Abstract
Background
T cells play an important role during the immune response that accompanies atherosclerosis. To date, the role for interleukin (IL)-17A in atherogenesis is not well defined. Here, we tested the hypothesis that atherosclerosis-prone conditions induce the differentiation of IL-17A-producing T cells, which in turn promote atherosclerosis.Methods and results
IL-17A was found to be elevated in the plasma and tissues of apolipoprotein E-deficient (Apoe(-/-)) mice. IL-17A-expressing T cells were significantly increased in the aortas, spleen, and lamina propria of aged Apoe(-/-) mice compared with age-matched C57BL/6 mice. IL-17A(+) T cells resided in both adventitia and aortas of aged Apoe(-/-) mice fed a chow diet. Elevated levels of IL-17A(+) T cells were also detected in the aortas of 21-week-old Apoe(-/-) mice fed a Western diet for 15 weeks. IL-17A(+) T cells were characterized as predominantly CD4(+) T helper 17 (Th17) cells and gammadelta(+) T cells. Blockade of IL-17A in Apoe(-/-) mice by use of adenovirus-produced IL-17 receptor A reduced plaque burden in Apoe(-/-) mice fed a Western diet for 15 weeks. In addition, the treatment diminished circulating IL-6 and granulocyte colony-stimulating factor levels and limited CXCL1 expression and macrophage content within the aortas. Conversely, IL-17A treatment of whole aorta isolated from Apoe(-/-) mice promoted aortic CXCL1 expression and monocyte adhesion in an ex vivo adhesion assay.Conclusions
These results demonstrate that atherosclerosis-prone conditions induce the differentiation of IL-17A-producing T cells. IL-17A plays a proatherogenic inflammatory role during atherogenesis by promoting monocyte/macrophage recruitment into the aortic wall.Free full text

Blockade of IL-17A results in reduced atherosclerosis in Apoe-deficient mice
Abstract
Background
T cells play an important role during the immune response that accompanies atherosclerosis. To date, the role for IL-17A in atherogenesis is not well-defined. Here, we tested the hypothesis that atherosclerosis-prone conditions induce the differentiation of IL-17A-producing T cells, which in turn promote atherosclerosis.
Methods and Results
IL-17A was found elevated in the plasma and tissues of apolipoprotein E-deficient (Apoe−/−) mice. IL-17A-expressing T cells were significantly increased in the aortas, spleen and lamina propria of aged Apoe−/− mice compared to age-matched C57BL/6 mice. IL-17A+ T cells resided in both, adventitia and aortas of aged Apoe−/− mice on chow diet. Elevated levels of IL-17A+ T cells were also detected in the aortas of 21 week old Apoe−/− mice fed western diet for 15 weeks. IL-17A+ T cells were characterized as predominantly CD4+ Th17 and γδ+ T cells. Blockade of IL-17A in Apoe−/− mice using adenovirus-produced IL-17RA reduced plaque burden in Apoe−/− mice fed western diet for 15 weeks. Also, the treatment diminished circulating IL-6 and G-CSF levels, and limited CXCL1 expression and macrophage content within the aortas. Conversely, IL-17A treatment of whole aorta isolated from Apoe−/− mice, using an ex vivo adhesion assay, promoted monocyte adhesion and CXCL1 expression.
Conclusions
- These results demonstrate that atherosclerosis-prone conditions induce the differentiation of IL-17A-producing T cells. IL-17A plays a pro-atherogenic inflammatory role during atherogenesis by promoting monocyte/macrophage recruitment into the aortic wall.
Introduction
Atherosclerosis is the leading cause of cardiovascular disease worldwide. Defined as chronic inflammation of the artery wall, its progression from fatty streaks to more complex lesions and plaque rupture involves a complicated interplay between many different cell types and cytokine networks. Both innate and adaptive immune responses have been shown to regulate local and systemic inflammation during atherogenesis.1,2 T cells are found within the adventitia of normal/non-inflamed vessels as a result of a constitutive T cell homing into the aorta.3 Atherosclerosis-prone conditions accelerate T cell recruitment into the aorta of Apoe−/− mice in both early and advanced stages of atherosclerosis.3 The majority of aortic T cells are TCRαβ+ CD4+ cells, with few CD8+ and γδ+T cells present.1,4 Of the CD4+ T cells, Th1 cells predominate over Th2 cells during early lesion formation and respond with an elevated production of IFN-γ and IL-6. In the later stages of the disease, a switch to a Th2 response and IL-4 production is evident in the atherosclerotic lesions of Apoe−/− mice.5
IL-17A is a member of the IL-17 family that includes: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F.6 Many lymphocyte subsets secrete IL-17A in response to cytokine and/or monoclonal antibody stimulation, including CD4+αβ+ (Th17 cells) CD8+, CD4−CD8−αβlow, NKT and γδ+ T cells.7 The expression of IL-17A is low under normal/non-inflamed conditions, where γδ+ T cells are the largest IL-17A-producing T cell subset.6 In several murine models of autoimmune diseases, including multiple sclerosis, inflammatory bowel disease and arthritis, serum IL-17A levels are elevated and the Th17 cell population is expanded and plays a highly pathogenic role.8 Conversely, IL-17A is a protective cytokine in host responses against extracellular pathogens through the induction of pro-inflammatory cytokines such as IL-6, TNF-α and G-CSF, and the chemokines CXCL8, CCL2, CXCL1 and CXCL2 in infected tissues.6 These downstream mediators of IL-17A are involved in granulopoiesis and induce the recruitment of neutrophils, eosinophils and monocytes into sites of inflammation.9
To date, the contribution of IL-17A to atherogenesis remains controversial, with different studies proposing either a pro-atherogenic or an atheroprotective role for IL-17A. In patients with unstable angina, levels of plasma IL-17A and the Th17-related cytokines IL-6 and IL-23 are elevated.10 Cultured human T cells isolated from atherosclerotic coronary arteries also produce a unique combination of IL-17A and IFN-γ after polyclonal stimulation compared to T cells extracted from non-diseased vessels.11 In murine models of atherosclerosis, deletion of IL-18 in Apoe−/− mice results in an increase in Th17 cells within the aorta and exacerbated atherosclerosis.12 Additionally, the absence of IL-17RA on bone marrow-derived cells in lethally irradiated low density lipoprotein receptor (Ldlr)-deficient mice also reduces atheroma formation.13 Together, these studies suggest a pro-atherogenic role for IL-17A and IL-17A-producing cells. More recently, a study using intravenous administration of recombinant IL-17A reduced atherosclerosis in young chimeric Ldlr−/− mice suggesting atheroprotection by IL-17A.14 However, the administration of anti-IL-17A Abs had no effects on atherosclerosis in this model.14
Here, we report that Apoe−/− mice have elevated plasma levels of IL-17A and show increased numbers of IL-17A-expressing T cells, in particular Th17 and IL-17A+ γδ+T cells, within the aorta (adventitia and intima) and spleen. Blockade of IL-17A by soluble IL-17RA causes a significant decrease in the plasma levels of IL-6 and G-CSF, diminishes aortic macrophage content, reduces aortic CXCL1 expression and leads to reduced plaque burden in Apoe−/− mice. IL-17A-treatment of isolated Apoe−/− aortas increases CXCL1 expression and monocyte adhesion. Taken together, these results demonstrate a pro-atherogenic role of IL-17A and highlight a new role for IL-17A-producing T cell subsets in the progression of atherosclerosis.
Materials and Methods
Animals
Female and male Apoe−/− mice on a C57BL/6 background and control C57BL/6 mice (Jackson Labs, Bar Harbor, ME), were kept on chow diet and used between 40 and 55 weeks of age. Alternatively, Apoe−/− mice were fed a western diet (WD; 21% fat and 0.15% cholesterol, Harlan Teklad) for 15 weeks and used at 21 weeks of age. All animals were kept in specific-pathogen-free conditions and animal experiments were approved by the Animal Care and Use Committees of the University of Virginia and Eastern Virginia Medical School. Blood counts were taken via tail bleed and analyzed by an automatic analyzer (Hemavet 850, CDC Technologies Inc., Oxford, CT). Additional materials and methods can be found in Supplemental Materials.
Statistical Analysis
For unpaired t tests, data were expressed as mean ± SEM. Statistical significance between Apoe−/− and C57BL/6 mouse groups was set at p<0.05.
Results
Levels of IL-17A are elevated in Apoe−/− mice
To test a role of IL-17A in the immune response that accompanies advanced atherosclerosis, we analyzed IL-17A mRNA within the aorta, spleen, peripheral lymph nodes (PLN, including inguinal, axillary, brachial, cervical), mesenteric lymph nodes (MLN) and lamina propria (LP) of 40–55 week-old female and males Apoe−/− and C57BL/6 mice. We have chosen to study aged Apoe−/− mice since they have been shown to develop advanced fibrous plaques at 40–45 weeks of age.15 We found a significant increase in IL-17A expression in the aorta, spleen, PLN and LP of Apoe−/− mice, but not in the MLN (Fig. 1A). As the IL-17 family consists of five additional members (IL-17B-F), we also examined the mRNA expression of IL-17C, D, E and F in Apoe−/− aortas. IL-17A mRNA expression was found to be approximately five-fold greater than the other IL-17 family members examined (Fig. 1B). No IL-17E expression was found in aortas isolated from C57BL/6 or Apoe−/− mice. Importantly, circulating levels of IL-17A were also elevated in the plasma of aged Apoe−/− compared to C57BL/6 mice (Fig. 1C). As circulating neutrophil levels correlate closely with plasma IL-17A and G-CSF levels,9 we analyzed neutrophil numbers in the blood of Apoe−/− mice. Circulating neutrophil counts (PMN) were also significantly elevated in Apoe−/− mice compared to C57BL/6 mice (Fig. 1D); however, no significant difference was seen in the numbers of white blood cells (WBC), peripheral blood lymphocytes or monocytes (Fig. 1D).
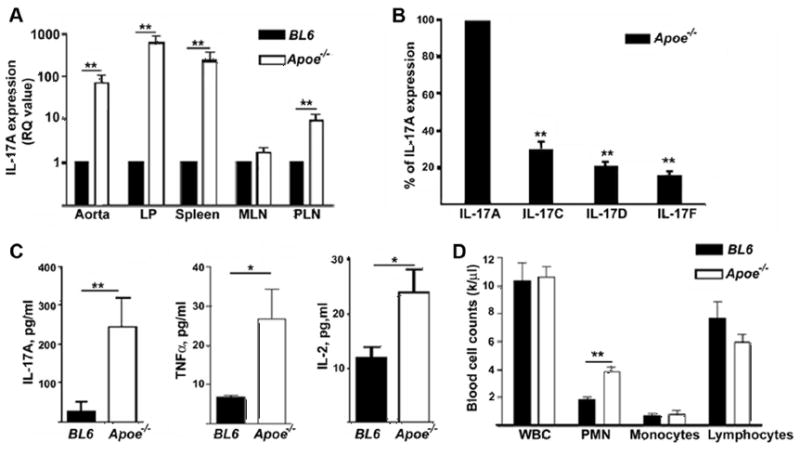
(A) IL-17A mRNA was found elevated in the aorta, spleen, LP and PLN of Apoe−/− mice (white bars, n=6) compared to C57BL/6 mice (black bars, n=6). (B) mRNA expression of IL-17C, D, and IL-17F was normalized compared to IL-17A (set as 100%) in the aortas of Apoe−/− mice (n=8). (C) Serum IL-17A, TNF-α, IL-2 were analysed in the plasma of Apoe−/− (white bars, n=10–11) and C57BL/6 mice (black bars, n=10–11), (D) Neutrophil (PMN) counts are elevated in Apoe−/− mice (white bars, n=8) compared to C57BL/6 mice (black bars, n=5); white blood cells (WBC). *P<0.05, **P<0.01 by unpaired Students t test.
IL-17A induces the release of many chemokines including CXCL1 and CCL2 as well as TNF-α, IL-1β and IL-6 from monocytes, epithelial cells and fibroblasts.6 Circulating levels of TNF-α and IL-2 were significantly elevated in Apoe−/− mice (Fig. 1C). Both Th1 (IL-12) and Th2 (IL-4, IL-5, IL-10 and IL-13) cytokines were also elevated in the plasma of Apoe−/− mice compared to C57BL/6 mice (Suppl. Fig. 1A–E). We next explored whether atherosclerosis-prone conditions induce elevated IL-17A levels through the Suppressor of cytokine signalling (SOCS-3)-dependent pathway. We found no difference in aortic mRNA SOCS-3 expression in 21 week old Apoe−/− mice fed WD or 40 week old Apoe−/− mice fed chow diet compared to C57BL/6 mice (data not shown).
Th17 and γδ+T cells are the major producers of IL-17A during atherosclerosis
As IL-17A was elevated at the RNA and circulating protein level in aged Apoe−/− mice, we attempted to identify the cellular source behind its production. Under normal conditions, the number of Th17 cells found in C57BL/6 mice is very small, with the main source of IL-17A-producing T cells being located in the LP.16,17 Here, we demonstrate elevated numbers of CD45+IL-17A+ cells in the aorta, LP and spleen of aged female and male Apoe−/− mice compared to C57BL/6 mice (Fig. 2). These IL-17A+ cells were CD45+ and CD3int or CD3+, indicating they were of T cell origin (Fig 2A and B). The percentages of T cells within the atherosclerosis-prone aortas consist of 35–40% from the leukocyte populaton3. Here, we demonstrated that about 5–7% of those cells in aortas are IL-17A-producing T cells. Further characterization of the IL-17A+ T cells revealed the presence of IL-17A-producing γδ+T cells as well as CD4+ (Th17) cells in the aorta and spleen of both Apoe−/− and C57BL/6 mice (Fig. 2C, D and E). Together, this data demonstrates a large elevation of IL-17A-producing T cells under atherosclerosis-prone conditions and suggests that both Th17 and γδ+T cells may be responsible for the elevated levels of IL-17A seen systemically in Apoe−/− mice. Large numbers of CD3+IL-17A+ leukocytes were also present in the aortas and spleen of Apoe−/− mice fed for 15 weeks WD compared to CD3+IL-17A+ cells in C57BL/6 mice (Fig. 2B), although the number of IL-17A+ T cells were not significantly different in Apoe−/− mice fed chow or WD, suggesting that the atherosclerosis-prone background rather than diet is responsible for the induction of IL-17A+ cells in vivo. We confirmed our data by RT-PCR, which also showed a significant increase of IL-17mRNA expression in aortas isolated from Apoe−/− mice fed WD for 15 weeks compared to C57BL/6 aortas (data not shown).
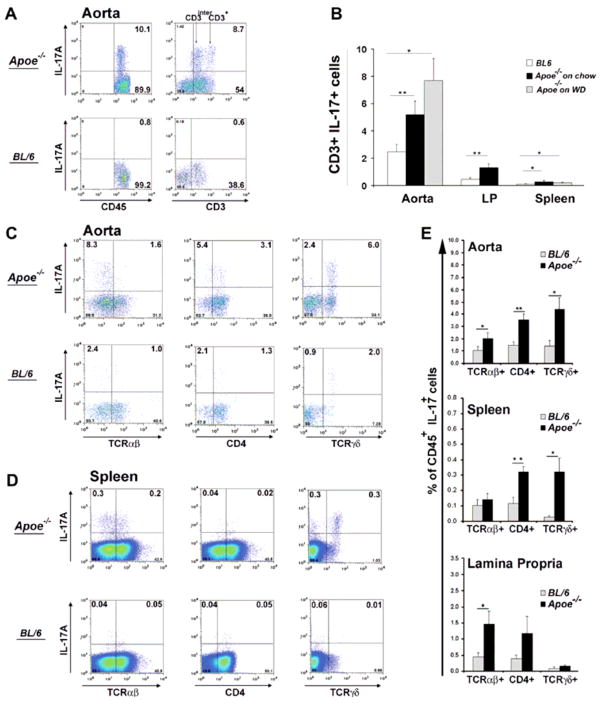
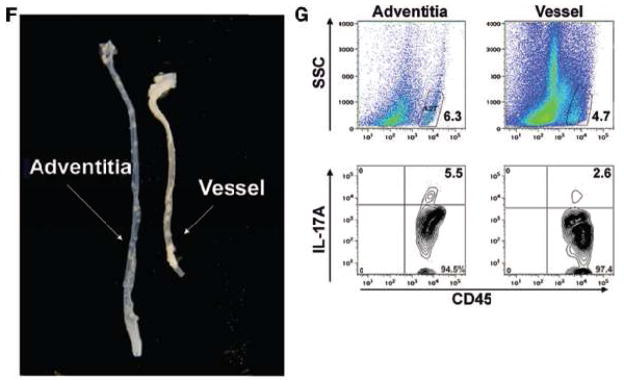
To examine leukocyte population within the analyzed tissues, a gate was set on CD45+ cells. (A) Representative FACS plot showing an elevation of IL-17A+CD45+CD3+ and IL-17A+CD45+ CD3int T cells in the aortas of aged Apoe−/− mice compared to C57BL/6 mice. (B) IL-17A+CD3+ T cells were found elevated in the aortas, spleen and LP of aged Apoe−/− (black bars, n=12) mice and 21 week old Apoe−/− mice fed WD (grey bars, n=4) compared to C57BL/6 mice (white bars, n=10). *p<0.05, **p<0.01 by unpaired Students t test with Bonferroni-Holm correction for multiple comparison (aorta and spleen). (C, D) Representative FACS plot of an aorta (C) and spleen (D) showing elevated IL-17A+ cells. The phenotype of (E) IL-17A-expressing cells in the aorta and spleen of C57BL/6 (grey bars) and Apoe−/− (black bars) mice. Results show mean ± SEM, n=6 mice from at least 3 independent experiments. (F) Representative image of isolated murine aortic adventitia and aorta. (G) Representative FACS plot showing the presence of IL-17A+ T cells in the adventitia and aortic wall of 45 week old Apoe−/− mice.
There are several reports demonstrating lymphocyte accumulation in the aortic adventitia in normal3 and atherosclerotic vessels3,18. To further analyze the location of IL-17A+ T cells within the artery, we isolated the surrounding adventitia from the aortas (Fig. 2F, Suppl. Fig. 3). Flow cytometry on the isolated adventitia and aortas from aged Apoe−/− mice showed that IL-17A+ cells are present in both, the adventitia and to a lesser extent, the vessel wall (Fig. 2G).
Blockade of IL-17A diminishes atherosclerosis in Ad-IL-17RA:Fc treated Apoe−/− mice
To identify the role for IL-17A in atherosclerosis, we used a fusion protein comprised of the IL-17RA extracellular domain fused with the murine IgG1 CH2 and CH3 domains (Ad-IL-17RA:Fc19) to block circulating IL-17A in Apoe−/− mice. To express IL-17RA:Fc in vivo, E1-deleted recombinant adenovirus (Ad-IL-17RA:Fc) was generated.19 In vivo, these adenoviruses are typically expressed in the liver and soluble products are detected in the plasma of recipient mice.20 Ad-IL-17RA:Fc significantly inhibits recombinant IL-17A-induced production of IL-6 by 3T3 fibroblasts.19 Released soluble IL-17RA (sIL-17RA) blocks the activities of IL-17A, but not IL-17F and acts as a soluble receptor for IL-17A.21 Importantly, Ad-IL-17RA:Fc does not induce leukocyte activation via surface Fc receptors (data not shown).
We experimentally developed a protocol that allows maintaining the expression of Ad-IL-17RA:Fc for 15 weeks (Suppl. Fig. 2). To accelerate atherosclerosis development, mice were fed Western diet.15 In order to promote tolerance to the adenovirus and minimize possible adenovirus-mediated immune responses, we performed retro-orbital intravenous injections of Ad-IL-17RA:Fc or Ad-Lu (which encodes firefly luciferase) in 4 day old Apoe−/− mice. This was followed by booster injections of Ad-IL-17RA:Fc or Ad-Lu at days 42 and 104 (Suppl. Fig. 2). To track the expression of Ad-IL-17RA:Fc over the time in vivo, we carried out western blots using plasma from Ad-IL-17RA:Fc, and as negative controls, plasma from Ad-Lu-injected mice (Fig. 3A, lane 3) and Il-17ra−/− mice (Fig. 3A, lane 4). Figure 3A shows the detection of plasma sIL-17RA in 6 week old Apoe−/− mice, 7 days after the second injection of Ad-IL-17RA:Fc (Fig. 3A, lanes 1, 2). To confirm the expression of control Ad-Lu, we analyzed Ad-Lu expression by bioluminescence imaging (data not shown). Soluble IL-17RA was also detectable in the plasma of Ad-IL-17RA:Fc-recipient mice at the day of termination (Fig. 3B). To confirm that tolerance was maintained in the adenovirus-treated mice, we analyzed IFN-γ concentration in peripheral blood of recipient mice and found plasma levels of IFN-γ did not reach more than ~22ng/ml in Ad-IL-17RA:Fc and Ad-Lu-treated recipients (Fig. 4B). These results suggest that an acute immune response did not occur in the adenovirus recipient mice cohorts. Apoe−/− mice in both groups had comparable levels of triglycerides (186±28 mg/dl and 190±40 mg/dl, for Ad-IL-17R-Fc and Ad-Lu-injected Apoe−/− mice, respectively). Interestingly, Apoe−/− mice that received Ad-IL-17RA:Fc showed an increase of total plasma cholesterol levels compared to Apoe−/− mice that received control Ad-Lu (591±61 mg/dl and 350±40 mg/dl, respectively, p< 0.001).
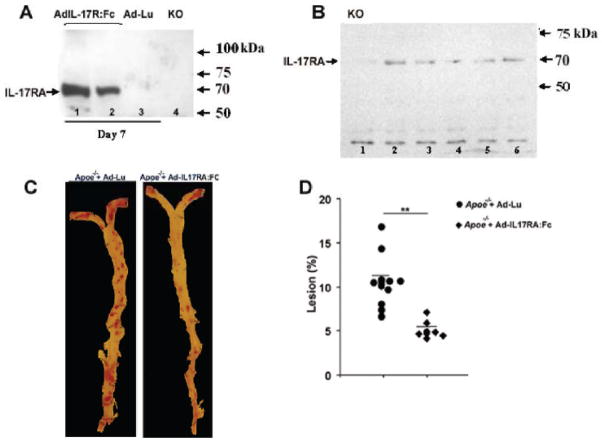
(A) Lane 1–3, Detection of soluble IL-17RA in the plasma of an Apoe−/− mouse 7 days after Ad-IL-17RA:Fc treatment by immunoprecipitaion and western blot; lane-4, KO, (plasma from Il17ra−/− mice). (B) Western blot demonstrating the detection of sIL-17RA in the plasma of Apoe−/− mice injected with Ad-IL-17RA:Fc on the day of termination (21 week old Apoe−/− mice). Lane 1, KO-negative control (Il17ra−/− plasma), lane 2–6-experimental Apoe−/− mice. (C) Representative en face staining of aortas excised from 21 week-old (15 weeks on WD) Apoe−/− mice treated with Ad-IL-17RA:Fc (right) or Ad-Luc (left). (D) Lesion sizes for Apoe−/− mice treated with Ad-IL-17RA:Fc (n=7) or Ad-Luc (n=11), (% of whole aorta). Each symbol represents one animal, horizontal bars represent means. **P<0.01.
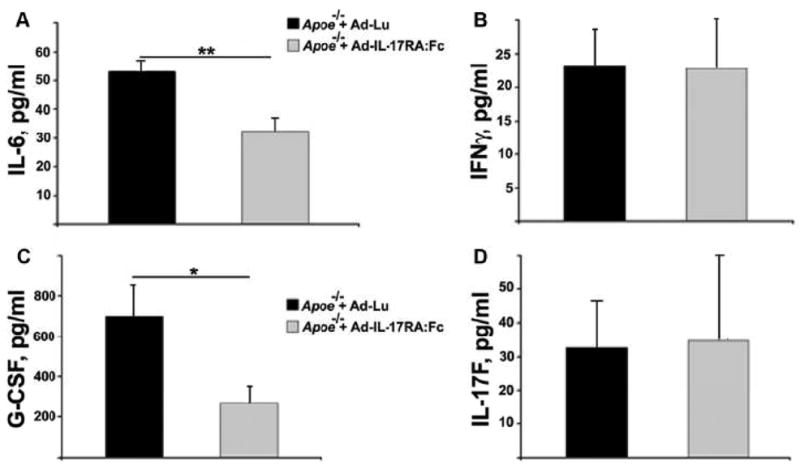
Collected plasma supernatants from Apoe−/− mice treated with Ad-IL-17RA:Fc or with control Ad-Lu were analyzed for the presence of (A) IL-6, (B) IFN-γ, (C) G-CSF and (D) IL-17F. Results show mean±SE from 5 to 10 mice. *, P<0.05, **, P<0.01.
Following the adenovirus treatments, the aortas of 21 week-old Apoe−/− mice were analyzed for plaque burden using en face Oil Red O staining. Apoe−/− mice that received Ad-IL-17RA:Fc developed on average 54% smaller lesions (5.7±0.4%) throughout the aorta compared to the mice that received control Ad-Lu construct (11.0±0.8%; Fig. 3C, D). The percentage of plaque area within the aortic roots was decreased by 18% in Ad-IL-17R:Fc-injected Apoe−/− mice compared with control Ad-Lu-injected Apoe−/− mice (45.8±2.1% and 54.5±1.4%, respectively, p<0.05). These results strongly implicate IL-17A as a pro-atherogenic cytokine in atherosclerosis.
To investigate the mechanisms by which the blockade of circulating IL-17A may lead to diminished atherosclerosis, we examined the circulating levels of key pro-inflammatory cytokines in the plasma of treated-Apoe−/− mice. We found a significant decrease in IL-6 plasma levels in Ad-IL-17RA:Fc- compared to Ad-Lu-treated Apoe−/− mice (Fig. 4A). Previous studies have shown that IL-17A drives the production of granulocyte colony-stimulating factor (G-CSF), which is directly responsible for the differentiation and proliferation of neutrophil precursors in the bone marrow9. Therefore, we hypothesized that the inhibition of IL-17A may lead to decreased levels of G-CSF in Ad-IL17-RA:Fc-treated mice. As shown in Fig. 4C, we detected diminished levels of G-CSF in the plasma of Ad-IL17-RA:Fc–treated compared to Ad-Lu-treated Apoe−/− mice. This data confirms that sIL-17RA neutralized plasma IL-17A activity and as a consequence decreased G-CSF production, which in turn may lower blood neutrophil numbers. Ad-IL-17RA:Fc treatment did not affect circulating IL-17F plasma levels in Apoe−/− recipient mice compare to Apoe−/− that received Ad-Lu (Fig. 4D).
IL-17A regulates macrophage accumulation within the aortas of Apoe−/− mice via CXCL-1 dependent mechanism
IL-17A promotes inflammation by the induction of pro-inflammatory chemokines such as CCL2 and CXCL1, enabling the recruitment of neutrophils and monocytes into inflamed tissue. Therefore, we examined the presence of CXCL1 and CCL2 in the aortas of Ad-IL-17RA:Fc or Ad-Lu-treated mice. A significant reduction in CXCL1 expression, but not CCL2 expression (not shown) was seen in aortic roots of Ad-IL-17RA:Fc Apoe−/− mice compared to control Ad-Lu Apoe−/− mice (Fig. 5A and C). A significant decrease in Mac-2+ area staining for macrophages was also found within the aortic roots Ad-IL-17RA:Fc Apoe−/− mice (Fig. 5B and D). The decrease in the total number of macrophages in Ad-IL-17RA:Fc Apoe−/− mice was also detected when we evaluated the number of plaque macrophages (78±7 per 104 μm2 and 285±10 per 104 μm2 macrophages for Ad-IL-17RA:Fc Apoe−/− and Ad-Lu Apoe−/− mice, respectively). These results suggest that IL-17A-producing T cells might promote atherogenesis by inducing monocyte migration into the atherosclerotic lesion.
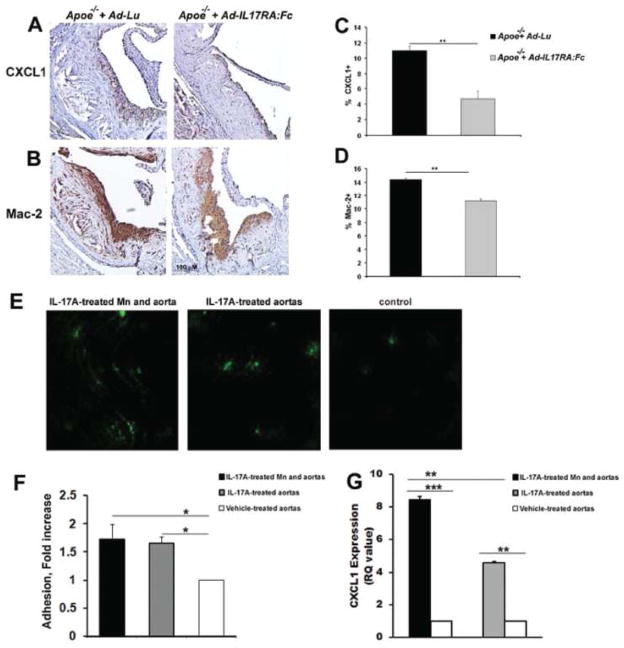
(A) Paraffin-embedded sections of aortic roots from 21 week old Apoe−/− mice that received either Ad-IL-17RA:Fc or Ad-Lu adenoviruses were stained with Abs against CXCL1 (A, brown) and Mac-2 (B, brown), and counterstained with hematoxylin (blue). Representative staining from one mouse is shown. Percentage of (C) CXCL1 and (D) Mac-2 positive staining relative to the total area of the aortic root. Values represent mean±SEM of 5 to 10 mice per group. (E) IL-17A-treated aortas show increased monocyte adhesion. Aortas were harvested from 25–30 week old Apoe−/− mice and treated with IL-17A as described in Materials and Methods. Fluorescently labeled monocytes (green) were added to aortas for an adhesion assay and adherent monocytes were counted by blinded observers using a fluorescent microscope. (F) Data represent the mean±SE of 3–4 counted aorta per group from 4 independent experiments. (G) Aortic mRNA expression. Total cellular RNA from vehicle-treated (white bars), IL-17A-treated (grey bars) and unwashed IL-17A-treated aortas (black bars) were examined for CXCL1 using quantitative real-time PCR. * P<0.05, ** P<0.01 by Student’s t test with Bonferroni-Holm correction for multiple comparison (for Fig. 5F and G). Data are from 3 independent experiments using 3–4 aortas per group.
To further test the hypothesis whether IL-17A is involved in the regulation of monocyte adhesion/migration, we performed ex vivo monocyte adhesion assays using IL-17A-treated or vehicle-treated isolated Apoe−/− aortas. We detected increased adhesion of CFSE-labeled monocytes to the IL-17A-treated luminal aortic wall in the presence of IL-17A compared to vehicle-treated aortas (Fig. 5E and F). Interestingly, elevated monocyte adhesion was also detected when both monocyte and isolated aortas were treated with IL-17A (Fig. 5E and F). To determine if the differences in monocyte adhesion could be explained by the induction of CXCL1, we further analyzed the aortas for CXCL1 expression by RT-PCR, and detected a significant increase in the CXCL1 expression in both IL-17A-treated monocytes/aortas and IL-17A-treated aortas in comparison to the vehicle-treated aortas (Fig. 5G). Together, these results suggest that IL-17A can activate vascular cells, induce CXCL1 production, and further accelerate monocyte recruitment into the aortic wall. In addition to this effect, IL-17A also elevates monocyte adhesion through the activation of monocytes in peripheral circulation.
Discussion
Our study reveals that IL-17A-producing T cells are present in the aortas of C57BL/6 mice and their numbers are greatly elevated under the atherosclerosis-prone conditions found in Apoe−/− mice fed chow diet or WD. Interestingly, not only Th17, but also γδ+ T cells are the source of IL-17A within atherosclerosis-prone aortas. The adenovirus mediated blockade of IL-17A resulted in reduced plasma levels of IL-6 and G-CSF, and diminished plaque burden with a concomitant reduction of aortic macrophages and CXCL1. Moreover, IL-17A elevates CXCL1-dependent monocyte adhesion to isolated aortas ex vivo. Thus, we demonstrate a pro-atherogenic role for IL-17A in the development of atherosclerosis in mice.
The association of IL-17A with human autoimmunediseases has been extensively shown in patients with rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease and psoriasis8. A growing body of evidence also suggests that IL-17A might be involved in the immune response during atherosclerosis.10,11,22,14 Soluble levels of IL-17A are elevated in the onset of acute coronary syndrome compared to stable angina10 and in a subset of aged patients with coronary atherosclerosis and referent outpatients.11 Recently, Xie et al. reported elevated levels of IL-17A in the plasma of Apoe−/− mice at 8–16 weeks of age23. Here, we confirm and extend these results and demonstrate elevated levels of plasma IL-17A in 40–50 week old Apoe−/− mice with a concomitant increase in IL-2, IL-6, and TNF-α. We also found that IL-17A is the most prevalent cytokine of IL-17 family in Apoe−/− mice. Indeed, the number of IL-17A+ T cells is increased in the artery wall, spleen, and LP of aged Apoe−/− mice suggesting that systemic inflammation found in Apoe−/− mice may initiate the differentiation of IL-17A-producing T cells in secondary lymphoid and non-lymphoid tissues. Recent reports have indicated an important role of adventitita in atherogenesis.1,24 Most CD3+ cells are located within the aortic adventitia of atherosclerosis-prone Apoe−/− mice.3,18 Our study clearly identifies IL-17A-producing T cells by flow cytometry and RT-PCR on preparations of total aorta that include artery wall and surrounding adventitia. Using a flow cytometry on the aortic adventitia, we also demonstrated that IL-17A+ T cells are located in the adventitia as well as within the aortas of Apoe−/− mice. The role of leukocytes that reside in different locations during atherogenesis are not well understood, and further studies will identify the specific role of IL-17A+ cells in these two different sites.
Recently, a study by Eid et al. showed that CD4+ cells isolated from human atherosclerotic coronary arteries produce IL-17A as well as IFN-γ in response to polyclonal stimulation in vitro.11 In this report, we further examine the phenotype of IL-17A+ cells and demonstrate that not only Th17 cells, but also IL-17A+γδ+ T cells are significantly elevated in atherosclerosis-prone murine aortas. The mechanisms behind human and murine Th17 cell differentiation are well established in vitro; however, little information is available about the induction of IL-17A+ cells during chronic inflammation in vivo. SOCS-3 is involved in the preferential promotion of Th17 cell induction.25 Interestingly, we found no significant changes in SOCS-3 expression between the aortas of C57BL/6 and Apoe−/− mice. These preliminary findings suggest that IL-17A is likely up-regulated independently of SOCS-3 in atherosclerosis-prone conditions. Further studies are necessary to determine the mechanisms behind IL-17A induction in atherosclerosis. Recently, CD27 was identified as a thymic determinant of the balance between IFN-γ and IL-17A-producing γδ+T cell subsets.26γδ+T cells also express RORγτ and produce IL-17A, IL-21, and IL-22 in response to IL-1β and IL-23, without T cell receptor (TCR) engagement.27 The existence of expanded IL-17A+γδ+T cells population in Apoe−/− mice suggest at least partial TCR-independent upregulation of IL-17A during atherosclerosis. Further studies will be necessary to investigate the mechanisms of the induction of IL-17A+ T cells during atherogenesis.
There are several pathways by which IL-17A might affect local inflammation. IL-17A induces the production of cytokines and chemokines in several cell types, including endothelial and vascular smooth muscle cells (VSMCs).28,29 IL-17A and IFN-γ synergistically initiate production of IL-6, IL-8, CCL5, CXCL1 and CXCL10 in cultured VSMCs.11 CXCL1 is a chemokine that triggers monocyte arrest on endothelium during atherogenesis and thus, plays a critical role in monocyte recruitment into artery wall.30 Monocytes express IL-17RA on their surface, and recent data suggests that IL-17A can also directly affect monocyte chemotaxis in vivo and in vitro.31,32 Antibody blockade of IL-17A in the synovial fluid of rheumatoid arthritis patients inhibits monocyte migration in vitro as well as macrophage accumulation in the bronchoalveolar lavage fluid during allergic airway inflammation.31,32
The role of IL-17A and IL-17A-expressing T cells in atherosclerosis remains unclear. Several studies with conflicting results have been published and more research on the role of IL-17A-dependent response in atherosclerosis is warranted. Our study, for the first time, characterizes the subpopulations of T cells that express IL-17A in atherosclerosis and highlight a possible role of IL-17A in the regulation of monocyte migration into aortas. We report that blocking IL-17A in vivo leads to decreased CXCL1 expression within atherosclerosis-prone aortas and diminished aortic macrophage content. We also demonstrate the pro-inflammatory role of IL-17A in the regulation of monocyte migration into the aortas via ex vivo monocyte adhesion assay. Together, these results indicate that IL-17A contributes to the pathogenesis of atherosclerosis through the regulation of monocyte recruitment to the aortic wall at least via CXCL1. It remains to be determined whether IL-17A also activates monocytes/macrophages within the plaque to induce the synthesis of TNFα, IL-1β, IL-6, CXCL2 and GM-CSF and further perpetuate inflammation.
IL-17A regulates the levels of G-CSF in circulation and thus, affects neutrophil numbers in the blood. We detected decreased levels of G-CSF in the plasma of Apoe−/− mice that received Ad-IL-17RA:Fc. It would be important to investigate the role of IL-17A-dependent involvement of neutrophils in atherogenesis. To further understand the cytokine profile upon blocking of IL-17A, we also analyzed plasma levels of IL-17F, a cytokine that can compensate for a deficiency in IL-17A in Il-17a−/− mice.33 We found no difference between Ad-IL-17RA:Fc- and Ad-Lu-treated groups of mice suggesting that the neutralization of IL-17A is not enough to induce compensatory elevation of IL-17F in Apoe−/− mice.
Regulation of Th17 cell differentiation by IFN-γ was shown in vitro assays,8 implicating the Th1 cell signals as potent inhibitors of Th17 cell differentiation. However, a population of cells that secrete both IFN-γ and lL-17A can be detected among cells isolated from inflammatory conditions in vivo.34,35,36 To date, it is also unclear whether IL-17A can regulate the Th1 response in vivo. Our results demonstrate that blocking of IL-17A with soluble IL-17RA has no effects on the levels of plasma IFN-γ in Apoe−/− mice. Further studies will be necessary to explored complex relationship between Th1 and Th17 cell differentiation programs in vivo.
In summary, the discovery of IL-17A-producing Th17 and γδ+T cells and their pro-atherogenic properties, challenges the Th1 and Th2 paradigm thought to be involved in the adaptive immune response of cardiovascular disease. Our findings suggest a new IL-17A-dependent pathway by which the immune system may influence the development and progression of atherosclerosis.
Acknowledgments
We thank Dr. Brent French and Dr. Sanford Feldman for technical advice with the adenovirus experiments.
Source of Funding.
This work was supported by National Institutes of Health grant: PO1 HL55798 (to K. Ley) and American Heart Association Scientist Development Grant 0525532U (to E. Galkina).
References
Full text links
Read article at publisher's site: https://doi.org/10.1161/circulationaha.109.924886
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.109.924886
Free after 12 months at intl-circ.ahajournals.org
http://intl-circ.ahajournals.org/cgi/reprint/121/15/1746.pdf
Free to read at intl-circ.ahajournals.org
http://intl-circ.ahajournals.org/cgi/content/abstract/121/15/1746
Free after 12 months at intl-circ.ahajournals.org
http://intl-circ.ahajournals.org/cgi/content/full/121/15/1746
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1161/circulationaha.109.924886
Article citations
Local adaptive immunity in atherosclerosis with T cell activation by aortic dendritic cells accelerates pathogenesis.
iScience, 27(11):111144, 10 Oct 2024
Cited by: 0 articles | PMID: 39502289 | PMCID: PMC11536043
Inflammation and all-cause mortality in patients undergoing peritoneal dialysis.
Einstein (Sao Paulo), 22:eAO0627, 09 Aug 2024
Cited by: 0 articles | PMID: 39140572 | PMCID: PMC11323835
Cross-talks between perivascular adipose tissue and neighbors: multifaceted nature of nereids.
Front Pharmacol, 15:1442086, 02 Aug 2024
Cited by: 0 articles | PMID: 39156105 | PMCID: PMC11327032
Review Free full text in Europe PMC
The Impact of Interleukin-17 Inhibitors on Major Adverse Cardiovascular Events in Psoriasis or Psoriatic Arthritis Patients Naive to Biologic Agents: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.
Cureus, 16(5):e60980, 24 May 2024
Cited by: 0 articles | PMID: 38910708 | PMCID: PMC11193915
Review Free full text in Europe PMC
Interactions Between HDL and CD4+ T Cells: A Novel Understanding of HDL Anti-Inflammatory Properties.
Arterioscler Thromb Vasc Biol, 44(6):1191-1201, 25 Apr 2024
Cited by: 3 articles | PMID: 38660807
Review
Go to all (278) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Protein structures in PDBe
-
(1 citation)
PDBe - 17RAView structure
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Smooth Muscle Cell-Derived Interleukin-17C Plays an Atherogenic Role via the Recruitment of Proinflammatory Interleukin-17A+ T Cells to the Aorta.
Arterioscler Thromb Vasc Biol, 36(8):1496-1506, 30 Jun 2016
Cited by: 28 articles | PMID: 27365405 | PMCID: PMC5242324
IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis.
J Immunol, 193(9):4344-4355, 26 Sep 2014
Cited by: 82 articles | PMID: 25261478 | PMCID: PMC4201987
The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment.
Circ Res, 110(5):675-687, 02 Feb 2012
Cited by: 110 articles | PMID: 22302786 | PMCID: PMC3337709
Interleukin 17A in atherosclerosis - Regulation and pathophysiologic effector function.
Cytokine, 122:154089, 26 Jun 2017
Cited by: 21 articles | PMID: 28663097
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (5)
Grant ID: P01 HL055798-110009
Grant ID: P01 HL055798-140009
Grant ID: P01 HL55798
Grant ID: P01 HL055798
Grant ID: P01 HL055798-070007





