Abstract
Free full text

The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells.
Abstract
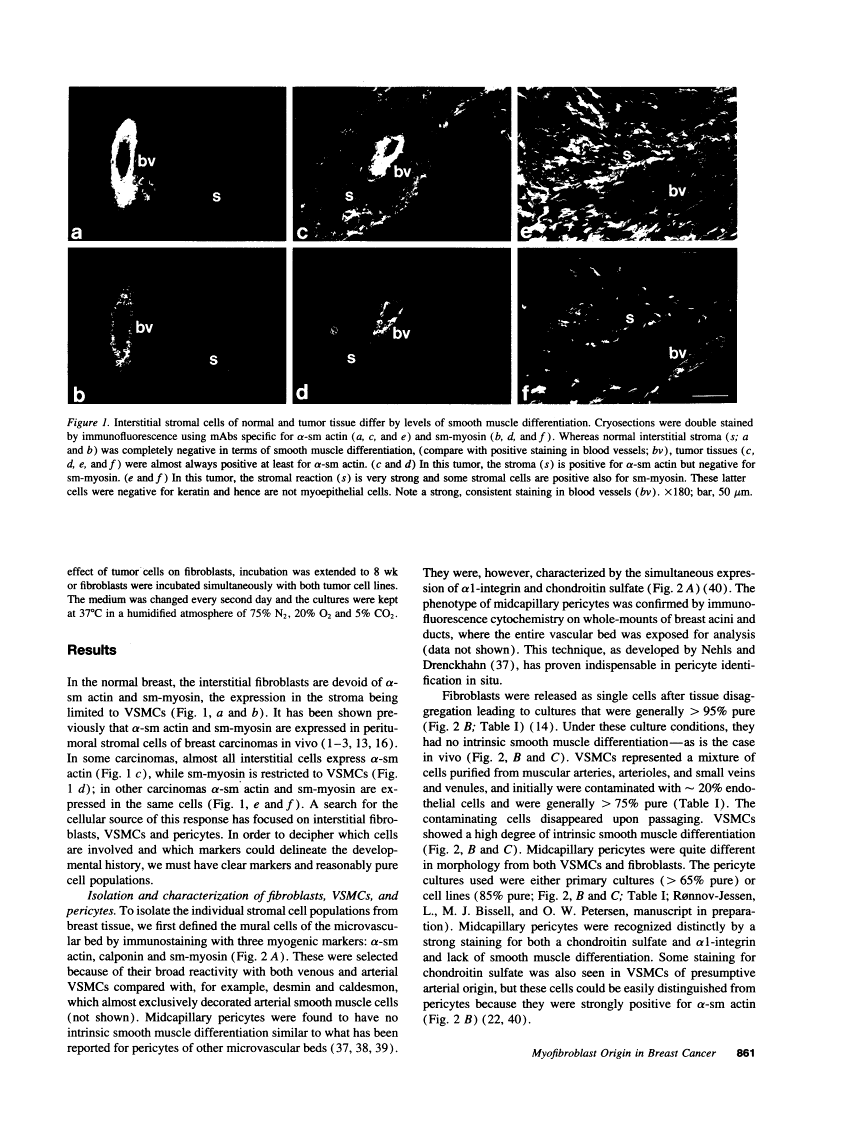
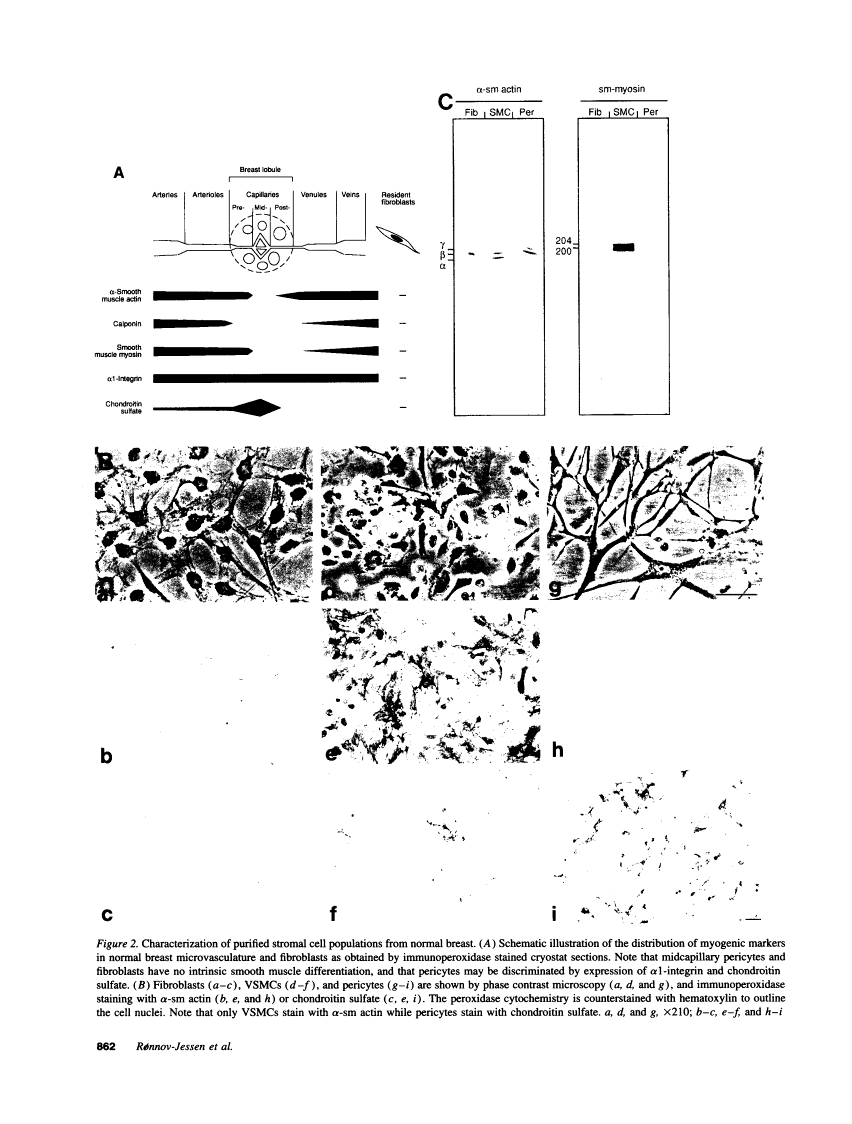
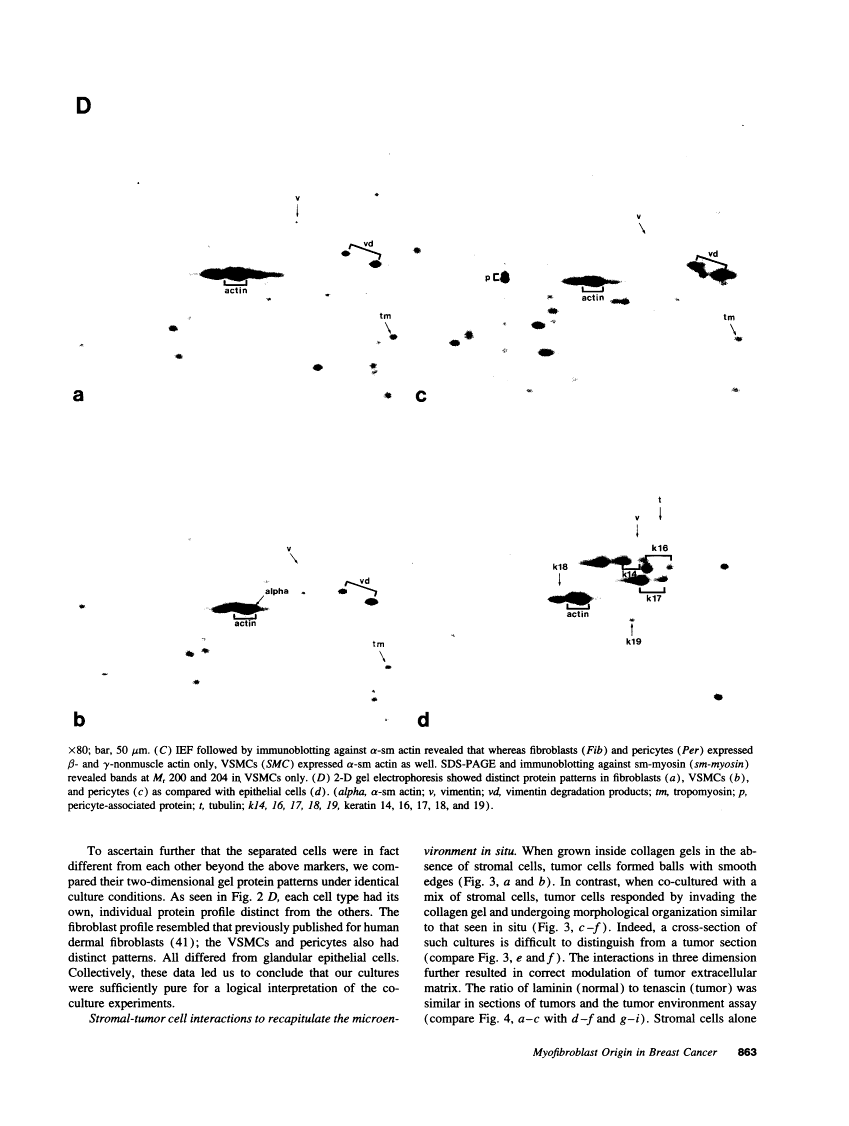
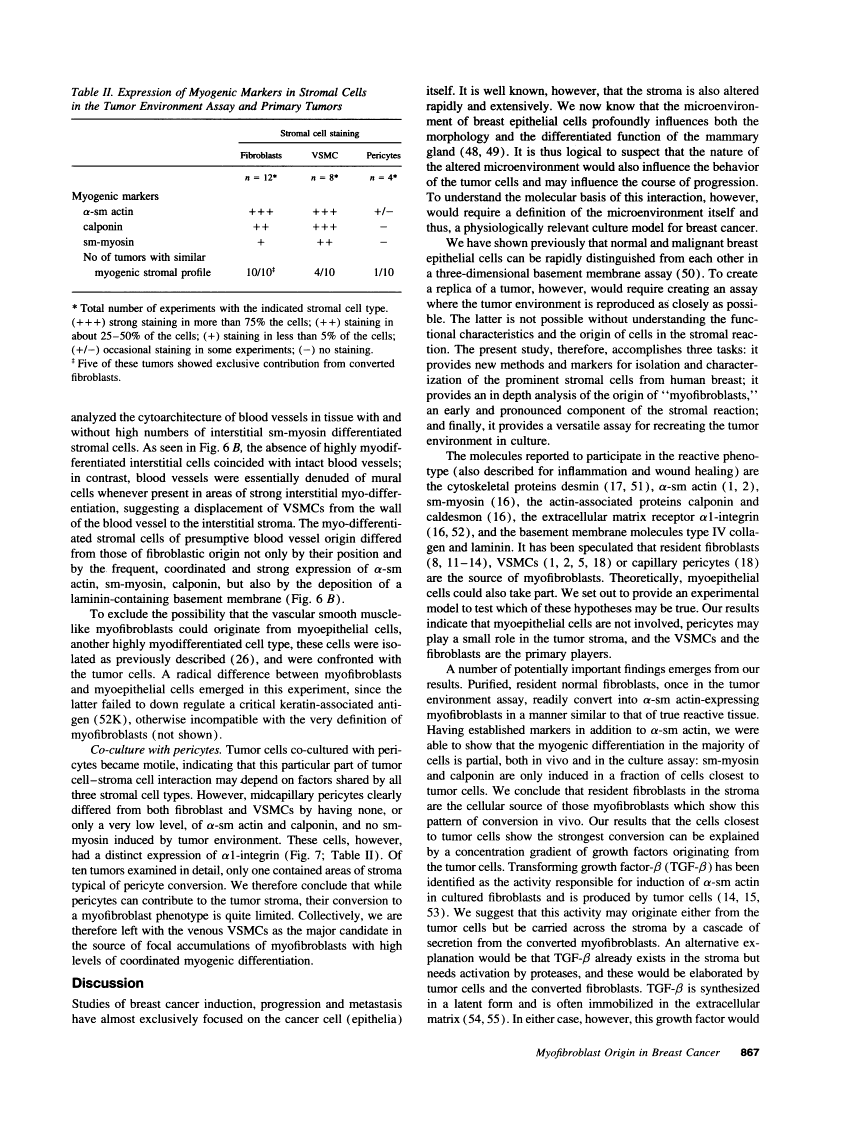
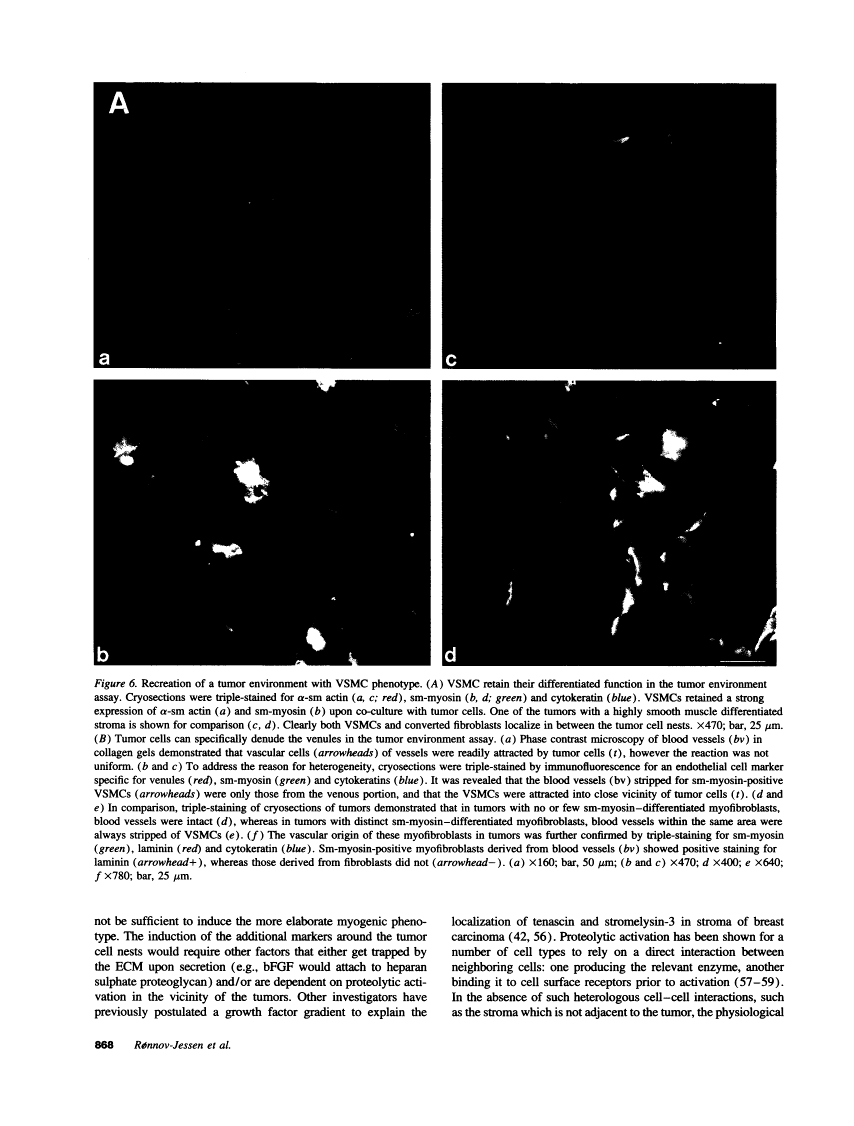
The origin of myofibroblasts in stromal reaction has been a subject of controversy. To address this question definitively, we developed techniques for purification and characterization of major stromal cell types. We defined a panel of markers that could, in combination, unequivocally distinguish these cell types by immunocytochemistry, iso-electric focusing, immunoblotting, and two-dimensional gel electrophoresis. We then devised an assay to recapitulate in culture, within two weeks of incubation, critical aspects of the microenvironment in vivo including the typical tissue histology and stromal reaction. When confronted with tumor cells in this assay, fibroblasts readily converted into a graded pattern of myogenic differentiation, strongest in the immediate vicinity of tumor cells. Vascular smooth muscle cells (VSMC), in contrast, did not change appreciably and remained coordinately smooth muscle differentiated. Midcapillary pericytes showed only a slight propensity for myogenic differentiation. Analysis of ten primary tumors implicated converted fibroblasts (10/10), vascular smooth muscle cells (4/10), and pericytes (1/10) in the stromal reaction. Tumor cells were shown to specifically denude the venules both in culture and in vivo, explaining the VSMC phenotype in the stroma. The establishment of this assay and clarification of the origin of these cells pave the way for further analysis of the mechanisms of conversion, and of the consequence of such heterogeneity for diagnosis and treatment.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (6.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. [Europe PMC free article] [Abstract] [Google Scholar]
- Tsukada T, McNutt MA, Ross R, Gown AM. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol. 1987 May;127(2):389–402. [Europe PMC free article] [Abstract] [Google Scholar]
- Sappino AP, Skalli O, Jackson B, Schürch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988 May 15;41(5):707–712. [Abstract] [Google Scholar]
- Eddy RJ, Petro JA, Tomasek JJ. Evidence for the nonmuscle nature of the "myofibroblast" of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol. 1988 Feb;130(2):252–260. [Europe PMC free article] [Abstract] [Google Scholar]
- Mitchell J, Woodcock-Mitchell J, Reynolds S, Low R, Leslie K, Adler K, Gabbiani G, Skalli O. Alpha-smooth muscle actin in parenchymal cells of bleomycin-injured rat lung. Lab Invest. 1989 May;60(5):643–650. [Abstract] [Google Scholar]
- Sappino AP, Dietrich PY, Skalli O, Widgren S, Gabbiani G. Colonic pericryptal fibroblasts. Differentiation pattern in embryogenesis and phenotypic modulation in epithelial proliferative lesions. Virchows Arch A Pathol Anat Histopathol. 1989;415(6):551–557. [Abstract] [Google Scholar]
- Czernobilsky B, Shezen E, Lifschitz-Mercer B, Fogel M, Luzon A, Jacob N, Skalli O, Gabbiani G. Alpha smooth muscle actin (alpha-SM actin) in normal human ovaries, in ovarian stromal hyperplasia and in ovarian neoplasms. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(1):55–61. [Abstract] [Google Scholar]
- Brouty-Boyé D, Raux H, Azzarone B, Tamboise A, Tamboise E, Béranger S, Magnien V, Pihan I, Zardi L, Israël L. Fetal myofibroblast-like cells isolated from post-radiation fibrosis in human breast cancer. Int J Cancer. 1991 Mar 12;47(5):697–702. [Abstract] [Google Scholar]
- Cintorino M, Bellizzi de Marco E, Leoncini P, Tripodi SA, Xu LJ, Sappino AP, Schmitt-Gräff A, Gabbiani G. Expression of alpha-smooth-muscle actin in stromal cells of the uterine cervix during epithelial neoplastic changes. Int J Cancer. 1991 Apr 1;47(6):843–846. [Abstract] [Google Scholar]
- Rønnov-Jessen L, van Deurs B, Celis JE, Petersen OW. Smooth muscle differentiation in cultured human breast gland stromal cells. Lab Invest. 1990 Oct;63(4):532–543. [Abstract] [Google Scholar]
- Rønnov-Jessen L, Celis JE, Van Deurs B, Petersen OW. A fibroblast-associated antigen: characterization in fibroblasts and immunoreactivity in smooth muscle differentiated stromal cells. J Histochem Cytochem. 1992 Apr;40(4):475–486. [Abstract] [Google Scholar]
- Rønnov-Jessen L, Van Deurs B, Nielsen M, Petersen OW. Identification, paracrine generation, and possible function of human breast carcinoma myofibroblasts in culture. In Vitro Cell Dev Biol. 1992 Apr;28A(4):273–283. [Abstract] [Google Scholar]
- Rønnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993 Jun;68(6):696–707. [Abstract] [Google Scholar]
- Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. [Europe PMC free article] [Abstract] [Google Scholar]
- Lazard D, Sastre X, Frid MG, Glukhova MA, Thiery JP, Koteliansky VE. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):999–1003. [Europe PMC free article] [Abstract] [Google Scholar]
- Skalli O, Schürch W, Seemayer T, Lagacé R, Montandon D, Pittet B, Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989 Feb;60(2):275–285. [Abstract] [Google Scholar]
- Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, Ravazzola M, Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989 Mar;37(3):315–321. [Abstract] [Google Scholar]
- Rockey DC, Friedman SL. Cytoskeleton of liver perisinusoidal cells (lipocytes) in normal and pathological conditions. Cell Motil Cytoskeleton. 1992;22(4):227–234. [Abstract] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. [Abstract] [Google Scholar]
- Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Res. 1986 Feb;46(2):467–473. [Abstract] [Google Scholar]
- Schlingemann RO, Rietveld FJ, Kwaspen F, van de Kerkhof PC, de Waal RM, Ruiter DJ. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol. 1991 Jun;138(6):1335–1347. [Europe PMC free article] [Abstract] [Google Scholar]
- Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. [Abstract] [Google Scholar]
- Betz E, Fallier-Becker P, Wolburg-Buchholz K, Fotev Z. Proliferation of smooth muscle cells in the inner and outer layers of the tunica media of arteries: an in vitro study. J Cell Physiol. 1991 Jun;147(3):385–395. [Abstract] [Google Scholar]
- Petersen OW, van Deurs B. Growth factor control of myoepithelial-cell differentiation in cultures of human mammary gland. Differentiation. 1988 Dec;39(3):197–215. [Abstract] [Google Scholar]
- Petersen OW, Hansen SH, Laursen I, van Deurs B. Effect of insulin on growth and expression of smooth muscle isoactin in human breast gland myoepithelial cells in a chemically defined culture system. Eur J Cell Biol. 1989 Dec;50(2):500–509. [Abstract] [Google Scholar]
- Frid MG, Shekhonin BV, Koteliansky VE, Glukhova MA. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev Biol. 1992 Oct;153(2):185–193. [Abstract] [Google Scholar]
- Pallesen G, Nielsen S, Celis JE. Characterization of a monoclonal antibody (BG3C8) that reacts with basal cells of stratified epithelia. Histopathology. 1987 Jun;11(6):591–601. [Abstract] [Google Scholar]
- Schlingemann RO, Dingjan GM, Emeis JJ, Blok J, Warnaar SO, Ruiter DJ. Monoclonal antibody PAL-E specific for endothelium. Lab Invest. 1985 Jan;52(1):71–76. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Celis JE, Gesser B, Rasmussen HH, Madsen P, Leffers H, Dejgaard K, Honore B, Olsen E, Ratz G, Lauridsen JB, et al. Comprehensive two-dimensional gel protein databases offer a global approach to the analysis of human cells: the transformed amnion cells (AMA) master database and its link to genome DNA sequence data. Electrophoresis. 1990 Dec;11(12):989–1071. [Abstract] [Google Scholar]
- Briand P, Lykkesfeldt AE. Long-term cultivation of a human breast cancer cell line, MCF-7, in a chemically defined medium. Effect of estradiol. Anticancer Res. 1986 Jan-Feb;6(1):85–90. [Abstract] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994 Feb;124(4):401–404. [Europe PMC free article] [Abstract] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991 Dec;115(5):1383–1395. [Europe PMC free article] [Abstract] [Google Scholar]
- Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991 Apr;113(1):147–154. [Europe PMC free article] [Abstract] [Google Scholar]
- Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992 Dec;270(3):469–474. [Abstract] [Google Scholar]
- Nehls V, Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry. 1993 Jan;99(1):1–12. [Abstract] [Google Scholar]
- Schlingemann RO, Rietveld FJ, de Waal RM, Ferrone S, Ruiter DJ. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990 Jun;136(6):1393–1405. [Europe PMC free article] [Abstract] [Google Scholar]
- Celis JE, Madsen P, Rasmussen HH, Leffers H, Honoré B, Gesser B, Dejgaard K, Olsen E, Magnusson N, Kiil J, et al. A comprehensive two-dimensional gel protein database of noncultured unfractionated normal human epidermal keratinocytes: towards an integrated approach to the study of cell proliferation, differentiation and skin diseases. Electrophoresis. 1991 Nov;12(11):802–872. [Abstract] [Google Scholar]
- Chiquet-Ehrismann R, Kalla P, Pearson CA. Participation of tenascin and transforming growth factor-beta in reciprocal epithelial-mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res. 1989 Aug 1;49(15):4322–4325. [Abstract] [Google Scholar]
- Schmitt-Gräff A, Pau H, Spahr R, Piper HM, Skalli O, Gabbiani G. Appearance of alpha-smooth muscle actin in human eye lens cells of anterior capsular cataract and in cultured bovine lens-forming cells. Differentiation. 1990 Apr;43(2):115–122. [Abstract] [Google Scholar]
- Desmoulière A, Rubbia-Brandt L, Abdiu A, Walz T, Macieira-Coelho A, Gabbiani G. Alpha-smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma-interferon. Exp Cell Res. 1992 Jul;201(1):64–73. [Abstract] [Google Scholar]
- Doane KJ, Birk DE. Fibroblasts retain their tissue phenotype when grown in three-dimensional collagen gels. Exp Cell Res. 1991 Aug;195(2):432–442. [Abstract] [Google Scholar]
- Buoro S, Ferrarese P, Chiavegato A, Roelofs M, Scatena M, Pauletto P, Passerini-Glazel G, Pagano F, Sartore S. Myofibroblast-derived smooth muscle cells during remodelling of rabbit urinary bladder wall induced by partial outflow obstruction. Lab Invest. 1993 Nov;69(5):589–602. [Abstract] [Google Scholar]
- Chiavegato A, Scatena M, Roelofs M, Ferrarese P, Pauletto P, Passerini-Glazel G, Pagano F, Sartore S. Cytoskeletal and cytocontractile protein composition of smooth muscle cells in developing and obstructed rabbit bladder. Exp Cell Res. 1993 Aug;207(2):310–320. [Abstract] [Google Scholar]
- Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993 Jun;7(9):737–743. [Abstract] [Google Scholar]
- Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9064–9068. [Europe PMC free article] [Abstract] [Google Scholar]
- Schürch W, Seemayer TA, Lagacé R. Stromal myofibroblasts in primary invasive and metastatic carcinomas. A combined immunological, light and electron microscopic study. Virchows Arch A Pathol Anat Histol. 1981;391(2):125–139. [Abstract] [Google Scholar]
- Duband JL, Belkin AM, Syfrig J, Thiery JP, Koteliansky VE. Expression of alpha 1 integrin, a laminin-collagen receptor, during myogenesis and neurogenesis in the avian embryo. Development. 1992 Nov;116(3):585–600. [Abstract] [Google Scholar]
- Arteaga CL, Coffey RJ, Jr, Dugger TC, McCutchen CM, Moses HL, Lyons RM. Growth stimulation of human breast cancer cells with anti-transforming growth factor beta antibodies: evidence for negative autocrine regulation by transforming growth factor beta. Cell Growth Differ. 1990 Aug;1(8):367–374. [Abstract] [Google Scholar]
- Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. [Europe PMC free article] [Abstract] [Google Scholar]
- Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992 Sep;202(1):1–8. [Abstract] [Google Scholar]
- Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. [Abstract] [Google Scholar]
- Pyke C, Kristensen P, Ralfkiaer E, Grøndahl-Hansen J, Eriksen J, Blasi F, Danø K. Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol. 1991 May;138(5):1059–1067. [Europe PMC free article] [Abstract] [Google Scholar]
- Blasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. Bioessays. 1993 Feb;15(2):105–111. [Abstract] [Google Scholar]
- Odekon LE, Blasi F, Rifkin DB. Requirement for receptor-bound urokinase in plasmin-dependent cellular conversion of latent TGF-beta to TGF-beta. J Cell Physiol. 1994 Mar;158(3):398–407. [Abstract] [Google Scholar]
- Benzonana G, Skalli O, Gabbiani G. Correlation between the distribution of smooth muscle or non muscle myosins and alpha-smooth muscle actin in normal and pathological soft tissues. Cell Motil Cytoskeleton. 1988;11(4):260–274. [Abstract] [Google Scholar]
- Barsky SH, Rao CN, Grotendorst GR, Liotta LA. Increased content of Type V Collagen in desmoplasia of human breast carcinoma. Am J Pathol. 1982 Sep;108(3):276–283. [Europe PMC free article] [Abstract] [Google Scholar]
- Krishnan R, Cleary EG. Elastin gene expression in elastotic human breast cancers and epithelial cell lines. Cancer Res. 1990 Apr 1;50(7):2164–2171. [Abstract] [Google Scholar]
- Bissell MJ. The differentiated state of normal and malignant cells or how to define a "normal" cell in culture. Int Rev Cytol. 1981;70:27–100. [Abstract] [Google Scholar]
- Blau HM, Baltimore D. Differentiation requires continuous regulation. J Cell Biol. 1991 Mar;112(5):781–783. [Europe PMC free article] [Abstract] [Google Scholar]
- Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. [Abstract] [Google Scholar]
- Watt FM. Cell culture models of differentiation. FASEB J. 1991 Mar 1;5(3):287–294. [Abstract] [Google Scholar]
- Horgan K, Jones DL, Mansel RE. Mitogenicity of human fibroblasts in vivo for human breast cancer cells. Br J Surg. 1987 Mar;74(3):227–229. [Abstract] [Google Scholar]
- Mukaida H, Hirabayashi N, Hirai T, Iwata T, Saeki S, Toge T. Significance of freshly cultured fibroblasts from different tissues in promoting cancer cell growth. Int J Cancer. 1991 May 30;48(3):423–427. [Abstract] [Google Scholar]
- Fabra A, Nakajima M, Bucana CD, Fidler IJ. Modulation of the invasive phenotype of human colon carcinoma cells by organ specific fibroblasts of nude mice. Differentiation. 1992 Dec;52(1):101–110. [Abstract] [Google Scholar]
- Dimanche-Boitrel MT, Vakaet L, Jr, Pujuguet P, Chauffert B, Martin MS, Hammann A, Van Roy F, Mareel M, Martin F. In vivo and in vitro invasiveness of a rat colon-cancer cell line maintaining E-cadherin expression: an enhancing role of tumor-associated myofibroblasts. Int J Cancer. 1994 Feb 15;56(4):512–521. [Abstract] [Google Scholar]
- Sieweke MH, Thompson NL, Sporn MB, Bissell MJ. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science. 1990 Jun 29;248(4963):1656–1660. [Abstract] [Google Scholar]
- Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest. 1993 Dec;92(6):2569–2576. [Europe PMC free article] [Abstract] [Google Scholar]
- Barsky SH, Gopalakrishna R. Increased invasion and spontaneous metastasis of BL6 melanoma with inhibition of the desmoplastic response in C57 BL/6 mice. Cancer Res. 1987 Mar 15;47(6):1663–1667. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci117736
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/117736/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1172/jci117736
Article citations
FGFR‑related phenotypic and functional profile of CAFs in prognostication of breast cancer (Review).
Int J Oncol, 65(4):94, 02 Sep 2024
Cited by: 0 articles | PMID: 39219285 | PMCID: PMC11374155
Review Free full text in Europe PMC
3D bioprinted breast cancer model reveals stroma-mediated modulation of extracellular matrix and radiosensitivity.
Bioact Mater, 42:316-327, 05 Sep 2024
Cited by: 0 articles | PMID: 39290339 | PMCID: PMC11405629
Exploring the multifaceted role of direct interaction between cancer cells and fibroblasts in cancer progression.
Front Mol Biosci, 11:1379971, 28 May 2024
Cited by: 2 articles | PMID: 38863965
Review
Evidence of steady-state fibroblast subtypes in the normal human breast as cells-of-origin for perturbed-state fibroblasts in breast cancer.
Breast Cancer Res, 26(1):11, 16 Jan 2024
Cited by: 1 article | PMID: 38229104 | PMCID: PMC10790388
The impact of tumor microenvironment: unraveling the role of physical cues in breast cancer progression.
Cancer Metastasis Rev, 43(2):823-844, 19 Jan 2024
Cited by: 1 article | PMID: 38238542
Review
Go to all (264) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tumor necrosis factor alpha and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor gamma: mechanism of desmoplastic reaction.
Cancer Res, 61(5):2250-2255, 01 Mar 2001
Cited by: 115 articles | PMID: 11280794
Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells.
Clin Cancer Res, 11(22):8006-8014, 01 Nov 2005
Cited by: 189 articles | PMID: 16299229
Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia.
Lab Invest, 68(6):696-707, 01 Jun 1993
Cited by: 342 articles | PMID: 8515656
Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues.
Mod Pathol, 18 Suppl 2:S61-79, 01 Feb 2005
Cited by: 248 articles | PMID: 15761467
Review