Abstract
Objective
We investigated the prevalence of mild cognitive impairment (MCI) in Olmsted County, MN, using in-person evaluations and published criteria.Methods
We evaluated an age- and sex-stratified random sample of Olmsted County residents who were 70-89 years old on October 1, 2004, using the Clinical Dementia Rating Scale, a neurologic evaluation, and neuropsychological testing to assess 4 cognitive domains: memory, executive function, language, and visuospatial skills. Information for each participant was reviewed by an adjudication panel and a diagnosis of normal cognition, MCI, or dementia was made using published criteria.Results
Among 1,969 subjects without dementia, 329 subjects had MCI, with a prevalence of 16.0% (95% confidence interval [CI] 14.4-17.5) for any MCI, 11.1% (95% CI 9.8-12.3) for amnestic MCI, and 4.9% (95% CI 4.0-5.8) for nonamnestic MCI. The prevalence of MCI increased with age and was higher in men. The prevalence odds ratio (OR) in men was 1.54 (95% CI 1.21-1.96; adjusted for age, education, and nonparticipation). The prevalence was also higher in subjects who never married and in subjects with an APOE epsilon3epsilon4 or epsilon4epsilon4 genotype. MCI prevalence decreased with increasing number of years of education (p for linear trend <0.0001).Conclusions
Our study suggests that approximately 16% of elderly subjects free of dementia are affected by MCI, and amnestic MCI is the most common type. The higher prevalence of MCI in men may suggest that women transition from normal cognition directly to dementia at a later age but more abruptly.Free full text

Prevalence of mild cognitive impairment is higher in men

Abstract
Objective:
We investigated the prevalence of mild cognitive impairment (MCI) in Olmsted County, MN, using in-person evaluations and published criteria.
Methods:
We evaluated an age- and sex-stratified random sample of Olmsted County residents who were 70–89 years old on October 1, 2004, using the Clinical Dementia Rating Scale, a neurologic evaluation, and neuropsychological testing to assess 4 cognitive domains: memory, executive function, language, and visuospatial skills. Information for each participant was reviewed by an adjudication panel and a diagnosis of normal cognition, MCI, or dementia was made using published criteria.
Results:
Among 1,969 subjects without dementia, 329 subjects had MCI, with a prevalence of 16.0% (95% confidence interval [CI] 14.4–17.5) for any MCI, 11.1% (95% CI 9.8–12.3) for amnestic MCI, and 4.9% (95% CI 4.0–5.8) for nonamnestic MCI. The prevalence of MCI increased with age and was higher in men. The prevalence odds ratio (OR) in men was 1.54 (95% CI 1.21–1.96; adjusted for age, education, and nonparticipation). The prevalence was also higher in subjects who never married and in subjects with an APOE ε3ε4 or ε4ε4 genotype. MCI prevalence decreased with increasing number of years of education (p for linear trend <0.0001).
Conclusions:
Our study suggests that approximately 16% of elderly subjects free of dementia are affected by MCI, and amnestic MCI is the most common type. The higher prevalence of MCI in men may suggest that women transition from normal cognition directly to dementia at a later age but more abruptly.
GLOSSARY
- AD
- = Alzheimer disease;
- a-MCI
- = amnestic mild cognitive impairment;
- CDR
- = Clinical Dementia Rating scale;
- CI
- = confidence interval;
- DSM-IV
- = Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- FAQ
- = Functional Activities Questionnaire;
- MCI
- = mild cognitive impairment;
- na-MCI
- = nonamnestic mild cognitive impairment;
- OR
- = odds ratio;
- STMS
- = Short Test of Mental Status;
- TICS-m
- = Telephone Interview for Cognitive Status-modified;
- WAIS-R
- = Wechsler Adult Intelligence Scale-Revised.
The field of aging and dementia is moving toward an earlier identification of clinical impairment, and the construct of mild cognitive impairment (MCI) has played a pivotal role.1,2 MCI is considered an intermediate state between the cognitive changes of aging and the earliest clinical features of dementia, particularly Alzheimer disease (AD).3 In recent years, the construct has been broadened to include other aspects of cognitive function beyond memory impairment.4,5
There have been several recent epidemiologic studies on MCI, but most investigators retrofitted the criteria for MCI to previously collected clinical information, used a variety of detection procedures, and implemented the MCI diagnostic criteria using different algorithms.6,7 By contrast, we evaluated in person a population-based sample specifically to detect MCI and its subtypes using published diagnostic criteria.
METHODS
Study sample.
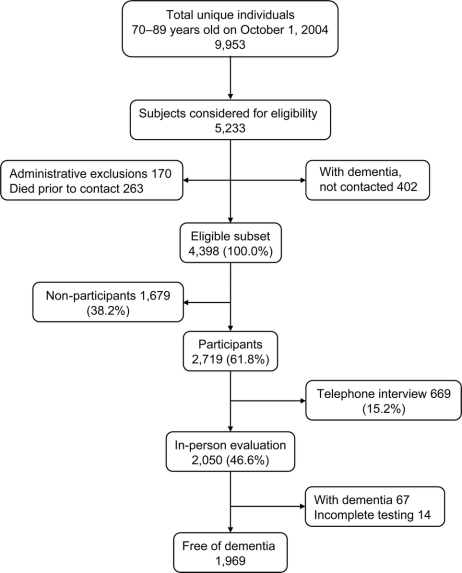
This was a prevalence study conducted during the baseline contact to establish a cohort for longitudinal study. The overall study design and methodology have been published in detail elsewhere.8 Briefly, all Olmsted County residents who were aged 70–89 years on October 1, 2004, were identified using the medical records-linkage system of the Rochester Epidemiology Project.9 We enumerated 9,953 persons aged 70–89 years and randomly selected 5,233 of them for recruitment. We excluded 263 subjects who died before they could be contacted, 56 subjects who were in hospice, and 114 subjects who could not be contacted (figure 1). Subjects with a preexisting diagnosis of dementia were identified by screening their medical record, and the clinical information was reviewed in detail by a neurologist (D.S.K.). Subjects confirmed to have dementia were not invited to participate in the study (n = 402); however, they were included in the denominator for some of the analyses. A total of 4,398 subjects were considered eligible for participation in the active evaluation (either an in-person evaluation or a telephone interview). The in-person evaluations were conducted from October 1, 2004, through July 31, 2007, and lasted on average approximately 2.5–3.0 hours (approximately 1 hour for cognitive testing).
Measurements of cognitive status.
Each participant completed an in-person evaluation including 3 components: 1) interview by a nurse or study coordinator to collect medical and neurologic history, to assess functional abilities, and to document risk factors; 2) a neurologic evaluation; and 3) neuropsychological testing. The interview included a short set of questions about memory administered directly to the participant, and the Clinical Dementia Rating Scale (CDR)10 and the Functional Activities Questionnaire (FAQ)11 administered to the informant. The neurologic evaluation was performed by a physician and included administration of the Short Test of Mental Status (STMS),12 medical history review, and a complete neurologic examination.
Neuropsychological testing was performed using 9 cognitive tests to assess 4 cognitive domains: memory (Logical Memory-II [delayed percent retention] and Visual Reproduction-II [delayed percent retention] from Wechsler Memory Scale-Revised and delayed percent retention from the Auditory Verbal Learning Test13); executive function (Trail Making Test B and Digit Symbol Substitution from Wechsler Adult Intelligence Scale-Revised [WAIS-R]); language (Boston Naming Test and category fluency); and visuospatial skills (Picture Completion and Block Design from the WAIS-R). We transformed the raw scores on each test into age-adjusted scores using normative data from the Mayo's Older American Normative Studies. These adjusted scores were scaled to have a mean of 10 and a SD of 3.13 We then obtained domain scores by summing the adjusted and scaled scores of the tests included within each domain. The domain scores were also scaled to allow comparisons across domains.
Subjects who refused the in-person evaluation but accepted a telephone interview were administered a structured questionnaire including the Telephone Interview for Cognitive Status-modified (TICS-m).14 However, because the TICS-m was found to perform only fairly well in separating MCI from either normal cognition or dementia, we excluded from this study the 669 subjects who only accepted a telephone interview.15
Demographic and clinical factors.
Date of birth, number of years of education, marital status, prior occupation, and history of diabetes, hypertension, coronary heart disease, and depression were obtained from the nurse interview and risk factors assessment, and a history of stroke and of other conditions possibly related to cognitive performance was obtained by the physician. Whenever possible, these comorbidities were confirmed with information from the medical records-linkage system.8,16
Diagnostic categories.
The performance of a person in a particular cognitive domain was measured by comparing the person's domain score with the score in normal subjects, available from normative work conducted in this same population.13,17 Subjects with scores of 1.0 SD or greater below the age-specific mean in the general population were considered for a possible cognitive impairment. However, the final decision about impairment in any cognitive domain was not based on a simple computer algorithm but rather on a consensus agreement among the examining physician, nurse, and neuropsychologist taking into account education, prior occupation, visual or hearing deficits, and other information.8
MCI was defined according to the following published criteria: 1) cognitive concern by subject, informant (from CDR), or nurse or physician; 2) impairment in 1 or more of the 4 cognitive domains (from cognitive battery); 3) essentially normal functional activities (from the CDR and the FAQ); and 4) absence of dementia (DSM-IV).4,18 Subjects with MCI were categorized as having amnestic MCI (a-MCI) if the memory domain was impaired or nonamnestic MCI (na-MCI) if there was no impairment in memory.
A diagnosis of dementia was based on the criteria in the DSM-IV.18 Subjects were characterized as cognitively normal according to published normative data developed on this community.13 The cognitive status of subjects as measured at the time of the in-person examination was assumed to be the same as on October 1, 2004. This retrodating of cognitive status allowed us to compute point prevalence figures using October 1, 2004, as the prevalence day.
Statistical analyses.
For the 4 age and sex strata included in the original sampling scheme, point prevalence was computed directly by dividing the number of prevalent cases of MCI by the population in the corresponding stratum (age- and sex-specific prevalence figures for men aged 70–79 and 80–89 years, and women aged 70–79 and 80–89 years). Whenever multiple strata were combined, the estimate for each stratum was weighted by its frequency in the total Olmsted County population (direct standardization to the Olmsted County population on October 1, 2004).19 Similarly, prevalence figures by education, marital status, and APOE genotype were standardized by age and sex. In our primary analyses, we estimated the prevalence of MCI considering only the population without dementia in the denominator. However, in secondary analyses, we also included subjects with dementia in the denominator. The denominators including dementia were obtained by incorporating and reproportioning both the subjects with previously diagnosed dementia who were excluded from the study and those found at the in-person evaluation.
To investigate possible biases caused by the subjects who did not participate in the study, we used the propensity score method.3,20 Information for nonparticipants was available from the medical records-linkage system for 97% of all subjects originally sampled.8 First, we used logistic regression models to estimate the effect of age, sex, and years of education on participation.20,21 Second, we used the reciprocal of the participation probabilities for each subject as weights to estimate MCI prevalence figures adjusted for nonparticipation. Although estimates adjusted for nonparticipation were similar to the unadjusted estimates, the primary results presented in tables and figures are all adjusted for nonparticipation.
We also conducted a set of case-control analyses comparing the 329 subjects found to have MCI (prevalent MCI) with the 1,640 subjects who were found to be cognitively normal. For several demographic variables, we computed prevalence odds ratios (OR) and 95% confidence intervals (CI) adjusted for age (≥80 vs <80 years), sex, years of education (≤12 vs >12 years), and nonparticipation (reciprocal probability weighting) using logistic regression models.
To explore the higher prevalence of MCI in men, we investigated the possible confounding effect of several clinical comorbidities (diabetes, hypertension, stroke, coronary heart disease, depression), the Charlson index of comorbidity (<2 vs ≥2), and APOE genotype (carriers vs noncarriers of ε4). We also assessed potential confounding effects of type of informant (spouse or offspring vs other [e.g., more distant relatives, caregivers]) and marital status (currently married vs previously married or never married). All models also included age and years of education. Finally, we assessed potential effect modification by these same variables by including interaction terms with sex in the logistic regression models.
Standard protocol approvals, registrations, and patient consent.
The study was approved by the institutional review boards of the Mayo Clinic and of Olmsted Medical Center. Written informed consent was obtained for all participants who were examined as part of the study.
RESULTS
Study sample.
Of the 4,398 subjects eligible to participate in the active evaluation, 2,719 agreed to participate (61.8% response) in an in-person evaluation (n = 2,050) or in a telephone interview (n = 669; figure 1). Comparison of participants and nonparticipants using information obtained from the medical records-linkage system showed that nonparticipants were older, more often men, less educated, and more likely to have diabetes or greater comorbidities.8 Subjects who accepted only the telephone interview were excluded from the analyses presented here.
Overall results.
Of the 2,050 subjects who participated in the in-person evaluation, 67 (3.3%) had a dementia that had not been detected by our review of the medical records, 14 (0.7%) had incomplete cognitive testing, 1,640 (80.0%) were cognitively normal, and 329 (16.0%) had MCI (figure 1). Among subjects with MCI, 237 (11.6%) had a-MCI (145 [7.1%] single domain; 92 [4.5%] multiple domain) and 92 (4.5%) had na-MCI (69 [3.4%] single domain; 23 [1.1%] multiple domain).
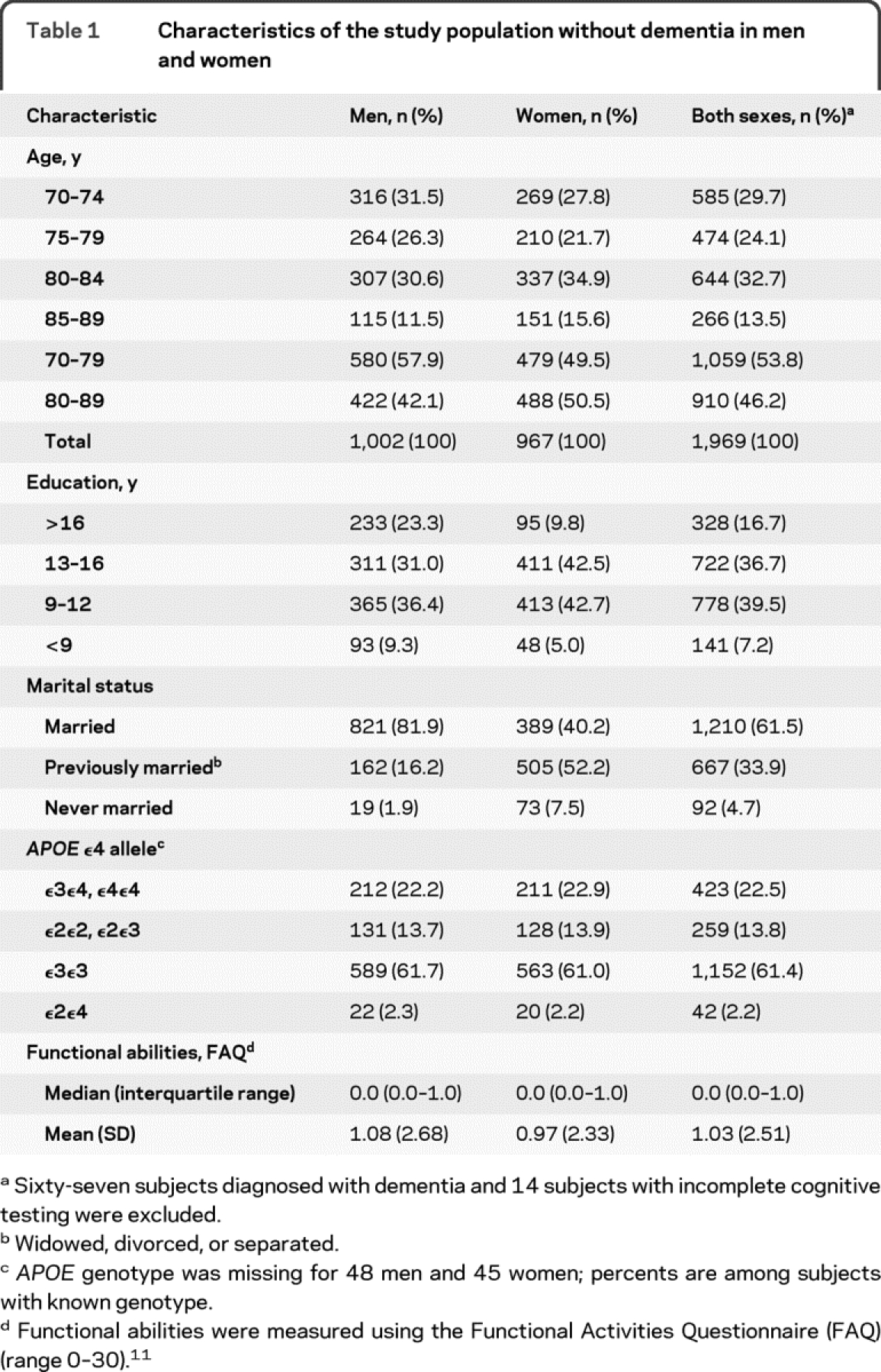
Table 1 shows the characteristics of the 1,969 subjects who received a cognitive evaluation and were free of dementia. Consistent with the study design, there were about equal proportions of men and women; 53.3% had >12 years of education, and 61.5% were currently married; a greater proportion of men (81.9%) compared with women (40.2%) were currently married. Approximately 23% of subjects had the APOE ε3ε4 or ε4ε4 genotype.
Table 1 Characteristics of the study population without dementia in men and women
The prevalence of MCI in the total population (including dementia cases in the denominator) and adjusted for nonparticipation was 14.3% for any MCI, 9.9% for a-MCI, and 4.4% for na-MCI (table 2, footnotes). The prevalence of dementia was 10.0% (95% CI 8.9–11.2), and 75.7% of subjects (95% CI 74.1–77.4) were cognitively normal (data not shown). The combined prevalence of either MCI or dementia was 24.3% (95% CI 22.6–25.9; data not shown). Table e-1 (on the Neurology® Web site at www.neurology.org) shows the distribution of the subjects with MCI by the type of concern about cognitive performance and by sex.
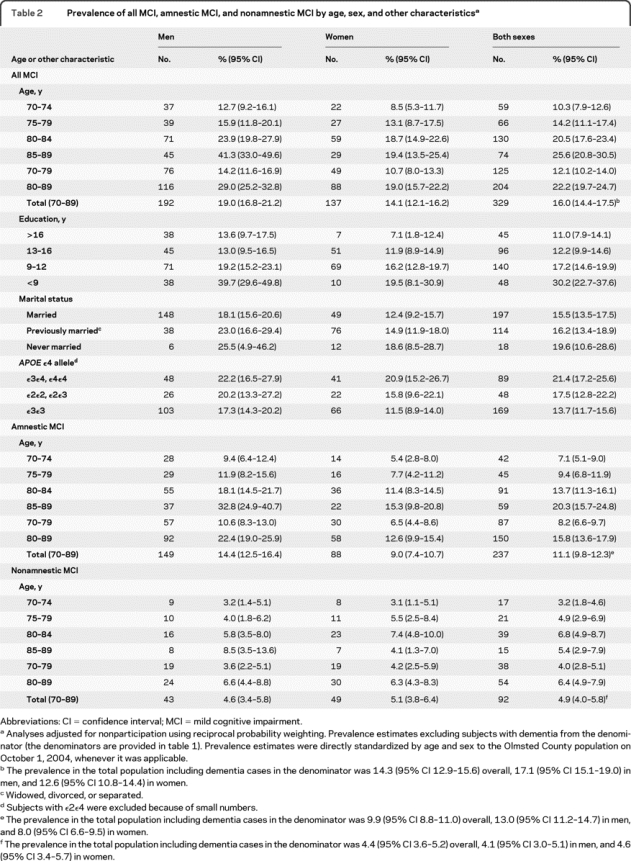
Table 2 Prevalence of all MCI, amnestic MCI, and nonamnestic MCI by age, sex, and other characteristics
MCI prevalence in the population without dementia.
Table 2 shows the prevalence, adjusted for nonparticipation, of all MCI, a-MCI, and na-MCI by age and sex among subjects without dementia. The overall age- and sex-adjusted prevalence of MCI was 16.0% for any MCI, 11.1% for a-MCI, and 4.9% for na-MCI. The prevalence of MCI increased with increasing age and was consistently higher in men than women across all ages (figure 2A). The age and sex patterns were similar for all MCI, a-MCI, and na-MCI. In addition, the increase with age was similar across subtypes of MCI (figure 2C and table e-2). The median FAQ score was 0.0 (interquartile range 0.0–1.0; mean 0.61; SD 1.53) in cognitively normal subjects and 1.0 (interquartile range 0.0–4.0; mean 3.10; SD 4.55) in subjects with MCI. Prevalence figures adjusted for nonparticipation (primary results in tables and figures) were somewhat higher than those unadjusted (e.g., the unadjusted overall prevalence of MCI was 14.9%; 95% CI 13.7–16.0).
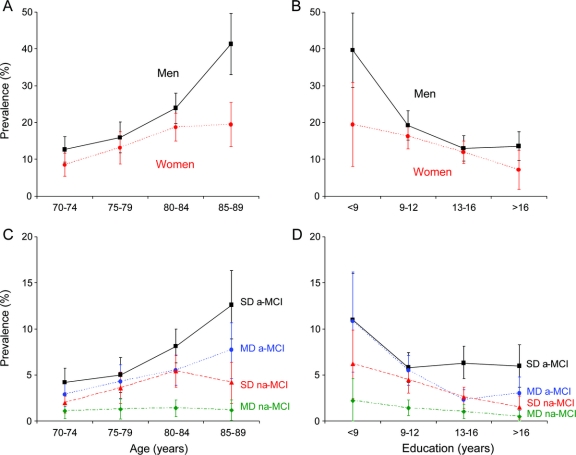
Figure 2 Patterns of mild cognitive impairment (MCI) prevalence by age, sex, education, and type of MCI
(A) Age- and sex-specific prevalence of MCI in Olmsted County, MN. Men had consistently higher prevalence than women at all ages. (B) Education- and sex-specific prevalence of MCI. The prevalence decreased with increasing education in both men and women. (C) Age- and type-specific prevalence of MCI. The increase of prevalence with age was consistent for single-domain amnestic MCI (SD a-MCI), multiple-domain amnestic MCI (MD a-MCI), single-domain nonamnestic MCI (SD na-MCI), and multiple-domain nonamnestic MCI (MD na-MCI). (D) Education- and type-specific prevalence of MCI. The decline in prevalence with increasing education was consistent across the 4 types of MCI.
Table 2 also shows the prevalence of MCI by years of education, marital status, and APOE genotype in men and women separately. The prevalence of MCI decreased markedly with increasing number of years of education from 30.2% in subjects with <9 years of education to 11.0% in subjects with >16 years of education. The pattern was similar for men and women (figure 2B) and across subtypes of MCI (figure 2D and table e-2). The prevalence of MCI was higher in never married subjects than in currently married or previously married subjects, and in subjects with the APOE ε3ε4 or ε4ε4 genotype (table 2).
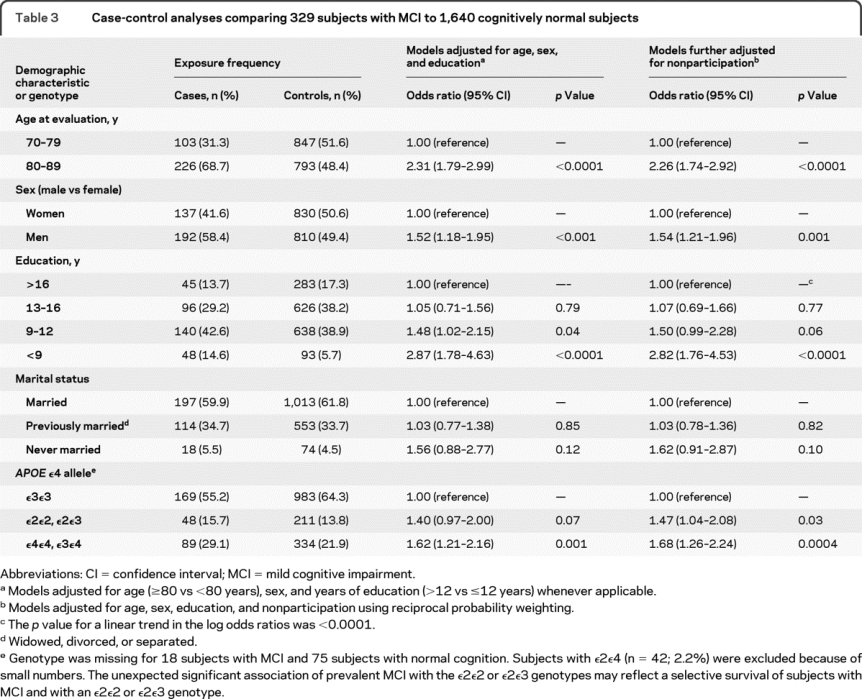
Case-control analyses.
Table 3 shows the results of our case-control analyses with and without adjustment for nonparticipation. The prevalence OR for MCI was increased in older subjects, in men, in subjects who never married, and in subjects with the APOE ε3ε4 or ε4ε4 genotype; however, the OR decreased with increasing number of years of education (table 3). The higher prevalence OR of MCI in men compared with women remained essentially unchanged after adjustment for several demographic variables (age, education, marital status, type of informant), clinical variables (diabetes, hypertension, stroke, coronary artery disease, depression, Charlson index), or APOE genotype. In addition, there were no significant interactions of sex with age, years of education, or APOE genotype (data not shown).
Table 3 Case-control analyses comparing 329 subjects with MCI to 1,640 cognitively normal subjects
DISCUSSION
This study provides estimates of the prevalence of MCI, a-MCI, and na-MCI in the community using the diagnostic criteria currently employed by the National Institute on Aging Alzheimer's Disease Center program and the Alzheimer's Disease Neuroimaging Initiative.22 This study documented a relatively high prevalence of MCI in the community at 16.0%. The prevalence increased with age, was higher in men, in never-married subjects, and in subjects with APOE ε3ε4 or ε4ε4 genotype, and decreased with higher levels of education.
We found that a-MCI was 2.3 times more common than na-MCI. Because a-MCI is considered a precursor of AD, this pattern is consistent with the pattern observed in the prevalence of AD vs other types of dementia. In addition, the combined prevalence of MCI and dementia at 24.3% highlights the public health impact of these conditions and the urgency for finding therapies.23,24
Prevalence estimates of MCI vary from studies around the world.25–27 This variability may be caused by the population studied, the age distribution and size of the sample, and the use of different implementations of the MCI criteria. In particular, studies used prospective vs retrospective fitting of diagnostic criteria, neuropsychological algorithms vs clinical consensus diagnosis, different types of cognitive instruments, different depth and breadth of in-person evaluations, and different normative data.28 A major limitation with retrofitting diagnostic criteria for MCI to previously collected neuropsychological data is the need to apply a rigid algorithm. By contrast, multiple data sources were used to make the diagnoses in our study.
The Kungsholmen Project in Sweden used global and domain-specific cognitive measures and yielded a prevalence of 11.1% in a sample of 379 subjects aged 75–95 years and free of dementia.27 A study from Leipzig, Germany, used a 55-point composite instrument and yielded an overall prevalence of 19.2% in subjects 75 years and older.25 The Cardiovascular Health Study yielded an overall prevalence of 19% in subjects aged 75 years and older.29 The North Manhattan Multi-Ethnic and Multicultural Study used retrospectively applied neuropsychological criteria and yielded prevalence estimates between 21.8% and 26.9%.6 The prevalence of MCI in the present study was also comparable with the prevalence of cognitive impairment no dementia in the Canadian Study of Health and Aging (16.8%),30 and in the Aging, Demographics, and Memory Study in the United States (22.2%).31 Despite some methodologic differences, most of the studies reported prevalence figures for MCI or for cognitive impairment no dementia in the 11%–20% range.
An interesting observation in this study was the higher prevalence of MCI in men compared with women. Other investigators have reported a similar pattern using different methods of implementation of the diagnostic criteria for MCI.7,32 By contrast, the higher prevalence of MCI in men was not observed in other studies.33–35 In addition, an Italian study and a French study yielded a higher prevalence of MCI in women compared with men.36,37 However, the diagnostic criteria for MCI relied on a few psychometric tests, in particular on the Mini-Mental State Examination.37
The higher OR of MCI in men remained essentially unchanged after adjustment for several demographic and clinical variables, and APOE genotype, suggesting that the association was not due to comorbid conditions or to a differential assessment of MCI in men and women. If the higher prevalence in men is confirmed, it may suggest the interplay of sex-specific risk factors, sex-specific disease course, and sex-specific survival. For example, men may experience cognitive decline earlier in life but more gradually, whereas women may transition from normal cognition directly to dementia at a later age but more abruptly. Sex differences in risk factors and outcomes have been reported for stroke and cardiovascular diseases.38–40
A strength of this study was the in-person assessment of MCI prevalence in subjects randomly selected from a complete enumeration of a defined population. This is in contrast to several previous clinic-based studies that showed a narrower spectrum of MCI severity.3 For example, the patients with MCI recruited into the Alzheimer's Disease Neuroimaging Initiative had more severe functional impairment at baseline (mean 3.9; SD 4.5; a clinical series of volunteers) than patients with MCI in our study (mean 3.1; SD 4.6; a population-based series of prevalent cases).22
Second, by using information from a medical records-linkage system, we were able to adjust our findings for nonparticipation.
Third, all subjects in this study underwent a detailed in-person assessment. Because subjects with MCI have essentially normal activities of daily living, it is difficult to make a clinical diagnosis of MCI without a thorough evaluation of the subject. We used independently developed normative data from the same community to interpret the psychometric tests, and clinical diagnoses were made by consensus after evaluation of all the data for a given person and involved experienced neuropsychologists. In contrast, studies that used a global measure of cognition to select subjects for further evaluation (e.g., a dementia screening test) may have missed subjects with MCI as was demonstrated in The Cardiovascular Health Study.29 Screening has been shown to yield lower estimates of MCI prevalence.33
An important limitation of the study was the relatively low participation rate. Despite the use of propensity scores to adjust for nonparticipation, we cannot exclude some residual bias. Second, the population of Olmsted County is predominantly white of European ancestry, and the finding may not apply to other ethnic groups. Finally, we will need longitudinal data to determine whether the sex differences observed in prevalence are due to differences in risk or in survival.
DISCLOSURE
Dr. Petersen serves on scientific advisory boards for Elan Corporation, Wyeth, and GE Healthcare; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH/NIA (U01 AG 06786 [PI], P50 AG 16574 [PI], U01 AG 024904 [coinvestigator], and R01 AG11378 [coinvestigator]). Dr. Roberts receives research support from the NIH (K01 AG028573 [PI] and U01 AG006786 [coinvestigator]). Dr. Knopman serves/has served on Data Safety Monitoring Boards for Sanofi-Aventis and Eli Lilly and Company; receives research support from Baxter Pharmaceuticals, Elan Corporation, and Forest Laboratories, Inc.; has served as a consultant to GlaxoSmithKline; and serves as Deputy Editor of Neurology®. Dr. Geda receives research support from the NIH (K01 MH68351 [PI]; AG06786 [coinvestigator]), Mayo CTSA (RR024150 [Career Transition Award]), the RWJ Foundation (Harold Amos Scholar), and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program. R.H. Cha reports no disclosures. Dr. Pankratz receives research support from the NIH (NCI P30 CA 15083 [statistician], NIAID N01 AI 40065 [coinvestigator], NIA P50 AG 16574 [core leader], and NIA U01 AG 06786 [coinvestigator]). Dr. Boeve receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge Medicine, 2009) and receives research support from Cephalon, Inc., the NIH (P50 AG16574 [coinvestigator], UO1 AG06786 [coinvestigator], and RO1 AG15866 [coinvestigator]), the Alzheimer's Association, and the Center for Inherited Disease Research (U24 AG026395 [coinvestigator]). Dr. Tangalos serves on a Data Safety Monitoring Board for Eli Lilly and Company; serves as a consultant for Purdue University and Amgen; serves on the editorial boards of MD Net Guide, Journal of the American Medical Directors Association, and IM News; has received honoraria for slide development form Takeda Pharmaceutical Company Limited, Novartis, and Ortho Biotech Products, L.P.; receives research support from Baxter International Inc. and Elan Corporation; and serves as a consultant to Novartis. Dr. Ivnik serves on the editorial boards of The Clinical Neuropsychologist and Aging, Neuropsychology, and Cognition; receives royalties from the publication of Clinical Interpretation of the WAIS-III and WMS-III (Academic Press, 2003); and receives research support from the NIH (NIA AG 06786 [coinvestigator] and NIA AG 16574 [coinvestigator Core B]). Dr. Rocca receives research support from the NIH (AR030582 [PI], AG006786 [coinvestigator], and ES010751 [coinvestigator]).
Notes
Address correspondence and reprint requests to Dr. R.C. Petersen, Department of Neurology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905 ude.oyam@8retep
Supplemental data at www.neurology.org
Study funding: Supported by the NIH (P50 AG016574, U01 AG006786, K01 MH068351, and K01 AG028573) and the Robert H. and Clarice Smith and Abigail van Buren Alzheimer's Disease Research Program. The study was made possible by the Rochester Epidemiology Project (R01 AR030582).
Disclosure: Author disclosures are provided at the end of the article.
Received December 11, 2009. Accepted in final form May 21, 2010.
REFERENCES
Articles from Neurology are provided here courtesy of American Academy of Neurology
Full text links
Read article at publisher's site: https://doi.org/10.1212/wnl.0b013e3181f11d85
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2938972?pdf=render
Subscription required at www.neurology.org
http://intl.neurology.org/cgi/content/full/75/10/889
Free to read at www.neurology.org
http://intl.neurology.org/cgi/content/abstract/75/10/889
Subscription required at www.neurology.org
http://intl.neurology.org/cgi/reprint/75/10/889.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1212/wnl.0b013e3181f11d85
Article citations
Non-rapid eye movement sleep slow-wave activity features are associated with amyloid accumulation in older adults with obstructive sleep apnoea.
Brain Commun, 6(5):fcae354, 07 Oct 2024
Cited by: 0 articles | PMID: 39429245 | PMCID: PMC11487750
Synergistic associations of CD33 variants and hypertension with brain and cognitive aging among dementia-free older adults: A population-based study.
Alzheimers Dement, 20(10):7193-7204, 30 Aug 2024
Cited by: 1 article | PMID: 39215505 | PMCID: PMC11485077
Bartonella spp. infection in people with Mild Cognitive Impairment: A pilot study.
PLoS One, 19(8):e0307060, 22 Aug 2024
Cited by: 0 articles | PMID: 39172940 | PMCID: PMC11340988
NODDI in gray matter is a sensitive marker of aging and early AD changes.
Alzheimers Dement (Amst), 16(3):e12627, 29 Jul 2024
Cited by: 0 articles | PMID: 39077685 | PMCID: PMC11284641
Can white matter hyperintensities based Fazekas visual assessment scales inform about Alzheimer's disease pathology in the population?
Alzheimers Res Ther, 16(1):157, 10 Jul 2024
Cited by: 0 articles | PMID: 38987827
Go to all (418) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association of prior stroke with cognitive function and cognitive impairment: a population-based study.
Arch Neurol, 66(5):614-619, 01 May 2009
Cited by: 48 articles | PMID: 19433661 | PMCID: PMC3050015
Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study.
Arch Gen Psychiatry, 65(10):1193-1198, 01 Oct 2008
Cited by: 227 articles | PMID: 18838636 | PMCID: PMC2575648
The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging.
Neurology, 78(5):342-351, 25 Jan 2012
Cited by: 160 articles | PMID: 22282647 | PMCID: PMC3280046
Conversion of mild cognitive impairment to dementia in elderly subjects: a preliminary study in a memory and cognitive disorder unit.
Arch Gerontol Geriatr, 44 Suppl 1:233-241, 01 Jan 2007
Cited by: 67 articles | PMID: 17317458
Funding
Funders who supported this work.
NIA NIH HHS (4)
Grant ID: R01 AG034676
Grant ID: K01 AG028573
Grant ID: U01 AG006786
Grant ID: P50 AG016574
NIAMS NIH HHS (2)
Grant ID: R01 AR030582
Grant ID: R01AR030582
NIMH NIH HHS (1)
Grant ID: K01 MH068351