Abstract
Free full text

Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation.
Abstract
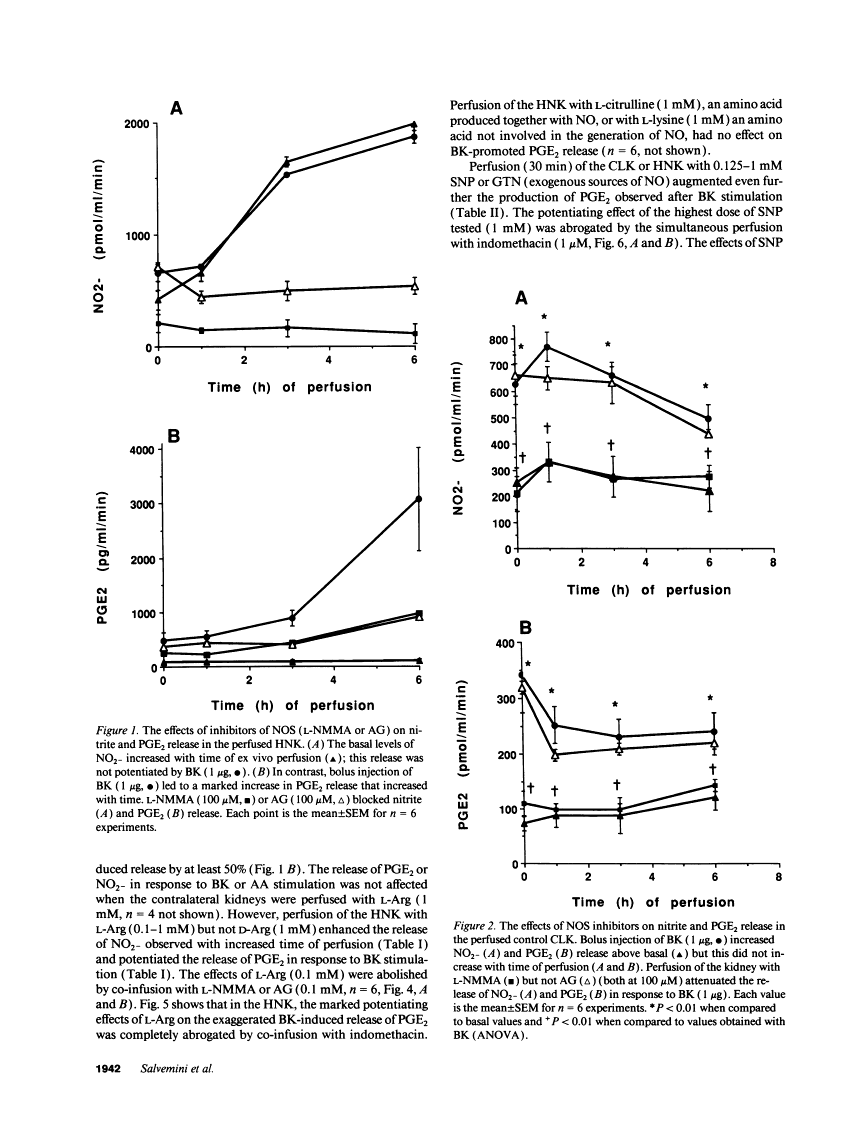
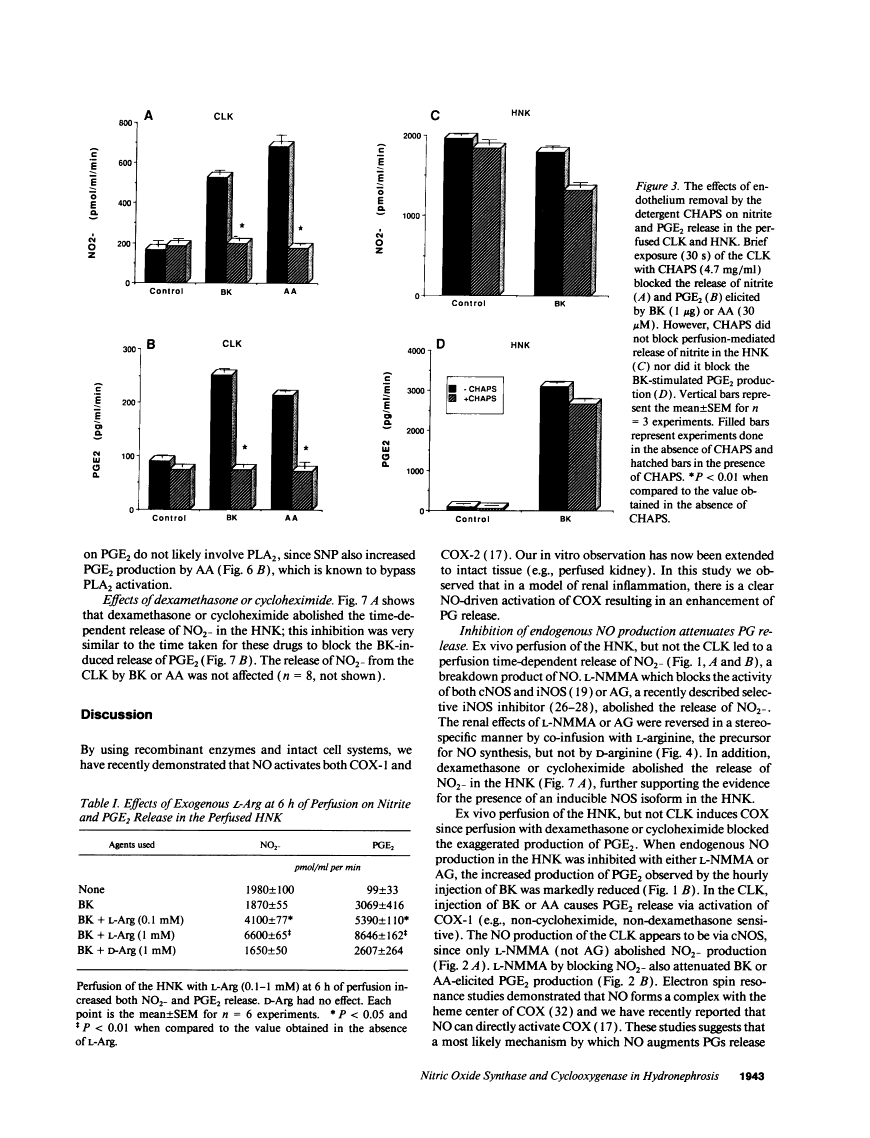
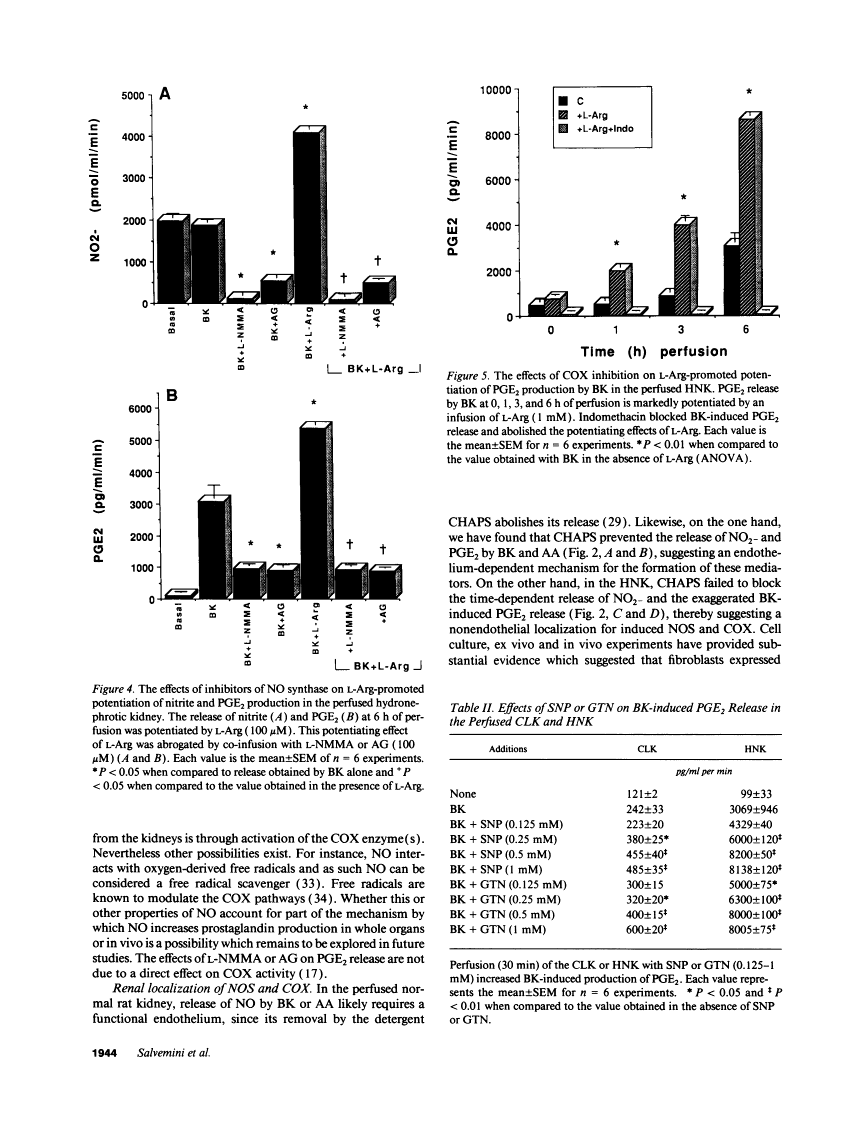
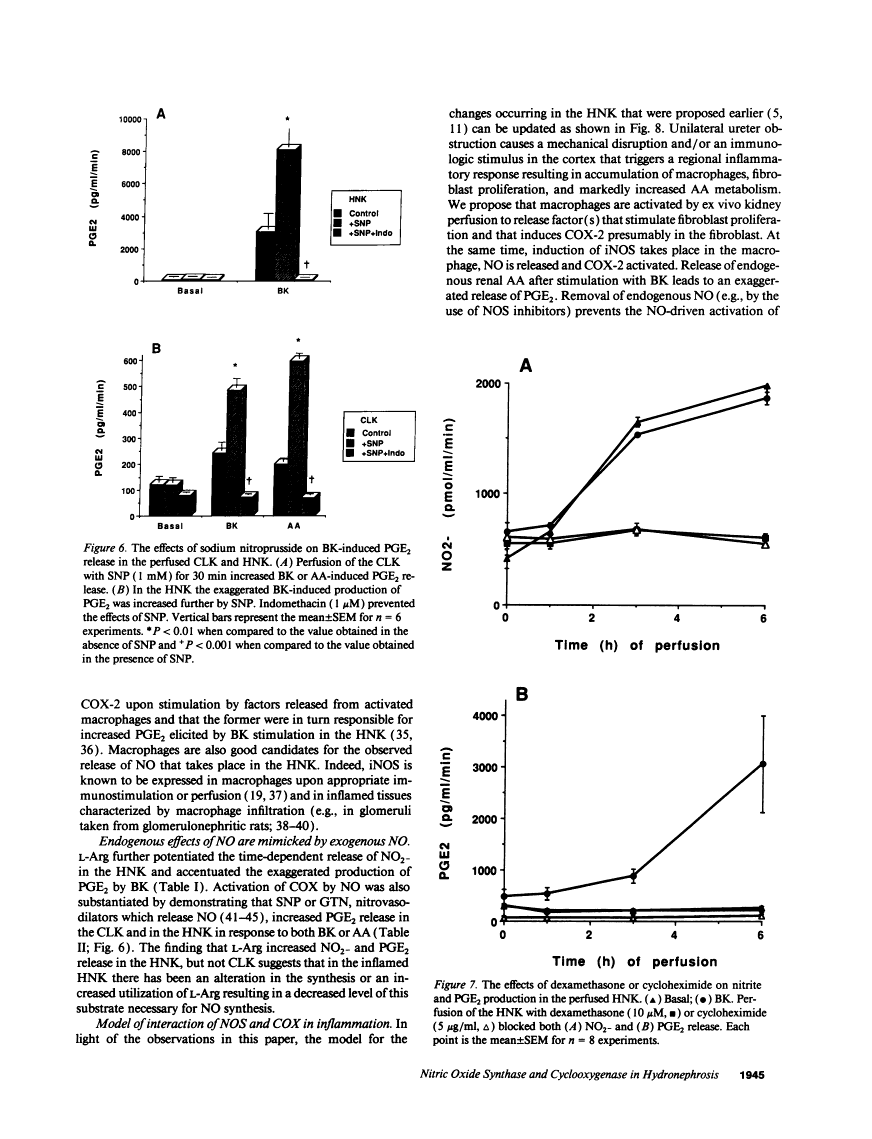
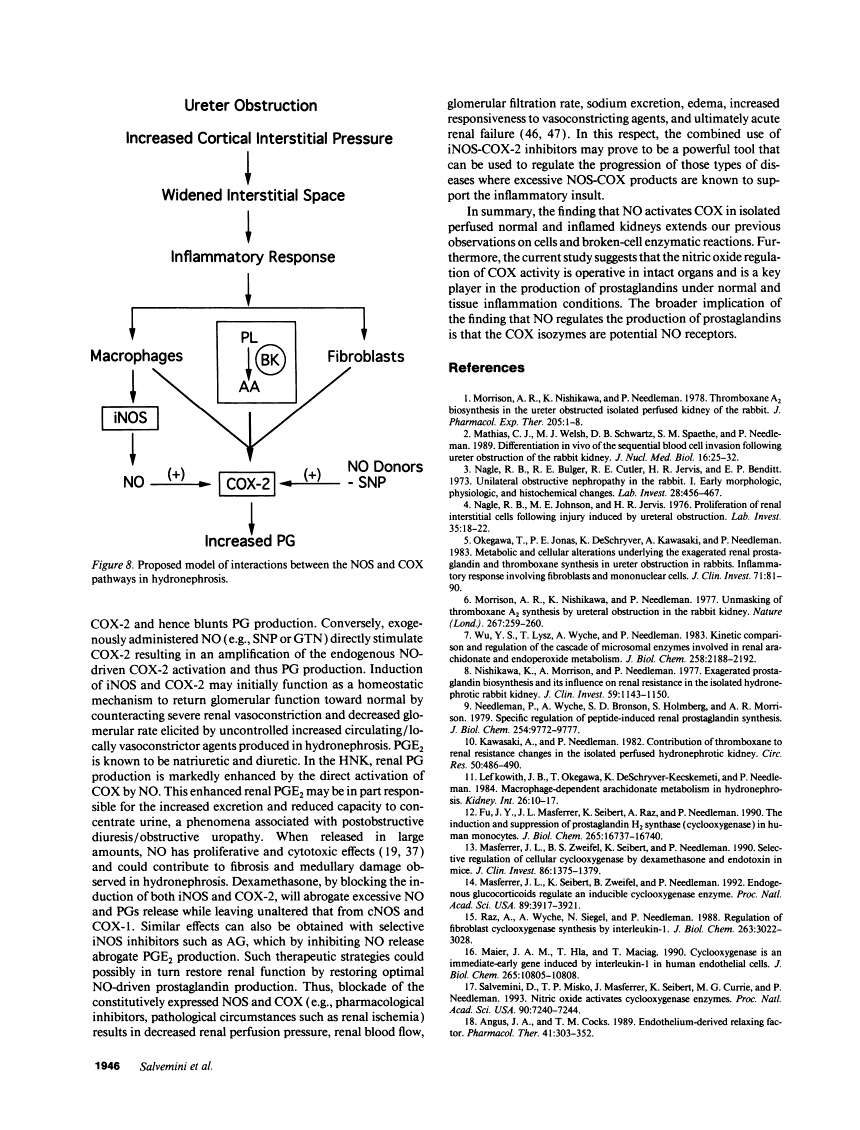
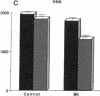
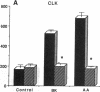
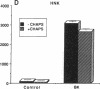
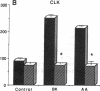
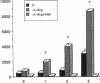
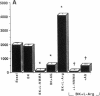
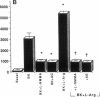
The interaction between nitric oxide (NO) and cyclooxygenase (COX) was studied in a rabbit model of renal inflammation, the ureteral obstructed hydronephrotic kidney (HNK). Ex vivo perfusion of the HNK but not the control kidney (e.g., unobstructed contralateral kidney, CLK), led to a time-dependent release of nitrite (NO2-), a breakdown product of NO. Stimulation of the HNK with bradykinin (BK) evoked a time-dependent increase in prostaglandin E2 (PGE2) production. NG-monomethyl-L-arginine (L-NMMA), which blocks the activity of both constitutive and inducible nitric oxide synthase (cNOS and iNOS), aminoguanidine, a recently described selective iNOS inhibitor, dexamethasone, or cycloheximide abolished the release of NO2- and attenuated the exaggerated BK-induced PGE2 production. This supports the existence of iNOS and COX-2 in the HNK. In the CLK, BK elicited release of both NO2- and PGE2 but this did not augment with time. L-NMMA but not aminoguanidine, dexamethasone, or cycloheximide attenuated NO2- and PGE2 release indicative of the presence of constitutive but not inducible NOS or COX. The current study suggests that the endogenous release of NO from cNOS in the CLK activates a constitutive COX resulting in optimal PGE2 release by BK. In addition, in the HNK, NO release from iNOS activates the induced COX resulting in markedly increased release of proinflammatory prostaglandin. The broader implication of this study is that the cyclooxygenase isozymes are potential receptor targets for nitric oxide.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.6M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Morrison AR, Nishikawa K, Needleman P. Thromboxane A2 biosynthesis in the ureter obstructed isolated perfused kidney of the rabbit. J Pharmacol Exp Ther. 1978 Apr;205(1):1–8. [Abstract] [Google Scholar]
- Mathias CJ, Welch MJ, Schwartz DB, Spaethe SM, Needleman P. Differentiation in vivo of the sequential blood cell invasion following ureter obstruction of the rabbit kidney. Int J Rad Appl Instrum B. 1989;16(1):25–32. [Abstract] [Google Scholar]
- Nagle RB, Bulger RE, Cutler RE, Jervis HR, Benditt EP. Unilateral obstructive nephropathy in the rabbit. I. Early morphologic, physiologic, and histochemical changes. Lab Invest. 1973 Apr;28(4):456–467. [Abstract] [Google Scholar]
- Nagle RB, Johnson ME, Jervis HR. Proliferation of renal interstitial cells following injury induced by ureteral obstruction. Lab Invest. 1976 Jul;35(1):18–22. [Abstract] [Google Scholar]
- Okegawa T, Jonas PE, DeSchryver K, Kawasaki A, Needleman P. Metabolic and cellular alterations underlying the exaggerated renal prostaglandin and thromboxane synthesis in ureter obstruction in rabbits. Inflammatory response involving fibroblasts and mononuclear cells. J Clin Invest. 1983 Jan;71(1):81–90. [Europe PMC free article] [Abstract] [Google Scholar]
- Morrison AR, Nishikawa K, Needleman P. Unmasking of thromboxane A2 synthesis by ureteral obstruction in the rabbit kidney. Nature. 1977 May 19;267(5608):259–260. [Abstract] [Google Scholar]
- Sheng WY, Lysz TA, Wyche A, Needleman P. Kinetic comparison and regulation of the cascade of microsomal enzymes involved in renal arachidonate and endoperoxide metabolism. J Biol Chem. 1983 Feb 25;258(4):2188–2192. [Abstract] [Google Scholar]
- Nishikawa K, Morrison A, Needleman P. Exaggerated prostaglandin biosynthesis and its influence on renal resistance in the isolated hydronephrotic rabbit kidney. J Clin Invest. 1977 Jun;59(6):1143–1150. [Europe PMC free article] [Abstract] [Google Scholar]
- Needleman P, Wyche A, Bronson SD, Holmberg S, Morrison AR. Specific regulation of peptide-induced renal prostaglandin synthesis. J Biol Chem. 1979 Oct 10;254(19):9772–9779. [Abstract] [Google Scholar]
- Kawasaki A, Needleman P. Contribution of thromboxane to renal resistance changes in the isolated perfused hydronephrotic rabbit kidney. Circ Res. 1982 Apr;50(4):486–490. [Abstract] [Google Scholar]
- Lefkowith JB, Okegawa T, DeSchryver-Kecskemeti K, Needleman P. Macrophage-dependent arachidonate metabolism in hydronephrosis. Kidney Int. 1984 Jul;26(1):10–17. [Abstract] [Google Scholar]
- Fu JY, Masferrer JL, Seibert K, Raz A, Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [Abstract] [Google Scholar]
- Masferrer JL, Zweifel BS, Seibert K, Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. [Europe PMC free article] [Abstract] [Google Scholar]
- Masferrer JL, Seibert K, Zweifel B, Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3917–3921. [Europe PMC free article] [Abstract] [Google Scholar]
- Raz A, Wyche A, Siegel N, Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [Abstract] [Google Scholar]
- Maier JA, Hla T, Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990 Jul 5;265(19):10805–10808. [Abstract] [Google Scholar]
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7240–7244. [Europe PMC free article] [Abstract] [Google Scholar]
- Angus JA, Cocks TM. Endothelium-derived relaxing factor. Pharmacol Ther. 1989;41(1-2):303–352. [Abstract] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [Abstract] [Google Scholar]
- Crack P, Cocks T. Thimerosal blocks stimulated but not basal release of endothelium-derived relaxing factor (EDRF) in dog isolated coronary artery. Br J Pharmacol. 1992 Oct;107(2):566–572. [Europe PMC free article] [Abstract] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. [Europe PMC free article] [Abstract] [Google Scholar]
- Mollace V, Salvemini D, Anggard E, Vane J. Nitric oxide from vascular smooth muscle cells: regulation of platelet reactivity and smooth muscle cell guanylate cyclase. Br J Pharmacol. 1991 Nov;104(3):633–638. [Europe PMC free article] [Abstract] [Google Scholar]
- Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. [Abstract] [Google Scholar]
- Corbett JA, Tilton RG, Chang K, Hasan KS, Ido Y, Wang JL, Sweetland MA, Lancaster JR, Jr, Williamson JR, McDaniel ML. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. [Abstract] [Google Scholar]
- Misko TP, Moore WM, Kasten TP, Nickols GA, Corbett JA, Tilton RG, McDaniel ML, Williamson JR, Currie MG. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. [Abstract] [Google Scholar]
- Griffiths MJ, Messent M, MacAllister RJ, Evans TW. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol. 1993 Nov;110(3):963–968. [Europe PMC free article] [Abstract] [Google Scholar]
- Bhardwaj R, Moore PK. The effect of arginine and nitric oxide on resistance blood vessels of the perfused rat kidney. Br J Pharmacol. 1989 Jul;97(3):739–744. [Europe PMC free article] [Abstract] [Google Scholar]
- Reingold DF, Watters K, Holmberg S, Needleman P. Differential biosynthesis of prostaglandins by hydronephrotic rabbit and cat kidneys. J Pharmacol Exp Ther. 1981 Mar;216(3):510–515. [Abstract] [Google Scholar]
- Karthein R, Nastainczyk W, Ruf HH. EPR study of ferric native prostaglandin H synthase and its ferrous NO derivative. Eur J Biochem. 1987 Jul 1;166(1):173–180. [Abstract] [Google Scholar]
- Kanner J, Harel S, Granit R. Nitric oxide as an antioxidant. Arch Biochem Biophys. 1991 Aug 15;289(1):130–136. [Abstract] [Google Scholar]
- Egan RW, Paxton J, Kuehl FA., Jr Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976 Dec 10;251(23):7329–7335. [Abstract] [Google Scholar]
- Lefkowith JB, Needleman P. Arachidonate metabolism in renal injury. Adv Prostaglandin Thromboxane Leukot Res. 1985;13:121–130. [Abstract] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [Abstract] [Google Scholar]
- Cattell V, Cook T, Moncada S. Glomeruli synthesize nitrite in experimental nephrotoxic nephritis. Kidney Int. 1990 Dec;38(6):1056–1060. [Abstract] [Google Scholar]
- Cook HT, Sullivan R. Glomerular nitrite synthesis in in situ immune complex glomerulonephritis in the rat. Am J Pathol. 1991 Nov;139(5):1047–1052. [Europe PMC free article] [Abstract] [Google Scholar]
- Jansen A, Lewis S, Cattell V, Cook HT. Arginase is a major pathway of L-arginine metabolism in nephritic glomeruli. Kidney Int. 1992 Nov;42(5):1107–1112. [Abstract] [Google Scholar]
- Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [Abstract] [Google Scholar]
- Rapoport RM, Draznin MB, Murad F. Endothelium-dependent vasodilator-and nitrovasodilator-induced relaxation may be mediated through cyclic GMP formation and cyclic GMP-dependent protein phosphorylation. Trans Assoc Am Physicians. 1983;96:19–30. [Abstract] [Google Scholar]
- Ignarro LJ, Gruetter CA. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochim Biophys Acta. 1980 Aug 13;631(2):221–231. [Abstract] [Google Scholar]
- Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [Abstract] [Google Scholar]
- Lüscher TF, Bock HA. The endothelial L-arginine/nitric oxide pathway and the renal circulation. Klin Wochenschr. 1991 Sep 3;69(13):603–609. [Abstract] [Google Scholar]
- King AJ, Brenner BM. Endothelium-derived vasoactive factors and the renal vasculature. Am J Physiol. 1991 Apr;260(4 Pt 2):R653–R662. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci117185
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/117185/files/pdf
Citations & impact
Impact metrics
Article citations
Suppression of Neuroinflammation by Coffee Component Pyrocatechol via Inhibition of NF-κB in Microglia.
Int J Mol Sci, 25(1):316, 25 Dec 2023
Cited by: 1 article | PMID: 38203488 | PMCID: PMC10778612
Levels of nitric oxide in smokers and nonsmokers with chronic periodontitis before and after nonsurgical periodontal treatment.
Natl J Maxillofac Surg, 14(3):450-453, 01 Sep 2023
Cited by: 0 articles | PMID: 38273932 | PMCID: PMC10806316
Nitric oxide mediates antimicrobial peptide gene expression by activating eicosanoid signaling.
PLoS One, 13(2):e0193282, 21 Feb 2018
Cited by: 7 articles | PMID: 29466449 | PMCID: PMC5821394
Nitric Oxide Mediates Insect Cellular Immunity via Phospholipase A2 Activation.
J Innate Immun, 10(1):70-81, 17 Oct 2017
Cited by: 18 articles | PMID: 29035888 | PMCID: PMC6757156
Riboflavin along with antibiotics balances reactive oxygen species and inflammatory cytokines and controls Staphylococcus aureus infection by boosting murine macrophage function and regulates inflammation.
J Inflamm (Lond), 13:36, 28 Nov 2016
Cited by: 24 articles | PMID: 27932936 | PMCID: PMC5126841
Go to all (184) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Nitric oxide activates cyclooxygenase enzymes.
Proc Natl Acad Sci U S A, 90(15):7240-7244, 01 Aug 1993
Cited by: 790 articles | PMID: 7688473 | PMCID: PMC47112
Pharmacological manipulation of cyclo-oxygenase-2 in the inflamed hydronephrotic kidney.
Br J Pharmacol, 117(6):1016-1020, 01 Mar 1996
Cited by: 29 articles | PMID: 8882591 | PMCID: PMC1909763
Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids.
Br J Pharmacol, 114(7):1335-1342, 01 Apr 1995
Cited by: 215 articles | PMID: 7541688 | PMCID: PMC1510271
The nitric oxide-mediated regulation of prostaglandin signaling in medicine.
Vitam Horm, 96:211-245, 01 Jan 2014
Cited by: 8 articles | PMID: 25189389
Review