Abstract
Free full text

Both Hematopoietic-Derived and Non–Hematopoietic-Derived β-Arrestin–2 Regulates Murine Allergic Airway Disease
Abstract
Allergic asthma, a major cause of morbidity and leading cause of hospitalizations, is an inflammatory disease orchestrated by T helper cells and characterized by the lung migration of eosinophils, which are important asthma effector cells. Lung migration of inflammatory cells requires, among other events, the chemokine receptor transduction of lung-produced inflammatory chemokines. Despite the widespread prevalence of this disease, the molecular mechanisms regulating chemokine production and receptor regulation in asthma are poorly understood. Previous work from our laboratory demonstrated that β-arrestin−2 positively regulates the development of allergic airway disease in a mouse model, partly through positive regulation of T-lymphocyte chemotaxis to the lung. However, β-arrestin−2 is expressed in many cell types, including other hematopoietic cells and lung structural cells, which are involved in the development and manifestation of allergic airway disease. To determine the cell types required for β-arrestin–2–dependent allergic inflammation, we generated bone marrow chimera mice. Using the ovalbumin murine model of allergic airway disease, we show that eosinophilic and lymphocytic inflammation is restored in chimeric mice, with expression of β-arrestin−2 exclusively on hematopoietic-derived cell types. In contrast, airway hyperresponsiveness is dependent on the expression of β-arrestin−2 in structural cells. Our data demonstrate that the expression of β-arrestin−2 in at least two divergent cell types contributes to the pathogenesis of allergic airway disease.
Asthma is a complex, heritable inflammatory disease that has been increasing in severity and prevalence (1). Currently, asthma affects ~11% of the United States population, and this disease accounts for $9.4 billion of direct healthcare costs (2). However, the mechanisms underlying the development of allergic asthma remain poorly understood, and there is a dearth of broadly effective new asthma treatments. We previously reported that mice deficient in β-arrestin−2 do not develop the hallmark features of allergic airway disease, thus identifying β-arrestin−2 as a potential novel therapeutic target. The G-protein–coupled receptors (GPCRs) have long been targeted in the treatment of asthma (3). Here we further explore the role of β-arrestin−2, a GPCR regulatory protein, in allergic airway disease, and comment on its potential importance as a therapeutic target for asthma.
Chemokine receptors are part of the GPCR family of seven transmembrane cell surface receptors (4). Classically, these receptors transduce extracellular signals into intracellular events by activating heterotrimeric G proteins. The dissociation of these G-protein subunits activates cell-signaling systems such as adenylate cyclases, phospholipases, and ion channels, ultimately resulting in a physiologic response. Although well-known for the induction of chemotaxis, chemokine-receptor activation can result in other physiologic cell functions, including chemokine production (5) and cell degranulation (6).
Chemokine receptor function is regulated by β-arrestin proteins (7). The β-arrestins, members of the arrestin family of proteins, are designated β-arrestin−1 or β-arrestin−2, are ubiquitously expressed, and regulate GPCR function through multiple mechanisms (8–10). As their name suggests, β-arrestin proteins were originally discovered to “arrest” G protein–mediated cell-signaling events (11). Since that time, our understanding of the mechanisms by which β-arrestin modulates GPCR function has expanded considerably. In addition to their classic role, β-arrestin proteins also act as adapters that couple GPCRs to a clathrin-coated pit endocytic mechanism, and that transduce GPCR ligand-binding into G protein–independent cell signaling, as reviewed elsewhere (12). Given the divergent function of β-arrestins, these proteins are likely to contribute to a number of common human diseases.
The inflammatory process in allergic airway disease is very complex. In brief, exposure of the lung to an allergen induces an adaptive immune response, characterized by a chemokine-directed migration of dendritic cells, T lymphocytes, eosinophils, mast cells, and neutrophils. Chemokines are produced by lung structural cells, such as airway epithelial and smooth muscle cells, as well as lung leukocytes, through the induction of inflammatory mediators (13). These inflammatory mediators constitute a broad range of molecules that can act at cytokine, and G protein–coupled, receptors. The β-arrestins functionally regulate both of these receptor families (12).
We previously demonstrated that mice lacking β-arrestin−2 do not develop the classic features of allergic airway disease. Specifically, allergen-sensitized and allergen-challenged β-arrestin−2−/− animals display a reduced level of Th2 cytokines, very low numbers of lung eosinophils/lymphocytes, and the absence of airway hyperresponsiveness (AHR) (14). Although our study showed that T lymphocyte chemotaxis was defective in the absence of β-arrestin−2, it was not abolished. Thus, it remains unknown whether β-arrestin−2 contributes to the function of other cell types required for the complete allergic phenotype. In this report, we address the possibility that β-arrestin−2 regulates the lung production of allergic inflammatory mediators and/or the lung migration of eosinophils. To focus on these questions, we used bone marrow chimera mice whose hematopoietic cells, but not structural cells, were replete with β-arrestin−2, and vice versa. In addition to the allergen, that is, ovalbumin (OVA), model of allergic inflammatory airway disease, we used an IL-13 model, in which eosinophil migration to the lung and other signs of allergic inflammatory airway disease occur in a manner independent of T lymphocytes (15).
MATERIALS AND METHODS
Inbred Mice
Genetically engineered β-arrestin−2 knockout mice were backcrossed onto a C57BL/6J background for at least six generations (14). The β-arrestin−2 heterozygotes were bred so that littermate wild-type and knockout mice were compared. Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Duke University Medical Center and the Durham Veterans Administration, and were performed in accordance with the standards established by the United States Animal Welfare Acts.
Bone Marrow Transplant
To examine the relative importance of β-arrestin−2 expression in hematopoietic and nonhematopoietic cells in the response to OVA and IL-13, we created bone marrow chimeric mice, as previously described (16). Mice expressing β-arrestin−2 in hematopoietic cells but not structural cells (H+/S−) were generated by transferring bone marrow from β-arrestin–replete wild-type mice (CD45.2) or B6.SJL mice (CD45.1) into irradiated β-arrestin−2–deficient mice (CD45.2). Conversely, mice expressing β-arrestin−2 in structural cells but not hematopoietic cells (H−/S+) were generated by transferring bone marrow from β-arrestin−2 deficient mice (CD45.2) into irradiated wild-type mice (CD45.2) or B6.SJL mice (CD45.1). Control groups were also generated by transferring bone marrow from B6.SJL mice (CD45.1) to wild-type mice (CD45.2) (H+/S+), and from β-arrestin−2–deficient mice (CD45.2) to β-arrestin−2–deficient mice (CD45.2) (H−/S−). These chimera animals were created by the lethal irradiation of 6–8-week-old recipient female mice with 10.5 Gy. Within 4 hours after irradiation, the mice were injected intravenously with 4 × 106 donor bone marrow cells. Engraftment was confirmed by flow cytometry in the peripheral blood at 5–6 weeks after transplantation. Flow-cytometry analysis of peripheral blood showed that after a single irradiation and bone marrow transplantation, peripheral engraftment was high and not significantly influenced by the absence of β-arrestin−2. Lymphocyte engraftment for each chimera was as follows: H+/S+, 89.7% with a range of 74.5 to 97.4%; H−/S+, 91.2% with a range of 88.5 to 98.1%; and H+/S−, 92.5% with a range of 87.8% to 94.6%. The average lymphocyte engraftment for all chimeras was 91.0%. Because the CD4+ T-cell response to allergen is dysfunctional in the absence of β-arrestin−2, we also show that the extent of CD4+ T-cell engraftment is not significantly different in the various chimeras. The CD4+ individual group engraftment entailed: H+/S+, 83.8%; range, 64.4–98.9%; H−/S+, 75.4%; range, 65.4–91.4%; and H+/S−, 90.3%; range, 85.0–98.9%. All animals were phenotyped 8–10 weeks after transplantation.
Flow Cytometry for Engraftment
Fifty microliters of heparinized peripheral blood were stained with monoclonal antibodies for 15 minutes at room temperature. The stained whole-blood samples were then processed in a Multi-Q-Prep (Coulter, Miami, FL) to lyse red blood cells. Fifty microliters of Flow-Count fluorospheres (Coulter) were added before flow cytometric analysis. The stained cells were analyzed using a Coulter EPICS XL equipped with System II software.
T-Lymphocyte Adoptive Transfer
Spleens were removed from CO2-sacrificed donor mice and mechanically disaggregated. Erythrocytes were lysed before the use of high-affinity negative selection (MCD4C-100; Research and Diagnostics, Minneapolis, MN) to isolate CD4+ T lymphocytes. We washed and resuspended 6 × 106 CD4+ T cells in a 250-μl volume of sterile RPMI with 10% FCS. The cells were injected retro-orbitally into recipient B6.129S7-Rag1tm1Mom/J mice (RAG) (Jackson Laboratories, Bar Harbor, ME). Control RAG mice were injected with vehicle.
OVA Sensitization and Challenge
Mice were sensitized on Days 0, 7, and 14 by intraperitoneal injection of 10 μg OVA (Sigma, St. Louis, MO), adsorbed to 2 mg of alum adjuvant (Pierce Biotechnology, Inc., Rockford, IL) diluted in saline, as previously described (14). On Days 21, 22, and 23, mice were exposed once for 60 minutes to an aerosol of 1% OVA (Sigma) in saline, generated using a six-jet atomizer (TSI, Inc., St. Paul, MN). The term “OVA treatment” is used hereafter to refer to mice treated according to the OVA sensitization and OVA aerosol challenge protocol described above. Animals were phenotyped 24 hours after their final aerosol challenge.
IL-13 Challenge
Mice received 50 μl of mouse recombinant IL-13 (3.9 μg; Biosource International, Carlsbad, CA) in endotoxin-free saline by oropharyngeal instillation on Days 1, 3, and 6 (17). On Day 7, all animals were phenotyped for AHR and cellular inflammation in whole-lung lavage.
Airway Physiology: Airway Pressure Time Index
Mice were anesthetized with pentobarbital sodium (60 mg/kg, intraperitoneal). Tracheal pressure was measured using a differential pressure transducer connected to the sideport of a surgically inserted tracheostomy cannula for ventilating the animals with 6–8 ml/kg of 100% oxygen at 125 breaths/minute. Neuromuscular blockade was accomplished with doxacurium chloride (0.25 mg/kg) and assessed by the appearance of spontaneous respiratory efforts. At 5-minute intervals, intravenous methacholine (25, 100, and 250 μg/kg) was administered into the jugular vein, and the time-integrated change in peak tracheal pressure (airway pressure time index) was calculated for the first 30 seconds of bronchoconstriction. The airway pressure time index is a measure that others have validated for its ability to provide a reasonable index of airway responsiveness, as assessed by the more specific mechanical variables of resistance and compliance (18, 19).
Analysis of Lung Lavage Cells and Cytokines
Whole-lung lavage cytokine concentrations and cell differentials were determined as previously described (14). Briefly, cytospin cell preparations (Cytospin 2; ThermoShandon, Pittsburgh, PA) were stained using a Hema-3 staining kit (Fisher Scientific, Springfield, NJ), and 200 cells were classified using standard morphologic criteria. Lavage-fluid cytokine levels were determined using commercially available cytokine ELISA kits (Quantikine; R&D Systems, Inc., Minneapolis, MN) and a Luminex instrument (BioRad, Hercules, CA). The detection limit for both eotaxin and regulated upon activation, normal T-cell expressed, and secreted (RANTES) was 16 pg/ml.
Cytokine Analysis of Lung Homogenates
Lung samples were homogenized (50 mM Tris-HCl, pH 7.5, 10% glycerol, 0.1 mM EDTA, and 1 mM DTT) and centrifuged at 13,000 rpm for 10 minutes at 4°C to remove tissue debris. The supernatant protein concentration of each sample was determined before use. All sample concentrations were adjusted so that equal amounts of protein were used in a Mouse IL-13 Quantikine ELISA kit (R&D Systems, Inc.), according to the manufacturer's instructions. The final concentration of lung homogenate IL-13 was expressed as picograms per gram of lung protein.
Histologic Semiquantitation of Lung Inflammation
Lung-tissue inflammation was semiquantitatively determined in a blinded examination of hematoxylin-and-eosin–stained sections, using a six-tiered scoring system of inflammation severity. Normal lung tissue was given a grade of 0 (no inflammation). Grade 1 (mild) referred to those sections with mild inflammation, as characterized by peribronchiolar/perivascular inflammation less than three cells in depth. Grade 2 was assigned when eosinophils were predominant in the grade 1 pattern. A score of grade 3 (moderate) was assigned to those sections with more disseminated peribronchiolar/perivascular inflammatory infiltrates, 3–5 cells in depth, and the grade was advanced to 4 when eosinophils were predominant. A score of grade 5 (marked) referred to those lung sections with diffuse peribronchiolar/perivascular inflammation, 5–15 cells in depth, and the grade was advanced to 6 when eosinophils predominated. To assess airway mucus production, lung sections were analyzed using the periodic acid-Schiff method. For each sample, 10 randomly chosen airways from one slide were graded to generate an average score. The scale ranged from 0–4, where 0 indicated no staining within the airway, 4 indicated greater than 75% airway staining, and scores 1, 2, and 3 represented up to 25%, 50%, and 75% staining, respectively.
Statistics
Data are expressed as mean ± SEM. Significant differences among groups were identified according to ANOVA. Individual comparisons between groups were confirmed by Student t test, unless otherwise stated (20). When variances were unequal, Welch's correction was applied. Statistical calculations were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). A P value of less than 0.05 was considered statistically significant.
RESULTS
Hematopoietic β-Arrestin−2 Is Sufficient for the Development of Antigen-Dependent Allergic Airway Inflammation
Previous work from our laboratory supports a critical role for the GPCR regulatory protein, β-arrestin−2, in the development of allergic inflammatory airway disease in an OVA murine model of allergic asthma. In the lung, β-arrestin−2 is ubiquitously expressed (21). Therefore, for a better understanding of the cell types required to express β-arrestin−2 in the OVA model of allergic asthma, we generated and then OVA-sensitized and OVA-challenged bone marrow chimeric mice. Chimeric mice express β-arrestin−2 exclusively on either hematopoietic (H+/S−) or nonhematopoietic structural cell types (H−/S+). Wild-type bone marrow completely restored eosinophil and T-lymphocyte recruitment into the lung, as measured by the number of cells present in the whole-lung lavage fluid (Figures 1A and 1B). Similarly, T-lymphocyte and eosinophil migration was impaired in mice transplanted with β-arrestin−2–deficient bone marrow (H−/S+). The histologic evaluation of lung tissue demonstrated robust peribronchiolar/perivascular inflammation in animals with wild-type hematopoietic cells (H+/S+ and H+/S−), but not β-arrestin−2–deficient hematopoietic cells (H−/S+ and H−/S−) (Figures 1C and 1D). Similarly, a trend for enhanced airway mucus content in mice receiving β-arrestin−2–replete hematopoietic cells was evident (Figures 1E and 1F). Although elevated in response to OVA, whole-lung lavage levels of eosinophilic chemokines, eotaxin (Figure 1G), and RANTES (data not shown) were independent of β-arrestin−2 expression. Lung homogenate levels of IL-13 were elevated in association with hematopoietic β-arrestin−2 (Figure 1H). These observations support a critical role of hematopoietic-derived β-arrestin−2, but not lung structural β-arrestin−2, in the biological response to OVA. This result was not entirely unexpected, because the chemotaxis of T lymphocytes, key regulators of antigen-dependent allergic airway inflammation, was previously shown to be dependent on β-arrestin−2 (14, 22).
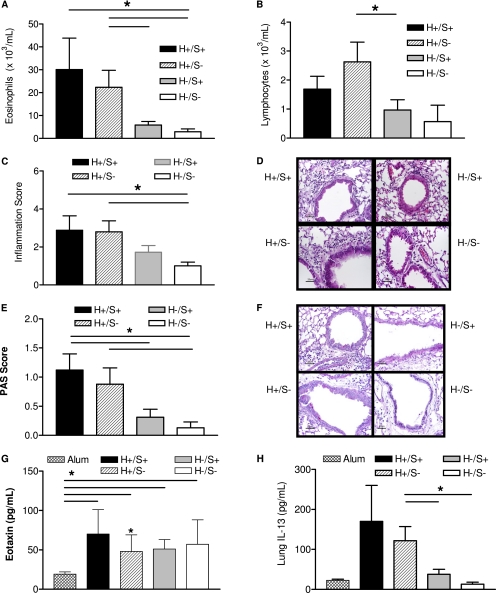
Hematopoietic-derived β-arrestin−2 and OVA challenge. Bone marrow chimeric mice express β-arrestin−2 exclusively in hematopoietic cells (H+/S−), structural cells (H−/S+), both (H+/S+), or neither (H−/S−). Chimeras were immunized and challenged with OVA. Hematopoietic-derived β-arrestin−2 is required for airspace recruitment of (A) eosinophils and (B) lymphocytes. (C, D) Hematopoietic-derived β-arrestin−2 is required for inflammatory cell recruitment into the airway subepithelial space. (E, F) Mucus staining is significantly elevated in the presence of hematopoietic β-arrestin−2 (PAS, periodic acid-Schiff). Lavage eotaxin is increased in all OVA-challenged animals compared with sham immunization (G), whereas tissue IL-13 is only elevated in association with hematopoietic β-arrestin−2 (H). Histology is representative of 4–11 mice in each group (original magnification, ×40) (*P < 0.05).
Hematopoietic β-Arrestin−2 Is Sufficient for Eosinophil Recruitment after IL-13 Exposure
For a better understanding of β-arrestin−2–dependent eosinophil recruitment in allergic airway inflammation, we exposed animals to recombinant murine IL-13 that induces eosinophil migration in a manner largely independent of lymphocytes (15). In these experiments, we observed that IL-13 leads to robust eosinophil recruitment into the lungs of wild-type but not β-arrestin−2–deficient mice (Figure 2A). In addition, eosinophil recruitment was completely restored in chimeric animals with hematopoietic-derived β-arrestin−2 expression (H+/S−), but not wild-type mice that received β-arrestin−2–deficient hematopoietic cells (H−/S+). Similarly, we observed that peribronchiolar/perivascular lung inflammation was significantly enhanced in wild-type mice relative to both β-arrestin−2–deficient mice and wild-type recipients of β-arrestin−2–deficient hematopoietic cells (H−/S+) (Figures 2C and 2D). This lack of lung inflammation was not a result of reduced lung eosinophilic chemokine production, since the lavage level of eotaxin (Figure 2G) and RANTES (data not shown) was the same for all chimeras and thus, independent of β-arrestin−2. Consistent with these findings, we observed that β-arrestin−2–replete hematopoietic cells are associated with airway mucus content (Figures 2E and 2F). As expected, the exogenous administration of IL-13 resulted in lung homogenate IL-13 levels some 10-fold greater than those produced endogenously in response to OVA treatment (data not shown). These observations further support the critical role of hematopoietic-derived β-arrestin−2 in eosinophil recruitment into the lung.
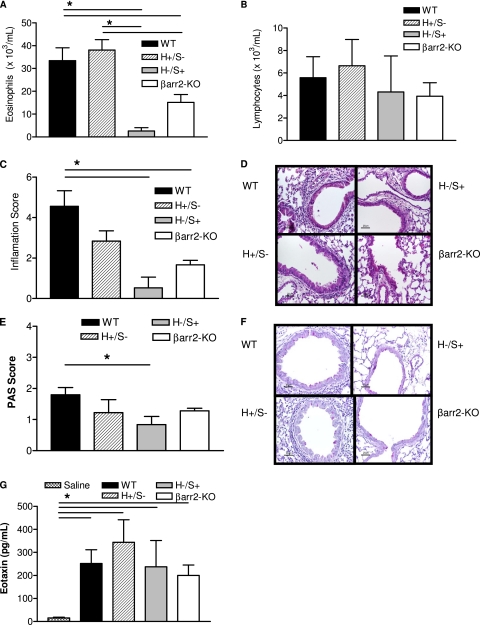
Hematopoietic-derived β-arrestin−2 and IL-13 challenge. Bone marrow chimeric mice express β-arrestin−2 exclusively in hematopoietic cells (H+/S−), structural cells (H−/S+), both (H+/S+), or neither (H−/S−). Chimeras were challenged with recombinant murine IL-13. β-arr2, β-arrestin−2; WT, wild-type; PAS, periodic acid-Schiff. (A) Hematopoietic-derived β-arrestin−2 is required for airspace recruitment of eosinophils. (B) No difference in airspace lymphocytes is evident. Ubiquitous expression of β-arrestin−2 is required for (C, D) inflammatory cell recruitment into the airway subepithelial space and (E, F) epithelial mucus staining. (G) Eotaxin is increased in all IL-13 challenged animals compared with sham challenge. Histology is representative of 4–11 mice in each group (original magnification, ×40) (*P < 0.05).
T-Lymphocytes Do Not Significantly Influence Eosinophil Recruitment in Response to IL-13
Others showed that IL-13–mediated lung eosinophil recruitment can occur in mice deficient in both B lymphocytes and T lymphocytes (recombinase-activating gene-1 knockout (RAG-KO) mice) (15). However, this does not preclude a contribution from T lymphocytes, when they are present, to eosinophil recruitment. Thus, to determine specifically whether lymphocyte-derived β-arrestin−2 significantly contributes to the eosinophilic response, we adoptively transferred T lymphocytes into RAG mice and exposed them to IL-13. These experiments demonstrated no significant effect of either wild-type or β-arrestin−2–deficient T lymphocytes on the lung eosinophilic response to IL-13 challenge (Figure 3). Given that the biological response to IL-13 in our model was not measurably influenced by T-lymphocytes, our observations suggest an important, direct, and positive regulatory role for β-arrestin−2 in eosinophil migration into the lung.
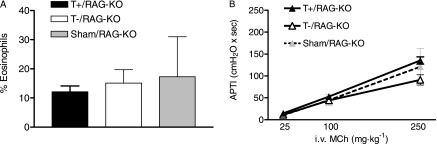
Effect of T lymphocytes on response to IL-13. The adoptive transfer of wild-type or β-arrestin−2–deficient T lymphocytes into recombinase activating gene-1 knockout (RAG-KO) mice had no significant effect on the IL-13–induced (A) percentage of eosinophils in lung lavage or (B) airway hyperresponsiveness. MCh, methacholine.
Nonhematopoietic β-Arrestin−2 Expression Is Required for OVA-Induced AHR
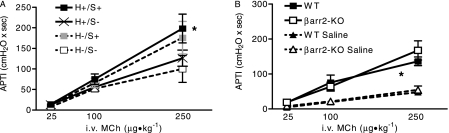
Airway hyperresponsiveness is a hallmark feature of allergic asthma. We characterized the cell types required to express β-arrestin−2 for the development of AHR in the murine OVA model. Surprisingly, we observed that hematopoietic-derived β-arrestin−2 is not sufficient for the development of AHR (Figure 4A, H+/S−). The development of AHR was dependent on the expression of β-arrestin−2 in hematopoietic and nonhematopoietic structural cell types (Figure 4A, H+/S+). Airway hyperresponsiveness in response to IL-13 instillation was independent of β-arrestin−2 (Figure 4B). These observations support the notion that non–hematopoietic-derived β-arrestin−2 is required for antigen-induced AHR, but is not required for the airway response to direct IL-13 challenge.
DISCUSSION
We previously demonstrated that genetically altered mice lacking β-arrestin−2 (β-arrestin−2–KO) do not develop allergic inflammatory airway disease, and that dysfunctional T-lymphocyte migration is at least partly responsible for the absent phenotype (14). However, the dramatic lack of signs of allergic inflammatory airway disease in β-arrestin−2–KO mice led us to hypothesize that β-arrestin−2 may regulate other cell types that participate in the pathogenesis of allergic inflammatory airway disease. GPCRs are recognized to mediate immune cell chemotaxis, lung structural cell contraction/relaxation, and the production/secretion of inflammatory mediators by both hematopoietic and nonhematopoietic cell types (3). The present study was specifically designed to improve our understanding of the cell-types required to express β-arrestin−2 in the development of allergic inflammatory airway disease.
Our data from OVA-treated chimeras (H+/S−) indicated the preserved migration of T lymphocytes and eosinophils replete with β-arrestin−2 to lungs with structural cells devoid of β-arrestin−2. This observation suggests that the lung structural cell production of allergic inflammatory chemokines is not regulated by β-arrestin−2. In further support of this conclusion was the finding that lavage levels of eosinophil and T-lymphocyte chemokines (CCL11 and CCL5) (13) were independent of β-arrestin−2 expression in both hematopoietic and nonhematopoietic cells.
However, lung eosinophilic inflammation in the OVA model is dependent on the function of T lymphocytes, which we previously demonstrated to be dysfunctional in β-arrestin−2–deficient mice (14). Therefore, to examine the direct impact of β-arrestin−2 on eosinophil migration, we challenged mice with recombinant IL-13, which can induce the signs of allergic inflammatory airway disease independent of lymphocytes (15). We showed that lung eosinophil accumulation and peribronchiolar/perivascular inflammation were significantly reduced in IL-13–treated β-arrestin−2–KO mice compared with wild-type mice, despite producing comparable levels of IL-3, IL-5, IL-9, granulocyte macrophage colony-stimulating factor GM-CSF (data not shown), eotaxin, and RANTES, that is, cytokines and chemokines known to play an important role in eosinophil maturation, recruitment, and survival during asthma (23). To rule out the possibility that β-arrestin−2–replete T lymphocytes, which were included in the bone marrow transplant, influenced eosinophil migration, we showed that eosinophil accumulation was not different in IL-13–challenged RAG-KO mice that received adoptively transferred wild-type or β-arrestin−2–deficient T lymphocytes. These findings suggest that lymphocyte-derived β-arrestin−2 is not sufficient for a complete response to IL-13. We cannot rule out the possibility that cross-talk between hematopoietic and nonhematopoietic cells, permissive regarding the response to IL-13, is β-arrestin−2–dependent. However, taken together, these data identify that the hematopoietic expression of β-arrestin−2 promotes lung eosinophil recruitment in a manner independent of lymphocyte-derived β-arrestin−2.
The finding that β-arrestin−2 directly promotes eosinophil migration to the lung in allergic inflammatory airway disease is consistent with our previously published conclusion regarding T cells (14). The present findings also indicate that the induction of an allergic inflammatory lung chemokine gradient is independent of hematopoietic or nonhematopoietic β-arrestin−2 expression. Thus, although we cannot rule out the possibility that β-arrestin−2 regulates the hematopoietic cell expression of chemokine receptors or cell adhesion molecules, we speculate that β-arrestin−2 positively regulates a chemotactic signaling pathway distal to chemokine receptor activation. Although β-arrestins were named for their ability to quench G-protein–mediated cell signaling, a relatively new paradigm describing GPCR function indicates that β-arrestins transduce cell signaling via a G-protein–independent pathway (24). Thus, upon agonist activation, GPCRs can signal via both G-protein–dependent and β-arrestin–dependent pathways, the activation of which may lead to similar, or diverse, functional cellular responses. In other words, a cell that lacks β-arrestin may respond to GPCR activation in an exaggerated manner if the G-protein–mediated physiologic response is measured, or in an inhibited manner if the β-arrestin–dependent cellular response is measured.
Several investigators established the existence of β-arrestin–dependent chemotaxis signaling pathways in lymphocytes and neutrophils (25–27). Direct studies of β-arrestin−2–mediated signal regulation in eosinophil chemotaxis are limited by the impaired ex vivo viability of isolated, activated primary murine eosinophils, and are beyond the scope of this work. However, CCR3, the primary chemokine receptor for eosinophils, expresses multiple C-terminus serine/threonine-rich regions that undergo agonist-induced phosphorylation (28) and are thus likely binding sites for β-arrestins. The translocation of β-arrestin to an agonist-activated GPCR is key to its ability to desensitize G-protein signaling and transduce β-arrestin–dependent signaling. Whether or not β-arrestin−2 transduces eosinophil chemokine receptor activation into a chemotactic response remains an area for future investigation.
In contrast to our finding that lung structural cell β-arrestin−2 is not required for lung inflammation in allergic inflammatory airway disease, our results demonstrate that the development of AHR is dependent on nonhematopoietic β-arrestin−2. We suggest that the β-arrestin−2 regulation of airway epithelial and/or smooth muscle cell function is crucial in the development of AHR. We observed attenuated airway responsiveness in H+/S− mice, despite the presence of lung inflammation. This attenuated airway responsiveness may occur because β-arrestin−2 regulates the relaxation of airway smooth muscle (ASM) in allergic inflammatory airway disease. In fact, we previously showed in untreated mice that β-arrestin−2 functionally constrains β2-adrenergic receptor–mediated airway smooth muscle relaxation, but not M3 muscarinic receptor-mediated bronchoconstriction (21).
The β2-adrenergic receptor, that is, the prototypic GPCR, plays a critical role in the regulation of pulmonary function in asthma through the cellular transduction of β-agonist bronchodilator therapy (29). The recruitment of β-arrestin−2 to activated β2-adrenergic receptor leads to the desensitization and internalization of this GPCR (30–32). The absence of β-arrestin−2 in H+/S− and H−/S− chimeras may reduce this β2-adrenergic receptor tachyphylaxis and manifest as less severe AHR.
Although ASM contractile and relaxant properties are obvious and important contributors to the pathobiology of asthma, a large body of literature clearly shows that the immunomodulatory role of ASM and epithelial cells is key to asthma pathogenesis (33, 34). Lung structural cells are a target for, and a source of, proasthmatic factors such as cytokines and chemokines that actively perpetuate airway inflammatory processes and contribute to AHR, via autocrine and paracrine actions. Airway hyperresponsiveness does not develop in chimeric mice lacking lung structural cell β-arrestin−2 (H+/S−), even in the presence of airway inflammation. Thus, we suggest that the lung structural cell utilization of a β-arrestin−2–dependent signaling pathway is required for the development of AHR in a mouse model of allergic inflammatory airway disease. Others showed that cytokine production and release can be mediated via a β-arrestin–dependent signaling pathway, and that this effect is cell-specific and receptor-specific (5, 6). Moreover, proinflammatory chemokines and cytokines act on receptor families that are regulated by β-arrestins, including GPCRs and cytokine receptors (24). Thus, although lung inflammatory cells are present in H+/S− mouse lungs, their AHR-inducing immunomodulatory message is not effectively transduced by lung structural cells that lack β-arrestin−2. The exact identity and combination of immunomodulatory mediators that contribute to AHR are unknown. However, we speculate that these immunomodulators act through a β-arrestin−2–dependent signaling pathway.
In conclusion, we demonstrate that the expression of β-arrestin−2 exclusively in hematopoietic-derived cell types is sufficient for the lung recruitment of eosinophils in two different models of allergic airways inflammation. Hematopoietic-derived β-arrestin−2 promotes eosinophil migration to the lung, whereas lung allergic inflammatory chemokine production is independent of β-arrestin−2 expression in both hematopoietic and nonhematopoietic cells. These findings suggest a direct effect of β-arrestin−2 on eosinophil migration. Conversely, AHR was dependent on the expression of β-arrestin−2 in lung structural cells in the OVA model. These findings support cell-type–specific roles of β-arrestin−2 in the development of allergic inflammatory airway disease, and indicate that the mechanisms involved in AHR can be different from those involved in allergic airway inflammation. A broader implication of our study is that, although ubiquitously expressed, β-arrestin−2 can selectively regulate multiple cell type functions in vivo. Our data demonstrate that β-arrestin−2 expression in at least two divergent cell types contributes to the pathogenesis of allergic inflammatory airway disease.
Acknowledgments
The authors are grateful to Robert J. Lefkowitz for his generosity in providing expert advice.
Notes
The authors gratefully receive support from the National Heart, Lung, and Blood Institute and the National Institute of Environmental Health Sciences (grants ES16659, ES11961, and ES16126 to J.W.H., and grant HL84123 to J.K.L.W.), and from the Durham Veterans Administration and the Sandler Program for Asthma Research (J.K.L.W.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0198OC on October 5, 2009
Author Disclosure: J.K.L.W. received an industry-sponsored grant from Sepracor, Inc. ($10,000–$50,000). M.S. has served on the National Institutes of Health (NIH) Advisory Board ($1,000), and has received NIH sponsored grants ($100,000+) and sponsored grants from the American Asthma Foundation ($100,000+). D.A.S. has served as an expert witness for Wallace and Graham ($10,000–$50,000), Brayton and Purell ($5,001–$10,000), Weitz and Luxemberg ($50,001–$100,000), and Waters and Kraus ($10,001–$50,000), has a patent pending from MedImmune for TLR4 hyporesponsive polymorphisms used in respiratory syncytial virus vaccine research (less than $10,000), and has received support from the NIH ($100,000+) and the National Jewish Health ($100,000+) for employment. None of the other authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
Articles from American Journal of Respiratory Cell and Molecular Biology are provided here courtesy of American Thoracic Society
Full text links
Read article at publisher's site: https://doi.org/10.1165/rcmb.2009-0198oc
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2933545?pdf=render
Free after 12 months at intl-ajrcmb.atsjournals.org
http://intl-ajrcmb.atsjournals.org/cgi/content/full/43/3/269
Free to read at intl-ajrcmb.atsjournals.org
http://intl-ajrcmb.atsjournals.org/cgi/content/abstract/43/3/269
Free after 12 months at intl-ajrcmb.atsjournals.org
http://intl-ajrcmb.atsjournals.org/cgi/reprint/43/3/269.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Mast cell MrgprB2 in neuroimmune interaction in IgE-mediated airway inflammation and its modulation by β-arrestin2.
Front Immunol, 15:1470016, 17 Oct 2024
Cited by: 0 articles | PMID: 39483467 | PMCID: PMC11524863
Altered ontogeny and transcriptomic signatures of tissue-resident pulmonary interstitial macrophages ameliorate allergic airway hyperresponsiveness.
Front Immunol, 15:1371764, 25 Jun 2024
Cited by: 0 articles | PMID: 38983858 | PMCID: PMC11231371
Arrestin beta 1 Regulates Alveolar Progenitor Renewal and Lung Fibrosis.
J Respir Biol Transl Med, 1(2):10006, 30 Apr 2024
Cited by: 0 articles | PMID: 38736470 | PMCID: PMC11087074
The multifaceted functions of β-arrestins and their therapeutic potential in neurodegenerative diseases.
Exp Mol Med, 56(1):129-141, 11 Jan 2024
Cited by: 1 article | PMID: 38212557 | PMCID: PMC10834518
Review Free full text in Europe PMC
Identification of a β-arrestin-biased negative allosteric modulator for the β2-adrenergic receptor.
Proc Natl Acad Sci U S A, 120(31):e2302668120, 25 Jul 2023
Cited by: 5 articles | PMID: 37490535 | PMCID: PMC10401000
Go to all (38) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Beta-arrestin-2 regulates the development of allergic asthma.
J Clin Invest, 112(4):566-574, 01 Aug 2003
Cited by: 132 articles | PMID: 12925697 | PMCID: PMC171386
β-Arrestin-2 mediates the proinflammatory effects of proteinase-activated receptor-2 in the airway.
Proc Natl Acad Sci U S A, 109(41):16660-16665, 25 Sep 2012
Cited by: 56 articles | PMID: 23012429 | PMCID: PMC3478622
Quantification of beta adrenergic receptor subtypes in beta-arrestin knockout mouse airways.
PLoS One, 10(2):e0116458, 06 Feb 2015
Cited by: 5 articles | PMID: 25658948 | PMCID: PMC4319755
Genetic Deletion of β-Arrestin-2 and the Mitigation of Established Airway Hyperresponsiveness in a Murine Asthma Model.
Am J Respir Cell Mol Biol, 53(3):346-354, 01 Sep 2015
Cited by: 15 articles | PMID: 25569510 | PMCID: PMC4566063
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: HL84123
NIEHS NIH HHS (4)
Grant ID: ES16126
Grant ID: R01 ES016126
Grant ID: ES11961
Grant ID: ES16659