Abstract
Background
Variability in human immunodeficiency virus (HIV) disease progression cannot be fully predicted by CD4(+) T cell counts or viral load (VL). Because central memory T (T(CM)) cells play a critical role in the pathogenesis of simian immunodeficiency virus disease, we hypothesized that quantifying these cells in early HIV infection could provide prognostic information.Methods
We measured expression of CD45RO, chemokine (C-C motif) receptor (CCR) 5, CCR7, CD27, and CD28 to enumerate naive and memory subsets in samples from recently infected individuals. We also quantified proliferation, CD127 expression, and cell-associated VL. Disease progression was compared across subgroups defined by these measurements, using Kaplan-Meier survival curves and multivariate Cox proportional hazards regression.Results
Four hundred sixty-six subjects contributed 101 events. The proportion or absolute count of T(CM) cells did not correlate with disease progression, defined as the time to AIDS or death. However, significant associations were observed for proliferation within CD4(+) or CD8(+) T cells, loss of naive or CD127(+) memory CD8(+) T cells, and CD4(+) T cell-associated VL.Conclusions
Our results demonstrate that the extent of the immunopathogenesis established early in HIV infection predicts the course of future disease. Because antiretroviral drug treatment reverses such defects in part, our study provides mechanistic clues to why early use of antiretrovirals may prove beneficial.Free full text

Immunological and Virological Events in Early HIV Infection Predict Subsequent Rate of Progression
Abstract
Background
Variability in HIV disease progression cannot fully be predicted by CD4 T-cell counts or viral load. Because central memory T cells (TCM) play a critical role in the pathogenesis of SIV disease, we hypothesized that quantifying these cells in early HIV-infection could provide prognostic information.
Methods
We measured expression of CD45RO, CCR5, CCR7, CD27, and CD28 to enumerate naïve and memory subsets in samples from recently-infected individuals. We also quantified proliferation, CD127 expression, and cell-associated viral load. Disease progression was compared across subgroups defined by these measurements, using Kaplan-Meier survival curves and multivariate Cox proportional hazards regression.
Results
466 subjects contributed 101 events. The proportion or absolute count of TCM did not correlate with disease progression, defined as the time to AIDS or death. However, significant associations were observed for: proliferation within CD4 or CD8 T cells, loss of naïve or CD127+ CD8 T cells, and CD4 cell-associated proviral load.
Conclusion
Our results demonstrate that the extent of the immunopathogenesis established early in HIV infection predicts the course of future disease. Since antiretroviral drug treatment reverses such defects in part, our study provides mechanistic clues to why early use of antiretrovirals may prove beneficial.
Introduction
Traditionally, infection by the human immunodeficiency virus type 1 (HIV) has been viewed as slow and progressive, characterized by a gradual decline in T helper (CD4+) cells; however, there is considerable variation in the rate of progression amongst individuals. This variability has been largely ascribed to differences in set-point plasma HIV RNA levels (plasma viral load, VL) and CD4+ cell counts, although other parameters such as CD8 T cell activation have also shown predictive power [1–3]. These measurements account for a relatively small fraction of the variation in CD4 decline and progression to AIDS; still, plasma VL and CD4 counts are currently the major criteria used for initiating treatment with highly-active antiretroviral therapy (HAART). Recent publications [4–8] underscore the uncertainty as to when treatment should be initiated; identification of correlates of progression measured during early infection would be highly valuable for identifying individuals most likely to benefit from early therapeutic intervention.
It remains difficult to identify and sample patients with acute/early HIV infection. Much of our current knowledge on early mucosal and systemic immune responses, as well as immunopathogenesis, is based on studies performed in non-human primate (NHP) models of Simian Immunodeficiency Virus (SIV) infection [9]. While overt immunodeficiency during the first few weeks of SIV (or HIV) infection is rare, there is be a dramatic depletion of the total body memory CD4 compartment during this time – typically more than half of all memory cells are destroyed [10]. Furthermore, data suggest that measuring the extent of this destruction during the first four weeks of infection predicts lifespan independently of setpoint VL [11, 12].
The results of experiments in NHP models suggest that the dynamics of memory T cell subsets play a role in the pathogenesis of disease. Memory CD4 T cells can be subdivided into phenotypically distinct subsets that are related by differentiation and have different functions. Central memory (TCM), which localize to blood and secondary lymphoid tissues, are capable of regeneration and long-term maintenance. These can differentiate to effector memory (TEM) cells, which are more prevalent in peripheral tissues and provide immediate effector functions at sites of inflammation. CCR5, the obligate co-receptor of most transmitted HIV, is highly expressed on TEM, making them a principal target for destruction during acute HIV/SIV infection. Following the major destruction of memory CD4 T cells, a dramatic proliferation of TCM cells occurs, likely part of the homeostatic mechanisms to compensate for the loss [13]. The proliferative response in the memory T cell compartment appears essential, in that its failure predicts rapid disease progression [13]. Elegant studies in NHP models suggest that homeostasis within the TEM compartment is dependent on the continued differentiation of TCM cells and failure of this delivery due to the declining numbers of TCM accelerates advancement to AIDS [14]. This association is further strengthened by the observation that among animals vaccinated against SIV, the absolute number of TCM cells better predict survival than either the absolute CD4 count or plasma VL [11, 12].
Based on these observations and the critical role of CD4 TCM in adaptive immune responses, we hypothesized that quantifying these cells early after HIV infection might provide additional prognostic value for defining subsequent rates of progression. To this end, we conducted a large cross-sectional analysis in a well-characterized racially diverse cohort of 466 individuals sampled during acute or early HIV infection. The a priori defined primary goal of this study was to determine the association between progression to AIDS or death and the absolute number or fraction of CD4 and CD8 TCM cells. We also performed exploratory analyses to determine the prognostic value of (i) markers of immune proliferation (Ki-67 expression) and long-lived memory potential (CD127 expression [15]), (ii) representation of naïve and multiple memory T cell subsets, and (iii) cell-associated viral load within subsets of CD4 cells.
Methods
Subjects
The United States Military HIV Natural History Study (NHS) is a prospective observational cohort that has followed HIV-infected Department of Defense (DOD) beneficiaries since 1985. As part of this study, subjects undergo semi-annual study visits and blood draws for routine laboratory testing including CD4 counts and HIV- viral RNA levels and storage within the NHS repository. Recruitment and follow up procedures for this cohort have been previously described [16]. In brief, during the study visits all interim medical history including medication use, AIDS events, and significant non-AIDS events are captured. Approximately half of enrolled subjects are seroconverters with both documented HIV negative and positive dates. Subjects eligible for inclusion in our study were defined as seroconverters with cryopreservered peripheral blood mononuclear cells (PBMC) stored within 18 months of their estimated date of seroconversion (EDSC). We estimated seroconversion as the midpoint between documented HIV negative and positive dates. All subjects provided written informed consent to participate in the parent protocol. Both this sub-study and the parent protocol were evaluated and approved by the Institutional Review Boards (IRB) of the participating sites.
Cryopreserved PBMC from 466 subjects were assayed for this analysis. Table 1 describes the baseline virologic, immunologic, and demographic characteristics of the study group. Most subjects were males (94%) and the study population was racially diverse. The median time from the estimated dated of seroconversion (EDSC) to cell sampling was 225 days (IQR, 162–296 days). Baseline was defined as the time of the cell sample used for our analyses. The median CD4 count at baseline was 552 cells/µL, and the median HIV- RNA level was 4.2 log10 copies/ml. Subjects were followed an average of 4 years post seroconversion. A total of 135 AIDS or death events occurred, with 34 of these events occurring after initiation of HAART. While all events met the 1993 definition of AIDS, 47 events satisfied the more rigorous 1987 definition of AIDS [17, 18].
Table 1
Baseline characteristics of subjects selected for the analysis
| Baseline Demographics1 (n=466) | Median (IQR) | Range |
|---|---|---|
 Age (years) Age (years) | 26.4 (23.0–31.4) | 17.9–53.5 |
 Caucasian (%) Caucasian (%) | 50 | |
 African-American (%) African-American (%) | 39 | |
 Hispanic (%) Hispanic (%) | 6 | |
 Others (%) Others (%) | 5 | |
 Male (%) Male (%) | 94 | |
 Time from EDSC to cell samples (days) Time from EDSC to cell samples (days) | 225 (162–296) | 16.5–797.5 |
| Baseline Immunologic Parameters1 | ||
 CD4+ T cell count2 (cells/µl) CD4+ T cell count2 (cells/µl) | 552 (421–728.3) | 73–2412 |
 Proportion of CD4+ central memory cells (%) Proportion of CD4+ central memory cells (%) | 17 (12–23) | 1.5–51.2 |
 Proportion of CD8+ central memory cells (%) Proportion of CD8+ central memory cells (%) | 2.8 (1.7–4.5) | 0.06–34.7 |
 Proportion of CD4+ naïve cells (%) Proportion of CD4+ naïve cells (%) | 53.8 (42.5–63.3) | 0.5–100 |
 Proportion of CD8+ naïve cells (%) Proportion of CD8+ naïve cells (%) | 38.4 (25–50.5) | 0–82.8 |
 Proportion of Ki-67 expressing CD4+ T cells (%) Proportion of Ki-67 expressing CD4+ T cells (%) | 0.4 (0.2–.6) | 0–48.3 |
 Proportion of Ki-67 expressing CD8+ T cells (%) Proportion of Ki-67 expressing CD8+ T cells (%) | 1 (0.5– 1.8) | 0–59 |
| Virologic characteristics | ||
 Plasma HIV-1 RNA level (log10 copies/ml3 Plasma HIV-1 RNA level (log10 copies/ml3 | 4.2 (3.5–4.7) | 1.7–6.1 |
 Baseline cell-associated viral load
(gag copies/100 CD4 cells) Baseline cell-associated viral load
(gag copies/100 CD4 cells) | 0.23 (0.06–0.67) | 0–16. 03 |
Notes:
Laboratory methods
Statistical methods
AIDS was defined using the 1993 CDC definition and disease progression was defined as either the occurrence of an AIDS defining event or death [18]. For the time-to-event analysis, subjects were divided into three groups based on quartiles (lowest: <25%, intermediate: 25–75%, or upper: >75%) of measured variables. Both Kaplan-Meier curves and Cox proportional hazards regression models were used to evaluate the predictive value of immunophenotypic characteristics, immune activation, and cell-associated viral load. Time zero (baseline) was defined as the time of the cell sample. For ~15% of the patients, HIV RNA measurements were missing or not within 30 days of baseline and consequently 34% of the events are missing in analyses that adjust for HIV VL. For this reason we also report analyses adjusted only for CD4 counts. There was no adjustment for multiple comparisons; p values should be interpreted with this in mind. Kaplan-Meier p values on graphs are based on the log-rank statistic.
To assess whether the relationship of the predictor variables varied with stage of infection, for selected variables, we used the median time from imputed seroconversion to procurement of cell-samples to divide subjects into two groups. The first group includes half of the subjects, sampled within 225 days (7.4 months) of their imputed seroconversion while the second group includes the other half, sampled after 225 days. If a test of interaction (i.e. that the effect of the predictor variable differed from those sampled early versus late) was significant (P < 0.05), we reported the results separately for the early and late subgroups.
For the primary analysis, all observations were censored either at the time HAART was initiated or at last follow up. While HAART is probably not an independent censoring mechanism, treating it as such likely results in slightly conservative estimates of hazard ratios. We also conducted additional sensitivity analyses where HAART use was treated as a time-varying covariate and analyses stratified by whether the baseline sample occurred before or after January 1st, 1996, the year HAART became universally available in the United States. Results from these analyses were similar to the primary analysis. Statistical analysis was performed using SAS version 9.1 and SPLUS version 6.2.
Results
T-cell phenotypes during early HIV infection
To determine if TCM or other subsets predict disease progression, we performed a comprehensive phenotypic analysis of T-cell subsets in early HIV-infection using 14-color flow cytometric analysis. Fig 1A–C shows representative staining patterns of the markers studied and how different subsets were defined. To simplify the presentation of this complex data, we grouped subpopulations in a manner synthesized from a number of reports. Specifically, CD45RO−CCR7+CD27+CD28+CCR5− cells were termed naïve (a strict definition that minimizes contamination with memory cells), while differential expression of CCR5, CCR7, CD27, and CD28 were used to identify central memory (TCM), transitional memory (TTM), and effector memory (TEM) cells.

(A) Immunophenotypic characteristics of T-cell subsets. Graphs illustrate the sequential gating to identify pure populations. The first gate eliminates doublets; the second restricts analysis to live, CD3+ T cells; within those, CD4 or CD8 T cells are identified. (B) Naïve cells were defined by defining gates within the total CD4+ or CD8+ T-cell populations, and then combining these gates with the Boolean function indicated. The resulting naïve cell population was CD45RO−CD28+CD27+CCR7+. (C) Subsets of memory CD4+ and CD8+ T cells were identified utilizing various combinations of cell-surface markers, as follows: Central memory cells (TCM) = CD45RO+ CD28+ CCR7+ CCR5−; transitional memory cells (TTM) = CD45RO+ CD28+ CCR7− CCR5+; effector memory cells (TEM) = CD45RO− CD28− CCR7− CCR5+. (D) Distribution of naïve and memory cell subsets in the CD4 and CD8 compartments. The box and whisker plot represents the relative frequencies of naïve, central, transitional, and effector memory cells in the CD4 (left panel) and CD8 (right panel) compartments. Interquartile ranges are shown.
Fig 1D shows the distribution of cells within these subpopulations for our cohort. At these early time points in HIV disease, naïve cells still comprise a large proportion of the T-cell compartments, similar to healthy controls. When we grouped patients by the estimated day since seroconversion (EDSC), we found no statistically significant difference in T cell composition among those subjects identified 0–3 months, 3–6 months, 6–9 months, or 9+ months after seroconversion (Supplemental Fig 1). Thus, in this cross-sectional study, T-cell composition was not influenced by the length of infection. However, there was substantial heterogeneity in the frequency of each cell type (Fig 1D) allowed us to examine whether differential representations were associated with disease progression.
Memory T cell representation and progression
Our primary hypothesis, based on NHP models, was that the preservation of TCM following acute infection would predict disease progression. We divided the cohort into three groups based on TCM levels: those with the highest (>75th percentile), intermediate (25th to 75th percentile), or lowest (< 25th percentile) levels (Supplemental Fig 2). Next, we compared the relative risk of disease progression across these groups. Based on our power calculations, 65 events would provide us with sufficient power to detect a significant difference (HR ≥ 2.0) among subjects with differing proportions of CD4 or CD8 TCM. Despite being adequately powered (a total of 101 events), neither the proportion (Fig 2A) nor the absolute count (data not shown) of TCM at early time points predicted disease progression using either adjusted or unadjusted analyses. Similar results were obtained for the subgroup of patients (50%) sampled within 225 days of their EDSC (data not shown).
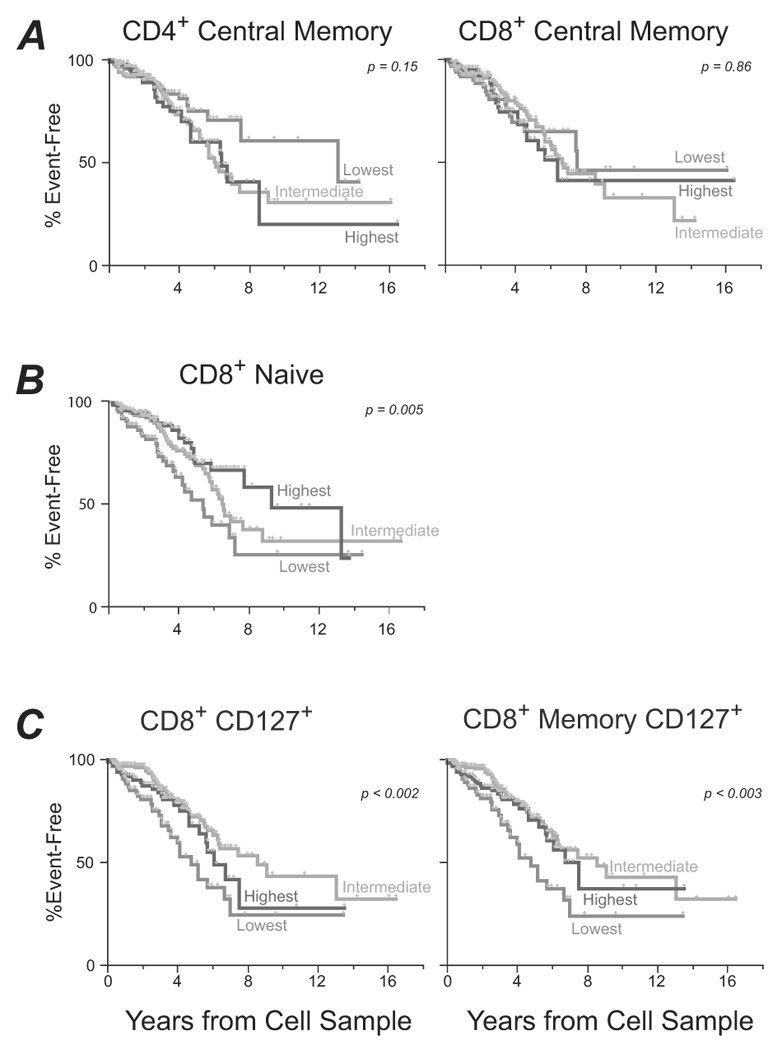
Subjects were grouped into three groups based on the relative proportion of any given T cell subset. Kaplan-Meier survival curves are shown for the groups. The red lines show data for those individuals with the lowest levels of a particular subset (<25th percentile), green lines for those with intermediate levels (25th–75th percentile), and blue lines for those with the highest levels (>75th percentile). (A) Disease progression according to baseline CD4 (left) or CD8 (right) TCM levels. (B) Disease progression according to baseline naïve CD8 T cell levels, for individuals measured within 225 days of imputed seroconversion (left) or after 225 days (right). (C) Disease progression according to baseline expression of CD127 on total CD8 T cells (left) or on memory CD8 T cells (right).
In secondary analyses, we examined a number of other phenotypic measurements, including absolute number and percentage of naïve, transitional, and effector memory cells, and found a number of parameters that were associated with progression. Notably, the proportion of CD8 T cells that were naïve strongly predicted slower disease progression (unadjusted P = 10−3). This measurement remains significant after adjusting for CD4 count (Table 2). This data is in agreement with studies of chronic infection [19], and suggests that individuals capable of maintaining naïve cells after the earliest stages of infection have a lower risk of AIDS.
Table 2
Factors associated with disease progression.
| Parameter | Hazard Ratio (95% CI) compared to lowest quartile group | ||
|---|---|---|---|
| Unadjusted | Adjusted for CD4 count | Adjusted for CD4 count + VL + age | |
| CD4+ TCM | N=426 | N=414 | N=348 |
| Middle half | 1.51 (0.89, 2.57) | 1.48 (0.86, 2.55) | 1.29 (0.65, 2.59) |
| Upper quartile | 1.51(0.83, 2.76) | 1.40 (0.76, 2.58) | 1.03 (0.47, 2.24) |
| CD8+ TCM | N=426 | N=414 | N=348 |
| Middle half | 0.92(0.56, 1.53) | 1.03 (0.62, 1.71) | 0.79 (0.41, 1.52) |
| Upper quartile | 1.11 (0.62- 2.00) | 1.38 (0.76, 2.52) | 1.17 (0.57, 2.40) |
| CD4+ Naïve | N=441 | N=429 | N=429 |
| Middle half | 0.74 (0.46, 1.20 | 0.78 (0.48, 1.27) | 0.73 (0.40, 1.32) |
| Upper quartile | 0.76 (0.44, 1.32) | 0.74 (0.42, 1.31) | 1.05 (0.52, 2.09) |
| CD8+ Naïve | N=441; P = 0.001 | N=430; P = 0.03 | N=430 |
| Middle half | 0.51 (0.33, 0.81) | 0.60 (0.38, 0.96) | 0.62 (0.35, 1.11) |
| Upper quartile | 0.37 (0.21, 0.64) | 0.47 (0.27, 0.84) | 0.56 (0.27, 1.18) |
| Ki-67+ CD4+ T | N=426; P = 0.001 | N=414; P = 0.009 | N=348 |
| Middle half | 1.74 (0.99, 3.07) | 1.62 (0.89, 2.96) | 1.51 (0.72, 3.19) |
| Upper quartile | 3.15 (1.68, 5.89) | 2.70 (1.39, 5.23) | 1.59 (0.63, 3.98) |
| Ki-67+ CD8+ T | N=426; P = 10−4 | N=414; P = 10−4 | N=348; P = 0.037 |
| Middle half | 1.81 (1.01, 3.26) | 2.18 (1.17, 4.04) | 1.80 (0.89, 3.77) |
| Upper quartile | 3.51 (1.89, 6.50) | 4.13 (2.15, 7.91) | 2.90 (1.25, 6.73) |
| Cell-associated VL | N=349; P = 6×10−4 | N=340 | N=286 |
| Middle half | 2.13 (1.15, 3.94) | 1.30 (0.69, 2.47) | 1.56 (0.62, 3.95) |
| Upper quartile | 3.69 (1.87, 7.30) | 1.78 (0.85, 3.74) | 2.29 (0.82, 6.45) |
| Cell-associated VL (EDSC < 225 days) | N=175; P = 10−4 | N=172; P = 0.004 | N=146; P = 0.027 |
| Middle half | 2.19 (0.91, 5.3) | 1.27 (0.50, 3.24) | 0.77 (0.21, 2.74) |
| Upper quartile | 11.38 (4.02, 32.3) | 5.27 (1.64, 16.94) | 3.59 (0.75, 17.09) |
| CD127+CD8 T | N=442; P = 0.009 | N=430; P = 0.01 | N=364 |
| Middle half | 0.47 (0.30, 0.75) | 0.49 (0.30, 0.79) | 0.61 (0.33, 1.15) |
| Upper quartile | 0.62 (0.37, 1.05) | 0.80 (0.47, 1.37) | 1.02 (0.51, 2.04) |
Notes:
Analysis of factors was performed by univariate or multivariate Cox proportional hazards regression. Initiation of HAART was treated as a censoring variable for this analysis. For each parameter, subjects were divided into three groups by quartile values (lowest, <25%, middle half, 25–75%, upper, >75%); for each analysis, the comparison group was the lowest quartile. The 95% confidence interval is shown in parentheses after the hazard ratio. “N” indicates the number of subjects for whom data was available for all covariates. (Note that the adjustment for VL was only performed for subjects for whom VL measurements were made within 30 days of baseline measurement.) A likelihood ratio was used to test whether the HR for both groups was 1.0; those comparisons resulting in P values less than 0.05 are indicated in the Table to indicate measurements that are significantly associated with disease progression.
We also investigated the association between CD127 expression on total or memory T cells and disease progression. CD127 expression is elevated on TCM compared to TEM, has been associated with homeostatic maintenance of memory cells [20], and is a marker for long-lived memory T cells [15]. As shown in Fig 2C, low levels of CD127+ CD8+ T-cells were significantly associated with faster progression.
Cell-associated viral load (CAVL) predicts progression
The destruction of CD4 T cells during acute infection is most likely due to infection of the cells by virus [10]; a fraction of infected cells survive and carry viral DNA. Quantification of the CAVL in early disease may reflect the extent of the viral replication (and hence associated immune destruction) during acute infection.
Based on the phenotypic measurements, we sorted four subsets of CD4 T cells by flow cytometry for every sample (Suppl Fig 1). We found HIV gag DNA in all cell types examined (surprisingly, including CCR5− naïve cells); TEM cells contain significantly higher levels than other cell types (Fig 3A). There was no significant difference in the predictive power of CAVL for any subset of CD4 T cells (data not shown); thus, we computed the total CD4 CAVL by summing the CAVL in each subset weighted by its representation. Total CAVL is a strong predictor of disease-free survival in subjects sampled within the first 225 days after seroconversion (Fig 3B, Table 2). The effect of CAVL differed for subjects sampled before or after the median EDSC of 225 days (interaction p = 0.04), with CAVL having a strong effect for subjects sampled in early infection (unadjusted p = 10−4, adjusted p = 0.03, Table 2, Figure 3B). For subjects sampled more than 225 days after EDSC, CAVL did not predict the rate of disease progression.
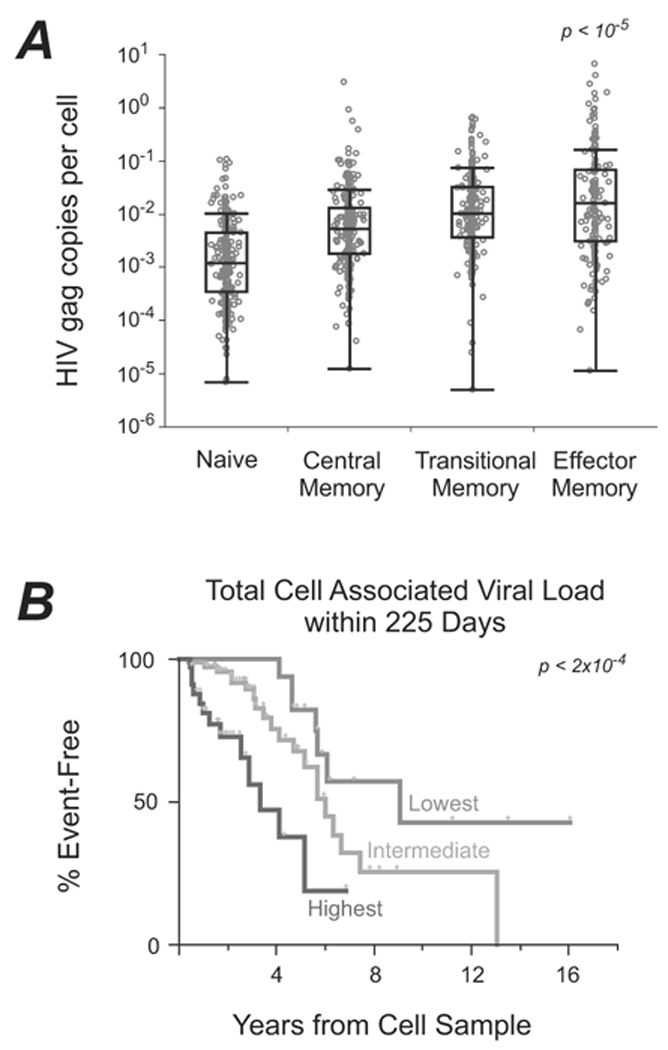
(A) Distribution of gag DNA within CD4+ T-cell subsets. This box and whisker plot depicts number of HIV gag DNA molecules per cell in each of the four sorted T-cell subsets. Interquartile ranges are shown. (B) Kaplan-Meier curves comparing the time to AIDS/death with levels of cell-associated DNA, for subjects identified within 225 days of imputed seroconversion.
T cell proliferation predicts progression
To determine the prognostic significance of immune proliferation, we measured the expression of Ki-67 (an antigen expressed during cellular proliferation). Ki-67 expression levels in both CD4 and CD8 T cells were predictors of progression (Table 2, Fig. 4). Specifically, subjects with the highest levels of Ki-67 expression exhibited faster progression to AIDS, for either CD4 or CD8 cells (Table 2). After adjustment for baseline CD4 count, age, and VL, CD8 Ki-67 levels continued to independently predict disease progression (Table 2).
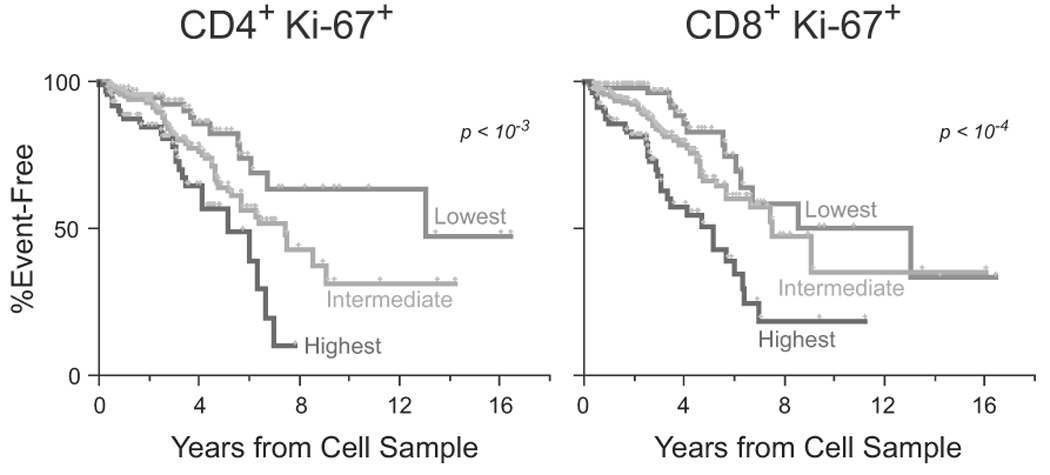
Kaplan-Meier curves comparing the time to AIDS/death among subjects with differing levels of Ki-67 expression in the CD4 compartment (left) or the CD8 compartment (right).
We repeated our analysis of the ability of Ki-67 to predict progression by treating HAART use as a time-varying covariate (rather than censoring upon initiation of HAART). In this analysis, the results were similar: the CD8 Ki-67 level was strongly associated with progression in a univariate analysis (HR-2.5, p < 0.001) and showed a trend towards significance in the multivariate model (HR = 1.81, p = 0.07).
Discussion
We performed a detailed evaluation of T cells and CAVL on a large, well-characterized cohort of individuals with long-term follow-up. The strength of this study is the comprehensive evaluation of T cells at early time points after seroconversion, with a large number of endpoints. Our primary objective was to evaluate whether central memory T cell (TCM) quantification predicts disease progression; secondary endpoints included evaluation of other T cell subsets, immune proliferation (activation), and CAVL.
TCM are a cornerstone of the adaptive immune response; the SIV model of infection points to the critical role played by these cells in the pathogenesis of disease [11, 14]. However, in our well-characterized cohort of recently-infected subjects, we did not observe an association between the proportion of TCM and disease progression. Nonetheless, we identified other potential correlates of progression including proportions of naïve or CD127+ memory CD8 T cells, Ki-67 expression in CD4 or CD8 T cells (correlating with immune activation), and the CD4 CAVL.
We considered several possible explanations for the lack of an association between TCM cells and disease progression. Since the proportions of memory cells vary with time since infection [10, 21], it is possible that mixing subjects identified during both acute and early HIV infection might mask associations. We therefore analyzed the predictive value of the proportion of TCM cells among subjects identified within 3 months, 3–6 months, etc., from imputed seroconversion; no significant association was observed for any group. While our study was powered to demonstrate differences overall, this subgrouping significantly limits statistical power. Longitudinal studies with repeated sampling are likely necessary to associate the dynamics of TCM cells with disease progression. Nonetheless, in humans, remodeling of the memory compartment is sufficiently variable during early infection such that enumeration of memory cells may not provide a predictor for progression.
As part of our secondary objectives we evaluated the predictive value of other T cell subset representation. After HIV infection, the naïve T cell pool progressively declines throughout the course of the disease [19, 22–24]. In our cohort, subjects who best maintained naïve CD8 T cells showed significantly slower progression of disease. This suggests that, while destruction of naïve T cells was thought to be a consequence of chronic disease, the loss of these cells starts early and may be related to the mechanisms accounting for progression throughout disease.
Within the CD8 T cells, another marker predictive of progression is expression of the IL7-receptor (CD127). IL7 plays an integral role in the differentiation and survival of TCM and naïve T cells [20, 25], and CD127 expression appears necessary for the generation of long-lived memory T cells, a hallmark of protective immunity [25]. Disturbances in the IL-7, IL-7 receptor axis have been reported during both acute and chronic HIV infection [26–28]; levels of CD127− CD8 T cells correlate with VL and inversely with CD4 counts [27, 29]. Here we show that decreased levels of CD127+ CD8 T cells during early disease are associated faster disease progression. Thus, the early depletion of long-lived cells in general (such as naïve or memory CD127+ cells, Fig. 2) may more accurately predict subsequent progression to AIDS than the loss of TCM as defined by commonly-used phenotypic markers.
Immune activation (IA) plays a key role in the pathogenesis of both SIV and HIV-infection [30], and correlates with loss of CD4 T cells and progression to AIDS. IA has been studied extensively in the setting of chronic HIV infection [2, 31, 32]; together with some small studies of early infection [1, 33, 34], it was suggested that early levels of IA may set the stage for future disease. In our study, the proportion of proliferating (Ki-67+) CD8 and CD4 T cells, a measure of IA, varied among individuals, but was low overall (mean = 1.7%). Nonetheless, having higher levels of proliferation was strongly predictive of progression. Specifically, the median time to AIDS and death among subjects with the highest levels (top quartile) of proliferation in CD8 T cells was 4 years, in contrast to 10 years for subjects with the lowest levels. Further, though proliferation correlated with plasma VL (data not shown), it still provides independent predictive power. While the mechanisms by which IA exerts deleterious effects remain unclear, they may be closely related to altered CD127 expression, since T cell activation is associated with reduced CD127 expression and altered T cell homeostasis [27, 35, 36].
In our study, Ki-67 expression in CD8 cells identified subjects that progressed faster to AIDS and death, even after adjusting for CD4 count and VL. This argues for the inclusion of a marker of proliferation and activation to the markers used to assess vaccine efficacy and prognostic significance following HIV infection. Similarly, measures of immune proliferation and activation could be used to identify subjects most at risk for progression and likely to benefit from early therapeutic intervention.
The basic impetus for our study was the finding that acute SIV infection is accompanied by a massive destruction of memory T cells. As discussed above, cross-sectional enumeration of TCM subsets in early HIV disease did not provide a correlate of subsequent progression. However, since the early destruction of CD4 T cells is mediated by viral infection, we hypothesized that a signature of the extent of the destruction during acute phase would still be found in the cell-associated viral load at early time points. Indeed, we found that CAVL was predictive of disease progression – in that individuals with higher rates of infection of CD4 T cells progressed more rapidly. Remarkably, we found relatively high rates of infection of naïve CD4 T cells (albeit 10-fold less than memory CD4 T cells). Naïve CD4 T cells do not express CCR5, the obligate HIV co-receptor for nearly all transmitted viruses. In this early-stage cohort, we expect that most circulating virus is CCR5-dependent. Since naïve CD4 T cells can be infected with CCR5-dependent viruses in vitro, with T cell activation [37–39], the infection in vivo that we observed may be a direct consequence of IA.
In otherwise asymptomatic individuals current practice guidelines recommend initiation of antiretroviral therapy at a CD4 count<350 [40, 41]. However, there is a growing body of literature that supports the initiation of ART at higher CD4 counts, demonstrating reductions in both AIDS and non AIDS-related morbidity and mortality [4, 5, 7, 42–44]. Our studies provide some mechanistic clues to why these beneficial effects with HAART are observed. Specifically, we suggest that immunological disturbances (altered homeostasis and increased proliferation/activation) are established early in HIV infection, and are observed even among subjects with relatively preserved CD4 counts. The magnitude of these disturbances correlate with progression. Since HAART has been shown to reduce immune activation, preserve naïve T cell populations, and maintain CD127 expression [26, 45–48], our data indirectly support early initiation of such therapy.
In conclusion, we find that quantification of TCM in early infection does not provide predictive power for progression. However, measures of homeostasis and activation, including CD127 expression and Ki-67 do provide such information, and should be studied further to determine their role in clinical monitoring of HIV-1 progression. In addition, CAVL provides predictive power, but is not as easily implemented on a routine basis. Future efforts at identifying markers of subsequent progression should focus on measures of activation and homeostasis during the earliest stages of infection.
Acknowledgments
We gratefully acknowledge the expert technical assistance and advice provided by Dr. Joanne Yu, Dr. Diane Bolton, Stephen Perfetto, Richard Nguyen, and Brenna Hill; we thank the members of the ImmunoTechnology Section and the VRC for critical discussion during the course of this project.
We would like to thank the patients without whom none of this work would be possible. We would also like to thank the research coordinators and support staff who diligently work on the DoD HIV NHS as well as the members of the IDCRP HIV Working Group, listed by site: National Naval Medical Center, Bethesda, MD: Catherine Decker, Timothy Whitman, Sybil Tasker; Walter Reed Army Medical Center, Washington, DC: Amy Weintrob, Glenn Wortmann, Michael Zapor; San Antonio Military Medical Center, San Antonio, TX: Mike Landrum, Vince Marconi, Jason Okulicz; Naval Medical Center San Diego, San Diego, CA: Nancy Crum-Cianflone, Mary Bavaro, Helen Chun; Naval Medical Center Portsmouth, Portsmouth, VA: Robert V Barthel; Tripler Army Medical Center, Honolulu, HI: Arthur Johnson; Uniformed Services University of the Health Sciences, Bethesda, MD: Brian Agan, Naomi Aronson, William Bradley, and Greg Gandits; Walter Reed Army Institute of Research, Rockville, MD: Linda Jagodzinski, Robert O'Connell, Connor Eggleston; National Institute of Allergy and Infectious Diseases, Bethesda, MD: John Powers.
Funding. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH; the National Cancer Institute, NIH, under contract No. HHSN261200800001E; the Military Infectious Disease Research Program, US Army Medical Research and Materiel Command; and the Infectious Disease Clinical Research Program (IDCRP) of the Uniformed Services University of the Health Sciences (USUHS).
Footnotes
Conflicts of interest. The authors declare that they have no financial conflicts of interest.
Disclaimer. The views expressed here are the opinions of the authors and are not to be considered as official or reflecting the views or policies of the Walter Reed Army Institute of Research, the National Naval Medical Center, the U. S. Department of Defense, the Vaccine Research Center / National Institutes of Health / Department of Health and Human Services, or the Uniformed Services University, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Presentations. Portions of these data have been presented at the Keystone HIV Pathogenesis Meeting (March 2008).
References
Full text links
Read article at publisher's site: https://doi.org/10.1086/649430
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/jid/article-pdf/201/2/272/18060681/201-2-272.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Impact of the first-line antiretroviral therapy on soluble markers of inflammation in cohort of human immunodeficiency virus type 1 in Moroccan patients: a prospective study.
Arch Microbiol, 205(6):223, 08 May 2023
Cited by: 0 articles | PMID: 37154966
Malignancy and viral infections in Sub-Saharan Africa: A review.
Front Virol, 3:1103737, 06 Mar 2023
Cited by: 3 articles | PMID: 37476029 | PMCID: PMC10358275
Exposure to Secreted Bacterial Factors Promotes HIV-1 Replication in CD4+ T Cells.
Microbiol Spectr, 11(2):e0431322, 28 Feb 2023
Cited by: 0 articles | PMID: 36853052 | PMCID: PMC10100953
Cocaine Self-Administration Influences Central Nervous System Immune Responses in Male HIV-1 Transgenic Rats.
Cells, 11(15):2405, 04 Aug 2022
Cited by: 2 articles | PMID: 35954251 | PMCID: PMC9368446
HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies.
J Immunol Res, 2021:7316456, 29 Sep 2021
Cited by: 61 articles | PMID: 34631899 | PMCID: PMC8494587
Review Free full text in Europe PMC
Go to all (54) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
T-cell dynamics during acute SIV infection.
AIDS, 18(1):13-23, 01 Jan 2004
Cited by: 52 articles | PMID: 15090825
CCR5 and CXCR4 expression on memory and naive T cells in HIV-1 infection and response to highly active antiretroviral therapy.
J Acquir Immune Defic Syndr, 27(2):105-115, 01 Jun 2001
Cited by: 27 articles | PMID: 11404531
Distribution of naïve (CD45RA+) and memory (CD45RO+) T-cells in HIV-infected Puerto Rican population.
P R Health Sci J, 21(3):195-201, 01 Sep 2002
Cited by: 1 article | PMID: 12243109
[Deep lung--cellular reaction to HIV].
Rev Port Pneumol, 13(2):175-212, 01 Mar 2007
Cited by: 3 articles | PMID: 17492233
Review
Funding
Funders who supported this work.
Intramural NIH HHS (2)
Grant ID: ZIA AI005021-08
Grant ID: Z99 AI999999
NCI NIH HHS (2)
Grant ID: HHSN261200800001E
Grant ID: HHSN261200800001C




