Abstract
Free full text

CHIMERISM AND DONOR-SPECIFIC NONREACTIVITY 27 TO 29 YEARS AFTER KIDNEY ALLOTRANSPLANTATION1
Abstract
Chimerism was demonstrated with immunocytochemical and/or polymerase chain reaction techniques in kidney allografts and in the native skin, lymph nodes, or blood of 5 of 5 patients who received continuously functioning renal transplants from 1 or 2 haplotype HLA mismatched consanguineous donors (4 parents, 1 aunt) 27–29 years ago. In the 4 cases where the kidney donor still was alive to provide stimulator lymphocytes for testing, these provoked no (n=2) or modest (n=2) MLR in contrast to vigorous MLR to third party lymphocytes. In all 4 cases, the donor cells failed to generate in vitro cytotoxic effector cells (cell-mediated lymphocytotoxicity). These findings are in accord with the hypothesis that cell migration, repopulation, and chimerism are seminal events that define graft acceptance and ultimately can lead to acquired donor-specific nonresponsiveness (tolerance).
We postulated recently that the acceptance of whole organ transplants connotes a state of long-term mixed allogeneic or xenogeneic chimerism after the migration from the graft and widespread seeding in the recipient of dendritic and other immune cells; simultaneous chimerism of the graft is produced by a reverse traffic of similar recipient cells (1). Our hypothesis further holds that the bidirectional cell migration and repopulation is the first step in the acquisition of donor-specific immunologic nonresponsiveness (tolerance). The changes in the graft and in recipient tissues has been easiest to prove after liver (2, 3) or intestinal (4, 5) transplantation because of the dense population of potentially migratory cells in these organs. However, we report here the same kind of chimerism on a smaller scale in 5 patients who have born kidney allografts for 27–29 years.
MATERIALS AND METHODS
Case Material
The 5 kidney recipients who now are 45.20±1.79 (SD) years old (range 43–47) underwent renal transplantation at the University of Colorado between July 1963 and July 1965 under AZA-PRED immunosuppression (6) (Table 1). They were selected for the present study in preference to our other survivors from this early era because: (a) 4 of the 5 donors still are alive, allowing their HLA retyping and use of their lymphocytes for studies of immunologic reactivity of the recipient; (b) known HLA incompatibilities between the foregoing 4 donors and their recipients permitted immunocytochemical and polymerase chain reaction (PCR*) distinction of donor from recipient cells in the biopsy tissues from the recipients; (c) in the fifth case (LD 52), in which the donor (father to daughter) had died, chimerism in tissues could be determined by sex typing.
TABLE 1
Clinical data
| LD numbers | |||||
|---|---|---|---|---|---|
| 17 | 33 | 51 | 52 | 90 | |
| Transplantation | 7-19-63 | 10-7-63 | 2-10-64 | 2-17-64 | 7-23-65 |
| Recipient sex | F | F | M | F | F |
| Donor relation | Mother | Mother | Aunt | Father | Mother |
| PRED (mg/day) | 2.5 | 0 | 10 | 7.5a | 7.5a |
| AZA (mg/day) | 75 | 100 | 125 | — | 50 |
| Creatinine mg% | 0.7 | 1.2 | 1.6 | 0.9 | 1.8 |
| BUN mg% | 6 | 15 | 19 | 13 | 21 |
The 5 recipients underwent bilateral nephrectomy and splenectomy at the time of transplantation. Their current medications and renal functions are shown in Table 1. Patient 52 has received no AZA for 12 years. The current steroid doses have been fixed > 25 years.
Determination of Chimerism
Each tissue biopsy specimen was obtained with separate sterile instruments to ensure against genetic contamination. The biopsy of the kidney allograft was performed in one room and the patient was then moved to a different operating room, where the skin and lymph node biopsies were obtained from a freshly sterilized field in the groin. Portions of the biopsies were fixed in 10% formalin for conventional staining, or else immediately frozen in optimal cold temperature compound or liquid nitrogen for immunocytochemistry or DNA typing, respectively.
Immunocytochemistry (ICC)
Both donors and recipients were freshly typed (Table 2). From the class I and class II HLA Ags present in the donor but not the recipient, mABs were chosen from a library and used for phenotyping of the kidney, skin, and lymph node biopsies (Table 3). Both indirect immunofluorescence and immunoperoxidase (avidin-biotin-complex) methods were used. Antibodies of the same Ig class that were reactive with the recipient but not the donor provided positive controls, and Ig class-matched anti-MHC antibodies that reacted with neither were negative controls. A tissue was considered chimeric when cells stained with both recipient-specific and donor-specific HLA antibodies, but not with nonspecific MHC antibodies.
TABLE 2
HLA phenotypes
| Recipient | Donor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | ||||||||||
| A | B | BW | DR | DQW | A | B | BW | DR | DQW | |
| 17 | 3,26 | 35,- | 6,- | 1,15 | 1,- | 1,3 | 35,44 | 4,6 | 7,15 | 1,2 |
| 33 | 2,3 | 7,27 | 4,6 | 1,13 | 1,- | 2,- | 18,27 | 4,6 | 1,4 | 1,3 |
| 51 | 24,29 | 44,55 | 4,6 | 4,7 | 3,- | 3,- | 7,- | 6,- | 4,2 | 1,3 |
| 52 | Female | Male | (Father) | |||||||
| 90 | 1,28 | 35,57 | 4,6 | 4,15 | 1,3 | 1,11 | 57,62 | 4,6 | 4,7 | 2,3 |
TABLE 3
Immunostaining for donor-specific chimerism
| Patient | Donor-specific HLA-mAbs tested | Positive Staining for donor HLA | Number of positive cells/ section | |
|---|---|---|---|---|
| Lymph nodesa | Skinb | |||
| 17 | 6 | B44 | <10 | 0 |
| 33 | 1 | B18 | <10 | <10 |
| 51 | 2 | B7,40 | 10–20 | 0 |
| A3 | <10 | <5 | ||
| 52 | Y probe | N/A | 0 | 2 |
| 90 | 5 | DQW2 | <10 | <5 |
The tissues from the female patient who received her father’s kidney were examined after in situ hybridization using a cocktail of cloned, biotinylated DNA probes directed against satellite DNA sequences specific for the Y chromosome (Oncor Inc., Gaithersburg, MD). Formalin-fixed, paraffin-embedded tissues were deparaffinized, partially digested, and hybridized overnight. High stringency washes were followed by an amplified avidin-fluorescein detection system (Oncor). Tissues of known sex served as controls. In addition, the male allograft organ served as a fixation and digestion control within each run. Tissues were examined and photographed using a Nikon Optiphot II epifluorescence microscope.
PCR
HLA-DRB molecular typing was performed according to the reference protocol of the XI International Histocompatibility Workshop in which preliminary generic amplification of the DRB gene is performed to decide which allele-specific amplifications must be done and which allele-specific oligonucleotide probes must be tested in the dot-blot hybridization analyses (7).
In the case where the paternal donor to a daughter recipient had died (LD 52), oligonucleotides specific for the satellite region of the Y chromosome centromere (8) and for the sex-determining region of the Y chromosome (9) were used as primers to determine the presence of DNA from the donor in the patient tissues. After Southern blotting, the amplified material was hybridized to radioactively labeled Y-specific probes to confirm the specificity of the bands visualized in an agarose gel after ethidium bromide staining.
Immunologic Studies
MLR
Unidirectional human MLR cultures were set up for 6 days with 5×104 responder and 5×104 irradiated (2000R) stimulator cells in a volume of 200 µl of tissue culture medium supplemented with 5% human serum. Proliferation was assessed by the degree of [3H]thymidine incorporation during the final 20 hr of incubation.
Cell-Mediated Lymphocytotoxicity (CML)
The cytolytic activity of recipient lymphocytes toward donor and third party targets was assessed in cell-mediated lympholysis assays. In these assays, effector lymphocytes were incubated with Cr-labeled target cells at various E:T ratios, ranging from 10:1 to 30:1.
RESULTS
Evidence of Chimerism
ICC
The donor HLA specificity of the renal allografts after 27–29 years was most easily demonstrated in the vascular endothelial cells (Fig. 1A). In contrast, the Y probe in case LD 52 showed the male marker in the renal tubules (Fig. 2A).
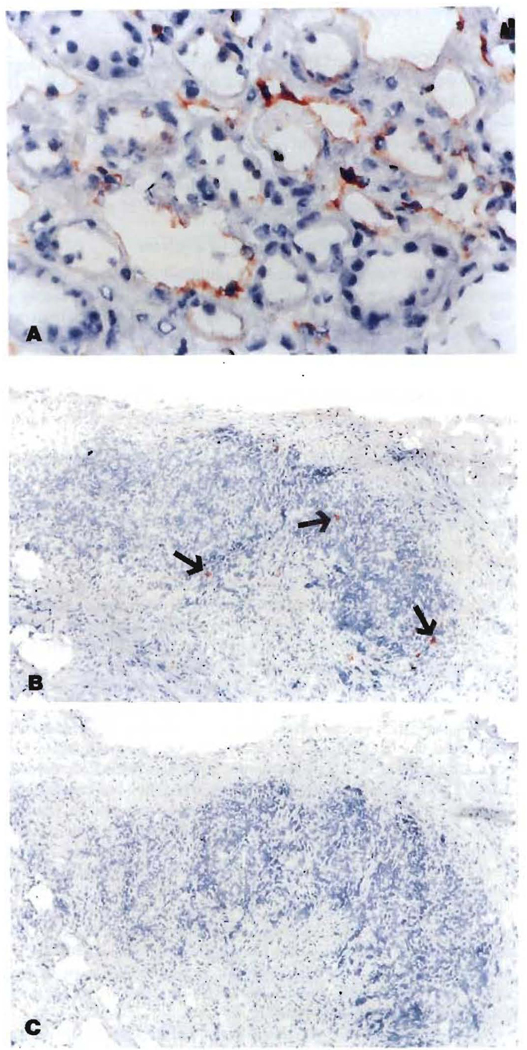
Kidney allograft (A, 450×) and recipient lymph node (B, 100×) stained with anti-HLA B 7,40 mAb using an avidin-biotin complex method, The rust-colored endothelial cells in the kidney and scattered cells in the lymph node are of donor origin, (C) (l00×) The negative control in which anti-A2, 28 (irrelevant class-matched antibody) was used as the primary antibody.
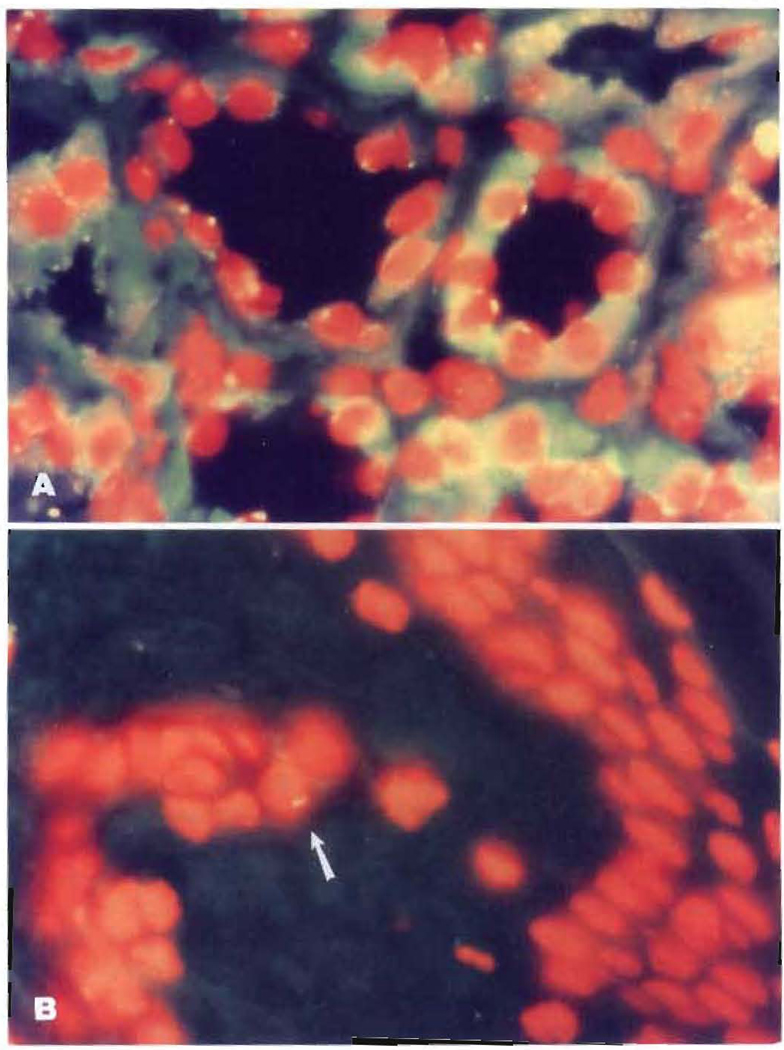
Fluorescent in situ hybridization for the Y chromosome. (A) Kidney allograft showing the male (Y) marker in over 50% of the tubular epithelial cells. (B) Y marker (arrow) in a skin biopsy (both photomicrographs 1000×).
All 5 of the recipients had small numbers of donor cells in their lymph nodes (Fig. 1B), skin (Fig. 2B), or both (Table 3). The smallest number of donor cells was in the female recipient of a paternal kidney. The number of donor cells in recipient tissues never exceeded 1 % and usually was estimated to be < 0.1%.
PCR
In the 4 cases in which blood was available from both the donor and recipient (LD17, 33, 51, 90), genomic DNA was extracted from the peripheral blood lymphocytes of the recipient. In addition, the blood of the respective donors, together with the biopsies of lymph nodes and skin of the recipients, were used for DNA extraction. The amplification with HLA-DRB “generic” primers was successful in all cases and allowed us to chose the primers for the allele-“specific” amplifications (Figs. 3 and and4).4). Tissue chimerism was demonstrated in all 4 cases, and in 2 there also was blood chimerism (Table 4).
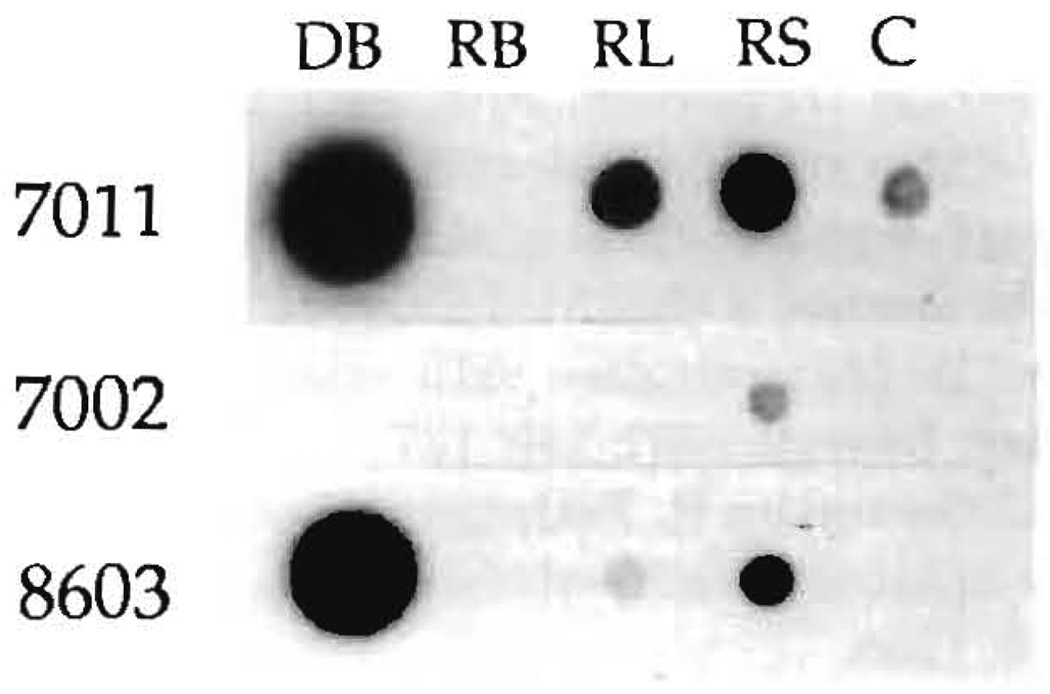
LD51: Chimerism demonstrated with PCR. After PCR amplification of HLA-DRB genes with “generic” oligo primers and hybridization of this material with allele-specific oligo probes, the recipient tissues were typed as DR4 (probe 1004) and DR7 (probe 1006). The donor blood was positive for probes 1004 (DR4), shared with the recipient, and 7011 (DR2). The amplified DNAs from donor blood (DB), recipient lymph nodes (RL), and recipient skin (RS) obtained using DR2 “specific” oligo primers were successfully hybridized with the allele-specific oligo probes 7011 and 8603, which recognize the DR2 allele of the donor. Probes 7002, 7003, and 8601 were negative. C is the negative control. In this experiment, the blood of the recipient (RB) was not positive.
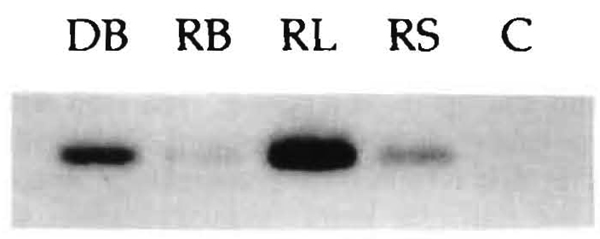
LD 90: PCR demonstration with Southern blot technique of chimerism in blood (RB), lymph node (RL), and skin (RS) of the recipient as detected with DR7 (donor) specific oligo probes after preliminary steps described in Figure 3. C is the negative control. DB is donor blood DNA diluted 100 times to avoid overwhelming the other bands.
TABLE 4
PCR
| Patient | Donor-specific allele | Donor-specific probe | Tissue distribution | ||
|---|---|---|---|---|---|
| Blood | L-node | Skin | |||
| 17 | DR7 | 0701 | − | + | − |
| 33 | DR4 | 0409 | + | + | − |
| 51 | DR2 | 1501 | − | + | + |
| 52 | Y chromosome | SRY | − | − | + |
| 90 | DR7 | 0701 | + | + | + |
In the woman whose paternal donor had died (LD 52), the detection of the Y chromosome in her skin showed the presence of donor (male) cells in the recipient (female) tissues. As in all the other cases, the allograft also was a chimeric structure.
Immunologic Studies
MLR
Lymphocytes from 2 of the 4 kidney recipients (33 and 51) whose donors are still alive failed to respond in vitro to irradiated donor cells while showing a normal proliferative response to third party unrelated control cells (Table 5). Lymphocytes from kidney recipients 17 and 90 showed normal and modest responses, respectively, to their mothers’ one haplotype mismatched donor cells and a good response to third party lymphocytes.
TABLE 5
MLR of recipient and donor lymphoid cells
| Stimulator cellsa,b | Responder cells | |||
|---|---|---|---|---|
| Recipient 17 (cpm) | Recipient 33 (cpm) | Recipient 51 (cpm) | Recipient 90 (cpm) | |
| Self | 845 | 193 | 163 | 968 |
| Donor (kidney) | 17,458 | 186 | 302 | 14,835 |
| Third party HLA-unrelated | 19,357 | 87,338 | 9,563 | 98,481 |
| Donor for 17 | Donor for 33 | Donor for 51 | Donor for 90 | |
| Self | 1,122 | 148 | 133 | 518 |
| Recipient | 8,168 | 2,789 | 138 | 26,244 |
| Third party HLA-unrelated | 15,041 | 57,062 | 5,760 | 122,347 |
When the MLR was done the other way around, using irradiated recipient cells to stimulate donor lymphocytes, the donor cells were totally nonreactive to the lymphocytes of recipient 51 or had reduced reactivity to the cells of recipients 17, 33, and 90, but reacted vigorously to third party control cells (Table 5). Both recipient and donor cells stimulated unrelated third party lymphocytes (Table 5, footnote).
CML
All 4 recipients generated significant cytotoxic activity (CML activity >15%) when cultured with third party control cells. However, these recipient lymphocytes failed to generate in vitro cytotoxic effector cells when stimulated by their donors (CML activity <10%), including recipient 90, whose cells had proliferated in the presence of donor cells (Table 5). This kind of CML nonresponsiveness has been documented during the first year after kidney transplantation under AZA and PRED (10), and was reported by Thomas et al. (11) to be a consistent finding in patients still bearing functional renal grafts up to 12 years after transplantation by Dr. David Hume of Richmond, VA.
DISCUSSION
Biopsies in all 5 of the long-surviving renal allograft recipients showed low level chimerism. This was identified by immunostaining of discrete cells of donor type in host lymph nodes and skin, and by confirmation in all 5 cases of donor DNA in the tissues with PCR techniques. In addition, 2 of the patients were blood chimeras by PCR. The subtlety of the chimerism in the skin and lymph nodes versus much more overt findings in liver transplant recipients (2,3) apparently reflected the relative paucity of the migratory leukocytes in the kidney compared to the liver. This could explain why tolerance of the renal allograft is harder to induce (1).
To increase the tolerogenicity of organs such as the kidney and heart that have a small migratory leukocyte constituency, the therapeutic possibility has been discussed elsewhere of augmenting cell migration to the level naturally enjoyed by the immunologically privileged liver (1). The most obvious expedient is the concomitant infusion of bone marrow from the organ donor, as has been advocated by Monaco et al. (12–15) and Thomas et al. (16, 17) for tolerance induction of kidneys and carried out clinically by Barber et al. (18), Van Twuyver et al. (19) have speculated that the tolerogenic effects of blood transfusion for conditioning of patients for kidney transplant recipiency could be due under certain conditions to the homing in of transfused lymphocytes to privileged sites, resulting in mixed allogeneic chimerism. All 5 of our kidney recipients had undergone splenectomy. However, the presence or absence of a spleen was not an important factor in the chimeric state of a series of long-surviving liver recipients, all of whom were chimeras (2, 3,20).
The critical migratory cells may be the dendritic cells (21) that previously were known as passenger leukocytes (22, 23). However, there are very few clues about how these cells of bone marrow orgin, whose life expectancy after maturation has been said to be days or weeks (24), have disseminated from the grafts and perpetuated themselves for nearly 30 years in their new environment. Inaba et al. (25) recently demonstrated stem cells in mouse blood that could be grown selectively in culture enriched with GM/CSF. However, it is unlikely that residual blood in allografts deliberately perfused to an asanguinous state could be a consistent source of stem cells that replicate in the recipients. The migratory cells, which generate progeny, could be hematopoetic stem cells that come in small numbers from the interstitium of the transplanted organ under conditions that are propitious in the perioperative period for migration, engraftment, and self-renewal. It was noteworthy that 2 of our 5 patients had circulating donor DNA demonstrated by PCR 27 and 29 years post-transplantation.
In the past, the clinical meaning of tolerance has been construed as permanent engraftment in the absence of continuing therapy. We have suggested that such drug-free classical tolerance and the “graft acceptance” achieved with chronic immunosuppression are both expressions of the cell repopulation (1). The achievement of a potentially drug-free state probably is not rare after kidney transplantation, particularly if well-matched family donors are used. Of the first 64 patients in the original University of Colorado series (26), 10, including the first 4 in the present study, still bear their original consanguineous transplants, which are among the 16 longest continuously functioning allografts in the world (27). Two of these 10 recipients who had HLA-compatible donors have perfect renal function after stopping all medications more than 26 years ago (28). In addition, Patient LD 52 of this report, who has a one haplotype mismatched graft, has been only on 7.5 mg/day PRED for more than 12 years.
The practical problem is how to determine when the need for treatment ends and the drug-free state begins. Strober et a1. (29) documented tolerance by discontinuance of treatment soon after transplantation in 3 cadaveric kidney recipients whose immunosuppression was with 20 Gy total lymphoid irradiation, a perioperative course of antithymocyte globulin, and maintenance PRED that was gradually weaned and eventually stopped. Donor-specific nonreactivity of lymphocytes from their drug-free patients was demonstrated with MLR and CML. In 4 of our 5 cases, the availability for blood sampling of the original kidney donors allowed these same in vitro immunologic tests to be done. The results showed absolute donor-specific nonresponsiveness with MLR in 2 cases, relative nonresponsiveness in the 2 others, and an inability in all 4 cases of the donor lymphocytes to stimulate a CML response. Unfortunately, donor-specific nonresponsiveness with either MLR or CML (or both) does not provide complete assurance of stable graft acceptance without immunosuppression in experimental animals (30) or humans (10, 11). Conversely, the demonstration of in vitro antigraft reactivity does not necessarily imply instability (31). This means that the late discontinuance of therapy still has an element of trial and error.
Footnotes
1This work was supported by Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MD.
*Abbreviations: CML, cell-mediated lymphocytotoxicity; ICC, immunocytochemistry; PCR, polymerase chain reaction.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1097/00007890-199306000-00012
Read article for free, from open access legal sources, via Unpaywall:
https://journals.lww.com/transplantjournal/Abstract/1993/06000/CHIMERISM_AND_DONOR_SPECIFIC_NONREACTIVITY_27_TO.12.aspx
Citations & impact
Impact metrics
Article citations
Efficacy of Engraftment and Safety of Human Umbilical Di-Chimeric Cell (HUDC) Therapy after Systemic Intraosseous Administration in an Experimental Model.
Biomedicines, 12(5):1064, 11 May 2024
Cited by: 1 article | PMID: 38791026 | PMCID: PMC11117770
Chimeric Cell Therapies as a Novel Approach for Duchenne Muscular Dystrophy (DMD) and Muscle Regeneration.
Biomolecules, 14(5):575, 13 May 2024
Cited by: 0 articles | PMID: 38785982 | PMCID: PMC11117592
Review Free full text in Europe PMC
Evidence for Microchimerism in Baboon Recipients of Pig Hearts.
Viruses, 15(7):1618, 24 Jul 2023
Cited by: 3 articles | PMID: 37515304 | PMCID: PMC10385208
Microchimerism, PERV and Xenotransplantation.
Viruses, 15(1):190, 10 Jan 2023
Cited by: 4 articles | PMID: 36680230 | PMCID: PMC9862020
Review Free full text in Europe PMC
Central tolerance promoted by cell chimerism.
Proc Natl Acad Sci U S A, 119(52):e2214989119, 19 Dec 2022
Cited by: 0 articles | PMID: 36534805 | PMCID: PMC9907097
Go to all (230) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Clinical transplantation tolerance twelve years after prospective withdrawal of immunosuppressive drugs: studies of chimerism and anti-donor reactivity.
Transplantation, 69(8):1549-1554, 01 Apr 2000
Cited by: 84 articles | PMID: 10836360
Induction of specific tolerance to class I-disparate renal allografts in miniature swine with cyclosporine.
Transplantation, 54(3):490-497, 01 Sep 1992
Cited by: 110 articles | PMID: 1412729
Status of microchimerism in recipients 15 years after living related kidney transplantation.
Transplantation, 62(1):126-128, 01 Jul 1996
Cited by: 27 articles | PMID: 8693527
Funding
Funders who supported this work.
NIDDK NIH HHS (2)
Grant ID: R01 DK029961-19
Grant ID: DK 29961





