, etc) was allowed. All patients gave written informed consent in compliance with state, federal, and institutional guidelines.
Study Plan
The Phase I dose-escalation portion of this study was designed to enroll cohorts of three to six patients, to determine the maximum tolerated dose (MTD) of single-agent AT-101. The MTD was defined as one dose level below that at which 33% of the patients within a cohort experienced a dose-limiting toxicity (DLT). DLTs were defined as any toxicity felt at least possibly related to AT-101, and met the following criteria (using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 3.022): grade ≥ 3 non-hematological toxicity (excluding nausea, vomiting or diarrhea unless uncontrolled by maximal antiemetic and/or antidiarrheal therapy), any elevation in serum troponin (outside of the institutional normal range), or any grade ≥ 3 granulocytopenia/thrombocytopenia lasting ≥ 7 days, or any grade thrombocytopenia if associated with bleeding.
At the recommended Phase 2 dose (RP2D), an additional 21 patients were planned to assess preliminary evidence of efficacy with the primary objective being PSA response, as defined by the PSA Working Group Criteria 123. Secondary objectives included determination of the rate, quality (depth), duration, and time to response, as well as objective tumor responses in patients with measurable disease using Response Evaluation Criteria in Solid Tumors (RECIST)24.
Treatment Plan
AT-101 was supplied as a 10 mg immediate release solid dosage tablet. AT-101 was administered orally, once daily starting at 30 mg, on a daily basis for 21 days of a 28 day cycle, and was taken at the same time each day on an empty stomach (at least one hour before or after a meal). Each patient continued at their assigned dose unless a dose-adjustment was made for safety or toxicity. No intra-patient dose-escalation was permitted. Patients continued treatment until they experienced progressive disease, unacceptable toxicity, or required alternative therapies. Compliance was monitored by counting unused medications during each visit.
Baseline and Treatment Assessments
At study entry, all patients underwent a complete history and physical exam, including a complete blood count, serum chemistries, and disease assessment (bone scan, computed tomography imaging, and PSA). Baseline electrocardiogram (ECG), serum testosterone and troponin level, and urine analysis were obtained as well.
Weekly laboratory assessments including a complete blood count (CBC), serum chemistries (sodium, potassium, chloride, calcium, glucose, BUN, creatinine, and phosphate), and liver function tests (AST, ALT, alkaline phosphatase, total bilirubin, albumin, total protein, and gamma glutamyl transferase) were obtained during the first four weeks of drug administration. Thereafter, physical examinations, complete blood counts, serum chemistries, serum troponin level, ECG, and PSA levels were evaluated every 4 weeks while the patient was on study. Radiographic disease assessments for soft tissue disease were repeated every 8 weeks and bone scans were repeated every 24 weeks, sooner if clinically indicated.
Post-study evaluations were conducted 30 days after the last dose and included a PSA and review of systems.
Toxicity and Dose Modifications
Patients with clinically significant grade 2-3 toxicity had their treatment held (up to 14 days) until the toxicity resolved to grade ≤ 1. Treatment was then restarted at the next lower dose level (20 or 10 mg). Grade 4, or recurrent clinically significant grade 2-3, toxicity required discontinuation of treatment. An elevation of serum troponin levels required discontinuation of therapy.
Response Criteria
Although all patients were included in the safety and toxicity analysis, only patients completing at least 8 weeks of AT-101 were considered evaluable (protocol-defined) for the treatment outcome assessment (disease progression prior to week 8 of treatment was counted as a treatment failure). The primary indicator of drug activity was the PSA response rate, defined as a ≥ 50% decrease in PSA from baseline, confirmed by a repeat value 4 weeks later (PSA Working Group Criteria 1). Patients may not have any evidence for clinical or radiographical progression during that time period. PSA progression was defined as a ≥ 25% increase from nadir, confirmed by a second value, with an absolute increase in PSA of at least 5 ng/mL. The radiologic response rate assessment used RECIST, and defined a complete response (CR) as the disappearance of all known disease during two observations at least 4 weeks apart, during which no new lesions develop. For patients with bone only disease, normalization of the bone scan was required. A partial response (PR) was defined as ≥ 30% decrease in sum of the greatest perpendicular tumor diameters of all measurable disease documented for ≥ 4 weeks. No new lesions or increased size of any existing lesion was allowed. Progressive disease included any unequivocal increase ≥ 20% in the size of any existing lesion, or the appearance of any new lesion. Stable disease (SD) was any other condition not met by the criteria outlined in CR, PR, or progression.
Statistical Analysis
The statistical analyses used SAS Version 8 or higher, and continuous variables were summarized with sample size, mean, standard deviation, median, and range values. Time-to-event parameters were summarized using Kaplan-Meier methods. The Phase II portion of the study used PSA response as the primary indicator of efficacy. Sample size was determined assuming that with a null hypothesis of 0.05, that the binomial probability of observing 4 or more responses is 0.019. With a response rate of 25% being of interest, 21 patients were required to achieve an estimate of power to detect 4 or more responses of 0.808. Days to response, duration of response, and time to progression were calculated from the start of therapy. Pretreatment rate of PSA progression was calculated using PSA values prior to, and including, day 1 of AT-101 treatment.
RESULTS
Patient Characteristics
From January 2006 to June 2006, a total of 23 patients were enrolled at the University of Wisconsin Carbone Comprehensive Cancer Center (Madison, WI), The West Clinic (Memphis, TN), and Yale University Medical Center (New Haven, CT). See Table 1 for patient characteristics. The median duration on treatment was 7.1 weeks (range, 4.6 – 30.6 weeks).
Table 1
Baseline Patient Demographics.
| Characteristics | No. of Patients | |
|---|---|---|
| Age, years | 23 | |
 Mean Mean | 70.2 | |
 Range Range | 52.3 – 85.9 | |
| ECOG PS | ||
 0 0 | 6 (26.1%) | |
 1 1 | 17 (73.9%) | |
| PSA | ||
 Mean (SD) Mean (SD) | 62.2 (55.5) | |
 Range Range | 8.4 – 195.5 | |
| Disease Status | ||
 Rising PSA Only Rising PSA Only | 4 (17.4%) | |
 Osseous Metastasis Only Osseous Metastasis Only | 7 (30.4%) | |
 Measurable Disease Measurable Disease | 12 (52.2%) | |
| Gleason Score | ||
 ≤ 6 ≤ 6 | 7 (30.4%) | |
 7 7 | 8 (34.8%) | |
 8 8 | 3 (13.0%) | |
 9 9 | 3 (13.0%) | |
 Unknown Unknown | 2 (8.7%) | |
| Prior Therapy | ||
 Hormonal Therapy Hormonal Therapy | 23 (100%) | |
 Immunotherapy Immunotherapy | 1 (4.3%) | |
 Radiotherapy1 Radiotherapy1 | 12 (52.2%) | |
 Surgery2 Surgery2 | 12 (52.2%) | |
 Other Other | 1 (4.3%) | |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; PSA, Prostate specific antigen; SD, Standard Deviation.
Phase I, Dose-Finding Safety Cohort
The Phase I portion of the study started at 30 mg/day on a continuous daily dosing but was terminated early based on emerging safety data from other ongoing Phase I trials of AT-101 showing gastrointestinal (GI) toxicities with continuous daily dosing. The protocol schedule was amended on February 22, 2006 to declare that AT-101 at 30 mg/day, given for 21 days, repeated in 28 day cycles as the RP2D.
Phase II, Clinical Outcomes
A total of 19 patients were evaluable for clinical outcomes as defined in the protocol (10 patients completed ≥ 8 weeks of therapy, 7 had confirmed disease progression before week 8, 2 had unconfirmed disease progression before week 8). Four patients were not evaluable for response as their treatment was discontinued within the first two cycle of therapy. Of these, three patients had developed a small intestinal obstruction in which AT-101 was discontinued, and one patient was taken off protocol by investigator discretion due to a rise in PSA which did not meet progression as defined by the protocol.
PSA Response
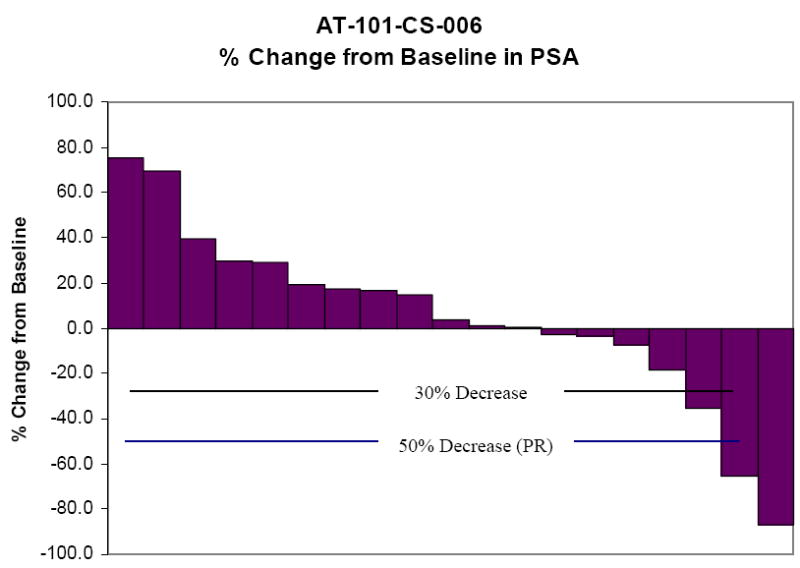
Two patients had confirmed PSA responses with a greater than 50% decrease from their baseline values. The relative change in PSA from baseline for these two patients is shown in Figure 1. The percent change in PSA for all patients is shown in Figure 2.
RECIST Response
No objective responses were seen. Stable disease for ≥ 24 weeks was seen in 2/19 patients with clinical benefit maintained for 148 and 172 days.
Toxicity
Table 2 lists the adverse events observed with frequency in at least 10% of patients. The most notable adverse event was the development of a small intestinal obstruction (SBO). Of the five reported cases of SBO on this study, four were felt by the treating physician to be related to AT-101. The first patient was started on AT-101 on March 8, 2006 at the 30 mg/day dose. On week 6 of treatment, the patient developed nausea and vomiting which was suspected to be either an ileus or partial distal small bowel obstruction. His past surgical history was remarkable for only prior lumbar surgery, as well as a prostatectomy. The patient did eventually improve with conservative therapy, but did not receive a rechallenge of AT-101. The second patient developed a SBO as a complication from an inguinal hernia that was treated surgically. This event was not felt to be related to AT-101. The third patient was treated at the 30 mg/day dose (21/28 day schedule) and developed nausea and vomiting within 3 weeks of starting AT-101. An exploratory laparotomy was eventually performed and adhesions were found at the terminal ileum. This event was considered possibly related to AT-101. The fourth case was a patient that was treated at 20 mg/day for 21 of 28 days. He had a history of obstructive prostate cancer resulting in urinary retention and peptic ulcer disease, and developed nausea, vomiting, and abdominal distension after 6 days of AT-101 at the reduced dose. He had 2 cycles of AT-101 at 30 mg/day for 21 of 28 days prior to the dose reduction. The symptoms resolved in 1 week with conservative treatment, and AT-101 was permanently discontinued. This event was felt related to AT-101. The last case was patient with remote history of colon cancer treated with a previous resection, who was admitted with an SBO after 5 ½ weeks of AT-101. This patient did require a surgical lysis of adhesions with small bowel resection. This event was considered possibly related to AT-101.
Table 2
Adverse Events Observed in at Least 10% of Patients.
| N = 23 | ||||||
|---|---|---|---|---|---|---|
| CTC Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | NR | Overall |
| Gastrointestinal | ||||||
 Diarrhea Diarrhea | 5 (21.7%) | 2 (8.7%) | 3 (13.0%) | 0 | 0 | 10 (43.5%) |
 Nausea Nausea | 3 (13.0%) | 2 (8.7%) | 0 | 0 | 0 | 5 (21.7%) |
 SBO SBO | 0 | 0 | 5 (21.7%) | 0 | 0 | 5 (21.7%) |
 Dry mouth Dry mouth | 4 (17.4%) | 0 | 0 | 0 | 0 | 4 (17.4%) |
 Vomiting Vomiting | 3 (13.0%) | 0 | 0 | 0 | 0 | 4 (17.4%) |
 Constipation Constipation | 0 | 0 | 0 | 0 | 1 (4.3%) | 3 (13.0%) |
 GERD GERD | 0 | 0 | 0 | 0 | 1 (4.3%) | 3 (13.0%) |
| Fatigue | 2 (8.7%) | 5 (21.7%) | 1 (4.3%) | 0 | 0 | 8 (34.8%) |
| Anorexia | 3 (13.0%) | 1 (4.3%) | 1 (4.3%) | 0 | 0 | 5 (21.7%) |
| Back pain | 1 (4.3%) | 2 (8.7%) | 0 | 0 | 0 | 3 (13.0%) |
| Bone pain | 0 | 2 (8.7%) | 1 (4.3%) | 0 | 0 | 3 (13.0%) |
| Dyspnea | 2 (8.7%) | 3 (13.0%) | 0 | 0 | 0 | 5 (21.7%) |
Abbreviations: CTC, Common Toxicity Criteria; NR, Not Reported; SBO, Small Bowel Onstruction; GERD, Gastroesophageal Reflux Disease.
Due to the incidence of gastrointestinal toxicity, the dose of AT-101 was reduced in all patients to 20 mg/day on a 21 of 28 day schedule. At this dose level, only one patient required discontinuation of therapy for an adverse event (Grade 3 weakness with ileus).
No clinically significant laboratory abnormalities were observed. Likewise, no changes in ECGs were considered clinically significant.
DISCUSSION
The purpose of this study was to assess the safety and tolerability of single-agent AT-101 in men with CRPC, as well as to evaluate for any preliminary evidence of clinical activity. During the implementation of this study, preliminary data from an ongoing, open label Phase I with AT-101 using doses up to 50 mg daily (QD) or 30 mg twice daily (BID) were available. At the 30 mg BID dose, 2 of 3 patients developed grade 4 AST/ALT elevation with associated nausea and vomiting after 1 week of drug. At the 40 mg QD dose, 1 of 6 patients was found to have Grade 4 hypokalemia and Grade 3 nausea. As a result, the RP2D was thought to be 30 mg QD on a continuous basis24. As ongoing data matured, including data on a Phase II trial in Chronic Lymphocytic Leukemia (CLL), nausea, vomiting, and ileus appeared to be related to continuous daily dosing, suggesting that an intermittent dosing schedule was needed to improve patient tolerance25. As a result, this trial was amended to administer AT-101 at 30 mg/day for 21 of 28 days.
During the conduct of this study, the incidence of gastrointestinal toxicities was initially high; with 4/5 cases of ileus/SBO observed that were felt at least possibly related to AT-101. As a result, the dose of AT-101 was reduced to 20 mg/day with significant improvements in toleration and incidence of significant toxicity. The RP2D for single agent AT-101 administered for repetitive cycles in CRPC is 20 mg/day given for 21 of 28 days.
The mechanism of small bowel obstructions seen in some patients treated with AT-101 at high doses or with repetitive cycles is unknown. Infrequent cases were observed following gossypol administration in patients with breast cancer, adrenal cancer and glioblastoma multiforme. Possible causes include direct myopathic or neuropathic effects, which could be evaluated in animal models. In addition, an inhibitor of Bcl-2 could result in a decrease in apoptosis of lymphocytes in the distal small intestinal lining (Peyer’s patches or gut-associated lymphoid tissue), resulting in an increased risk for bowel obstruction. (similar to the risk of intussusception associated with hypertrophy of Peyer’s patches)25. Nevertheless, a decrease in dose of AT-101 in this study did result in a reduction in the incidence and severity of serious GI toxicities.
Evidence for single-agent drug activity was seen with 3 patients achieving a PSA decline of 50% from baseline, of which two were confirmed PSA responses. Likewise, minor PSA responses were observed in many other patients as shown in Figure 2. Although the prespecified endpoint used for statistical purposes was not achieved, the data suggests that AT-101 has modest clinical activity. Mechanistically, this effect could enhance the effects of cytotoxic therapies, such as docetaxel.
Currently, there are three ongoing studies assessing AT-101 in prostate cancer. All of these trials use an intermittent dosing schedule of AT-101 to maximize the proapoptotic effects during the dosing period and minimize the risk of GI toxicities seen with the daily administration schedules. A Phase II trial of docetaxel/prednisone with AT-101 has recently been completed in men with chemotherapy naïve metastatic CRPC in which a PSA partial response rate of 67% was observed in 36 patients26. This has resulted in an ongoing, placebo controlled trial comparing docetaxel/prednisone +/- AT-101 with PFS as the primary endpoint. Lastly, a Phase II trial assessing AT-101 in combination with androgen deprivation therapy in men with metastatic, androgen-dependent prostate cancer has recently been activated through the Cancer Therapy Evaluation Program. The rationale for this trial is supported by preclinical data showing that AT-101 delays the development of CRPC by disrupting the antiapoptotic activity of Bcl-2 upregulation during the transition to androgen independence27.
In summary, there is preclinical and clinical evidence to suggest that upregulation of Bcl-2 family members is associated with resistance to chemotherapy, radiotherapy, and is associated with the progression to CRPC. In this trial, we have demonstrated modest single agent activity of AT-101 in CRPC at tolerable daily doses. Targeting the apoptosis network through stimulation of Noxa and Puma and inhibiting the Bcl-2 protein family using AT-101 has many potential implications in prostate cancer including use as a chemosensitizer or in combination with androgen-deprivation therapy and these studies are ongoing.
Acknowledgments
Sponsored by: Ascenta Therapeutics, Inc.
Supported by: Prostate Cancer Clinical Trials Consortium
References
Full text links
Read article at publisher's site: https://doi.org/10.1158/1078-0432.ccr-08-2985
Read article for free, from open access legal sources, via Unpaywall:
http://clincancerres.aacrjournals.org/content/15/9/3172.full.pdf
Free to read at clincancerres.aacrjournals.org
http://clincancerres.aacrjournals.org/cgi/content/abstract/15/9/3172
Free after 12 months at clincancerres.aacrjournals.org
http://clincancerres.aacrjournals.org/cgi/content/full/15/9/3172
Free after 12 months at clincancerres.aacrjournals.org
http://clincancerres.aacrjournals.org/cgi/reprint/15/9/3172.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1158/1078-0432.ccr-08-2985
Article citations
Prostate cancer microenvironment: multidimensional regulation of immune cells, vascular system, stromal cells, and microbiota.
Mol Cancer, 23(1):229, 12 Oct 2024
Cited by: 0 articles | PMID: 39395984 | PMCID: PMC11470719
Review Free full text in Europe PMC
Clinical trials of R-(-)-gossypol (AT-101) in newly diagnosed and recurrent glioblastoma: NABTT 0602 and NABTT 0702.
PLoS One, 19(1):e0291128, 29 Jan 2024
Cited by: 2 articles | PMID: 38285688 | PMCID: PMC10824421
Advances in the study of aerobic glycolytic effects in resistance to radiotherapy in malignant tumors.
PeerJ, 11:e14930, 16 Feb 2023
Cited by: 0 articles | PMID: 36811010 | PMCID: PMC9939019
Review Free full text in Europe PMC
Gossypol and Its Natural Derivatives: Multitargeted Phytochemicals as Potential Drug Candidates for Oncologic Diseases.
Pharmaceutics, 14(12):2624, 28 Nov 2022
Cited by: 4 articles | PMID: 36559116 | PMCID: PMC9787675
Review Free full text in Europe PMC
Comparison of the efficacy of gossypol acetate enantiomers in rats with uterine leiomyoma.
J Nat Med, 77(1):41-52, 19 Aug 2022
Cited by: 2 articles | PMID: 35984592
Go to all (101) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer.
Clin Cancer Res, 13(4):1208-1215, 01 Feb 2007
Cited by: 50 articles | PMID: 17317831
Phase II trial of weekly ixabepilone in men with metastatic castrate-resistant prostate cancer (E3803): a trial of the Eastern Cooperative Oncology Group.
Clin Genitourin Cancer, 10(2):99-105, 03 Mar 2012
Cited by: 14 articles | PMID: 22386239 | PMCID: PMC3535436
Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial.
Lancet Oncol, 15(9):975-985, 25 Jun 2014
Cited by: 99 articles | PMID: 24974051
Effect of docetaxel in patients with hormone-dependent prostate-specific antigen progression after local therapy for prostate cancer.
J Clin Oncol, 23(15):3352-3357, 28 Feb 2005
Cited by: 21 articles | PMID: 15738531
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: P30 CA014520-34
Grant ID: P30 CA014520