Abstract
Background
The GABRA2 gene has been implicated in addiction. Early life stress has been shown to alter GABRA2 expression in adult rodents. We hypothesized that childhood trauma, GABRA2 variation, and their interaction would influence addiction vulnerability.Methods
African-American men were recruited for this study: 577 patients with lifetime DSM-IV single and comorbid diagnoses of alcohol, cocaine, and heroin dependence, and 255 control subjects. The Childhood Trauma Questionnaire (CTQ) was administered. Ten GABRA2 haplotype-tagging single-nucleotide polymorphisms (SNPs) were genotyped.Results
We found that exposure to childhood trauma predicted substance dependence (p < .0001). Polysubstance dependence was associated with the highest CTQ scores (p < .0001). The African Americans had four common haplotypes (frequency: .11-.30) within the distal haplotype block: two that correspond to the Caucasian and Asian yin-yang haplotypes, and two not found in other ethnic groups. One of the unique haplotypes predicted heroin addiction, whereas the other haplotype was more common in control subjects and seemed to confer resilience to addiction after exposure to severe childhood trauma. The yin-yang haplotypes had no effects. Moreover, the intron 2 SNP rs11503014, not located in any haplotype block and potentially implicated in exon splicing, was independently associated with addiction, specifically heroin addiction (p < .005). Childhood trauma interacted with rs11503014 variation to influence addiction vulnerability, particularly to cocaine (p < .005).Conclusions
Our results suggest that at least in African-American men, childhood trauma, GABRA2 variation, and their interaction play a role in risk-resilience for substance dependence.Free full text

The Influence of GABRA2, Childhood Trauma and their Interaction on Alcohol, Heroin and Cocaine Dependence
Abstract
Background
The GABRA2 gene has been implicated in addiction. Early life stress has been shown to alter GABRA2 expression in adult rodents. We hypothesized that childhood trauma, GABRA2 variation and their interaction would influence addiction vulnerability.
Methods
African American men were recruited for this study: 577 patients with lifetime DSM-IV single and comorbid diagnoses of alcohol, cocaine and heroin dependence, and 255 controls. The Childhood Trauma Questionnaire (CTQ) was administered. Ten GABRA2 haplotype-tagging SNPs were genotyped.
Results
We found that exposure to childhood trauma predicted substance dependence (p < 0.0001). Polysubstance dependence was associated with the highest CTQ scores (p < 0.0001). The African Americans had four common haplotypes (frequency: 0.11 – 0.30) within the distal haplotype block: two that correspond to the Caucasian and Asian yin-yang haplotypes and two not found in other ethnic groups. One of the unique haplotypes predicted heroin addiction whereas the other haplotype was more common in controls and appeared to confer resilience to addiction after exposure to severe childhood trauma. The yin-yang haplotypes had no effects. Moreover, the intron 2 SNP rs11503014, not located in any haplotype block and potentially implicated in exon splicing, was independently associated with addiction, specifically heroin addiction (p < 0.005). Childhood trauma interacted with rs11503014 variation to influence addiction vulnerability, particularly to cocaine (p < 0.005).
Conclusions
Our results suggest that at least in African American men, childhood trauma, GABRA2 variation and their interaction play a role in risk-resilience for substance dependence.
INTRODUCTION
Alcoholism and drug dependence are common, chronic disorders with considerable personal and societal costs (1). The 12 month prevalence for substance use disorders (abuse plus dependence) in the United States is: alcohol 8.5%, cannabis 1.5%, opioids 0.4% and cocaine 0.3% (2,3). Alcoholism comorbidity in drug dependent individuals is high: 90% for cocaine, 74% for opioids and 68% for cannabis whereas only 13% of current alcoholics have a current drug use disorder (3). It has been shown that one common genetic factor has a strong influence on the risk for dependence on opiates, cocaine, cannabis and other illicit drugs and that most of the genetic and shared environmental risk factors are non-specific (4,5). Therefore there may be substantial shared vulnerability to addiction among substance dependent individuals although there is also evidence for substance-specific transmission factors (6).
A meta-analysis of studies in thousands of twin pairs has shown that the heritability of alcoholism is around 50%, and the heritability of cocaine and opiate addiction is around 60 – 70% (7). Therefore genetic and environmental influences on the development of addictive disorders are equally important. Preclinical studies indicate that GABRA2, the gene that encodes the GABAA α2 receptor subunit, may play a role in drug dependence (8–11). In human studies, the GABRA2 distal haplotype block has been robustly associated with alcoholism, at least in Caucasians (12–21). The first findings came from the Collaborative Study on the Genetics of Alcoholism (COGA) (12). A subsequent re-analysis of the dataset showed that the association signal derived from alcoholics with comorbid illicit drug dependence (13). However, another study found that the association was strongest in alcoholics without drug dependence (19). The two published studies in African Americans were negative (22,23). HapMap data shows that, unlike Caucasian and Asian populations who have only two common GABRA2 yin yang haplotypes, African populations have two unique haplotypes in addition to the yin yang haplotypes (24).
Early life stress has been shown to influences the expression of GABRA2 in rats by permanently altering GABAA α2 subunit distribution in the hippocampus (25). Moreover, early life stress has been shown to affect ethanol consumption in adult rhesus monkeys and alcohol, cocaine and morphine consumption in rodents (26–28). Numerous studies in humans have provided support for a relationship between childhood trauma and the development of alcohol and drug dependence (29–36). For example, Widom et al (37) showed in a prospective cohort community study that middle aged men and women who had been abused in childhood were at greater risk not only for illicit drug use but also polysubstance abuse. Taken together, it seems reasonable to hypothesize that early life stress might interact with GABRA2 variation to predict alcohol and drug dependence in humans.
Our study was therefore designed to test our hypothesis that GABRA2, childhood trauma and their interaction would contribute to vulnerability to substance dependence. To this end, the study sample included African American men recruited from a Veterans’ Affairs substance abuse treatment program, the majority of whom had comorbid alcohol, heroin and cocaine dependency, and African American male controls. The sample had been exposed to considerable childhood trauma. In investigating GABRA2 variation we focused both on the distal GABRA2 haplotype block that has previously been associated with substance dependence (12–21)and on a novel intron 2 SNP, rs11503014, that is not located in any haplotype block and is potentially implicated in exon splicing.
METHODS AND MATERIALS
Participants
Originally, 635 African-American substance dependent men were recruited: 590 from the Substance Abuse Treatment Program (SATP) at the Department of Veteran Affairs New Jersey Healthcare System (VANJHCS), East Orange Campus and 45 men originally screened as controls (see below) who were found to have lifetime substance dependence. Most of the participants recruited from the SATP were inpatients on a 21 day residential treatment ward, however some were recruited from the outpatient clinic or from the methadone clinic. Criteria for inclusion in the study were that participants were ≥ 18 years of age, met DSM-IV criteria for substance dependence, self-identified as African American and had been abstinent for at least two weeks. Exclusion criteria included mental retardation, dementia and acute psychosis. Patients were interviewed by a psychiatrist (A.R.) with the substance abuse section of the Structured Clinical Interview for DSM-IV (SCID) (38) to determine lifetime substance dependence diagnoses. The mean (SD) age of the patients was 45.6 (7.8) years.
Three hundred and twenty African American male controls were recruited from churches and a blood bank in Newark, NJ, (46%) and from among insulin-dependent diabetic outpatients seen at an ophthalmology clinic (54%) at the University of Medicine and Dentistry: New Jersey Medical School (UMDNJ, Newark, NJ). All controls had a semi-structured psychiatric interview and were without a lifetime history of any substance abuse or dependence or major Axis 1 psychiatric disorder. Their mean (SD) age was 34.0 (10.1) years.
After a full description of the study was provided, all participants gave written informed consent to the study that was approved by the Institutional Review Boards of the VANJHCS and UMDNJ.
Childhood Trauma Questionnaire (CTQ)
The CTQ (28 item version) (39,40) was completed by 495 patients with substance dependence and 145 controls. The CTQ yields scores for five traumas experienced in childhood: physical abuse, physical neglect, emotional abuse, emotional neglect and sexual abuse, as well as a total score. Reliability and validity of the CTQ has been demonstrated, including in drug abusers and African American populations (40–42). The total CTQ score ranges from 25 to 125. The CTQ was used as a continuous measure in all logistic regression analyses.
A dichotomous total CTQ score was derived for use in secondary analyses. A total CTQ score greater than or equal to one standard deviation above the mean CTQ score of controls (36.5 (10.3), i.e. ≥ 47) was designated ‘high adversity’ (N = 243); lower CTQ scores were designated ‘low’ adversity (N = 402).
Genotyping
A genomic region containing sequence 5 kb upstream and 1 kb downstream of GABRA2 was retrieved from NCBI Human Build 35.1. Haplotype tagging SNPs were identified using a previously described design pipeline (43). Ten GABRA2 SNPs were genotyped using the Illumina GoldenGate platform (43). Rs numbers for the 10 SNPs, the bases for alleles 1 – 2, together with the allele 2 frequencies, are shown in Figure 1. For each SNP, alleles 1 and 2 are located on opposite DNA strands.
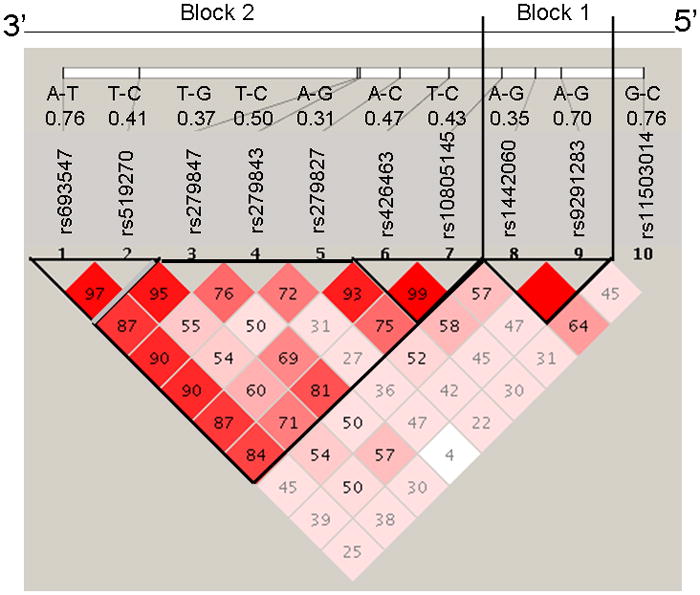
The rs numbers of the 10 SNPs and the bases for alleles 1 – 2 are given, together with the allele 2 frequencies. For each SNP, alleles 1 and 2 are located on opposite DNA strands. The numbers in the squares refer to linkage disequilibrium (LD) measured as D′ between each pair of SNPs. Haplotype blocks were defined using a setting of average pairwise D′ within-block of ≥ 0.80.
Final Dataset Summary
The dataset is summarized in Figure 2. Missing DNA and CTQ data was random and showed no selection bias.
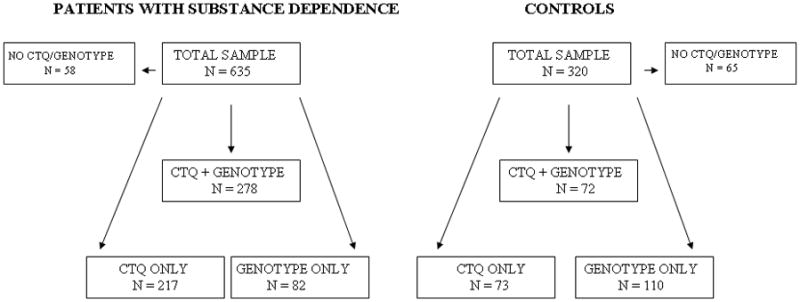
Description of dataset: patients with substance dependence (alcohol, cocaine, heroin) and controls (no addiction). CTQ: childhood trauma questionnaire. Genotype data: 542 men; 360 patients and 182 controls. CTQ data: 640 men; 495 patients, 145 controls. Genotype + CTQ data: 350 men; 278 patients, 72 controls.
Assessment of Population Stratification Using Ancestry Informative Markers
The samples were genotyped for 186 ancestry markers (AIMS) (43). The same AIMs were genotyped in 1051 individuals from the 51 worldwide populations represented in the HGDP-CEPH Human Genome Diversity Cell Line Panel (http://www.cephb.fr/HGDP-CEPH-Panel). Structure 2.2 (http://pritch.bsd.uchicago.edu/software.html) was run simultaneously using the AIMS genotypes from our sample and the 51 CEPH populations to identify population substructure and compute individual ethnic factor scores. This ancestry assessment identifies seven ethnic factors (43). In our study sample the predominant mean (median) ethnic factor scores were: African: 0.77 (0.81); European: 0.09 (0.04); Mid East and Asian: 0.06 (0.04).
Statistical Analyses
Logistic regression analyses were undertaken using JMP 7 software. Backward stepwise regression was performed with variables being eliminated from the model in an iterative process. CTQ scores and ethnic factor scores were therefore included as covariates in the final model if they had significant effects (the European factor had a significant effect on heroin addiction in most analyses). The interaction term was included in the final model when significant. Logistic regression models with nominal variables yielded likelihood ratio χ2 results. The Fit Least Squares method was performed to determine differences in CTQ scores between patients with substance dependence and controls.
There were significant differences between the mean (SD) age of the patients (45.6 (7.8)) and the controls (34.0 (10.1)), F = 211, p< 0.0001. Nevertheless, there was no correlation between age and CTQ score in the patients (r = 0.07, p = 0.211) or the controls (r = 0.09, p = 0.276). Inclusion of age as a covariate had no effect on outcomes.
Haplotype frequencies were estimated using a Bayesian approach implemented with PHASE (44). Haploview version 2.04 Software (Whitehead Institute for Biomedical Research, USA) was used to produce LD matrices.
In the logistic regression analyses for the effects of CTQ scores and (a) haplotypes and (b) rs11503014 on substance dependence, the only tests that were independent were the tests where the outcomes were single diagnoses of alcohol, cocaine and heroin dependence, resulting in a Bonferroni corrected significant p value of p < 0.008 for the whole model tests. A more stringent correction for all 14, non-independent logistic regression analyses would result in a Bonferroni corrected significant p value of p < 0.004 for each of the logistic regression whole model tests.
RESULTS
Effects of Childhood Trauma on Addiction
The total group of patients with substance dependence had a significantly higher mean total CTQ score than the controls: 48.7 (16.8) vs 36.5 (10.3); F(1,643) = 72, p < 0.0001. Further analysis showed that CTQ scores (mean (SD)) were higher in men with at least two addictions: no addiction (i.e. controls): 36.5 (10.3); one addiction: 46.1 (16.5); two addictions: 50.9 (16.8); three addictions: 49.2 (16.8); F(3,641) = 27, p < 0.0001. For further details see Supplementary Table 1.
GABRA2 Haplotype Block Structure
The GABRA2 haplotype block structure is shown in Figure 1. Although the African American haplotype block structure is not as well defined as in Caucasians, African Americans nevertheless have the same two haplotype blocks with a region of recombination in intron 3. Logistic regression analyses were performed with the distal block (block 2) haplotypes, since this is the GABRA2 region that has previously been associated with alcohol and drug dependence (12–21).
Block 2 Haplotype Analyses
There were 12 haplotypes with ≥ 0.01 frequency that accounted for 0.95 of the haplotype diversity. Only four haplotypes had a frequency ≥ 0.05 and these accounted for 0.79 of the haplotype diversity in the total sample: (a) 2222111 (0.30); (b) 1111222 (0.23); (c) 2112122 (0.15); and (d) 2111111 (0.11). The yin-yang haplotypes 2222111 and 1111222 correspond to the two haplotypes found in Caucasians and Asians (24). The 2112122 and 2111111 haplotypes are unique to individuals of African descent. Haplotype frequencies for each substance dependence group are given in Table 1. There were no differences in CTQ scores between the haplotypes (p = 0.34).
TABLE 1
GABRA2 block 2 haplotype frequencies in heroin, cocaine and alcohol dependent patients and controls
| Subjects | N | GABRA2 Block 2 Haplotype Frequencies | |||
|---|---|---|---|---|---|
| 2222111 | 1111222 | 2112122 | 2111111 | ||
| Controls | 308 | 0.34 | 0.28 | 0.19 | 0.19 |
| Total group of patients | 563 | 0.38 | 0.32 | 0.20 | 0.11 |
| All patients with heroin dependence | 250 | 0.36 | 0.31 | 0.23 | 0.10 |
| Patients with only heroin dependence | 66 | 0.41 | 0.26 | 0.26 | 0.07 |
| All patients with alcohol dependence | 357 | 0.38 | 0.32 | 0.19 | 0.11 |
| Patients with only alcohol dependence | 99 | 0.41 | 0.33 | 0.16 | 0.10 |
| All patients with cocaine dependence | 341 | 0.38 | 0.31 | 0.19 | 0.11 |
| Patients with only cocaine dependence | 88 | 0.40 | 0.33 | 0.18 | 0.09 |
Since these are haplotype analyses the N’s are the number of chromosomes (2 per individual). The four haplotypes for which frequencies are given here account for 0.79 of block 2 haplotypes in the total sample.
Logistic regression analyses were performed to determine the effects of all four GABRA2 haplotypes and childhood trauma (total CTQ score) on alcohol, cocaine and heroin dependence. The results are presented in Table 2. Childhood trauma had a significant effect on patients with alcohol dependence and cocaine dependence but there was no gene effect. There was a significant effect of childhood trauma and the 2111111 haplotype in the total group of patients with substance dependence. Childhood trauma had no effect in the total group of individuals with heroin dependence however haplotypes 2111111 and 2112122 both had significant effects. The direction of the haplotypic effects can be discerned from Table 1: haplotype 2112122 was more abundant in individuals with heroin dependence whereas haplotype 2111111 was more common in controls compared with individuals with alcohol, heroin or cocaine dependence. The full model contributed to 10% of the variance in any addiction and up to 13% of the variance for heroin addiction alone: in both of these models, genetic and environmental effects had a significant impact.
TABLE 2
The influence of GABRA2 block 2 haplotypes and childhood trauma on heroin, alcohol and cocaine dependence
| Patient groups | N’s for group comparisons | European Effect | Haplotype 21111111 | Haplotype 2112122 | Childhood Trauma | Whole | Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | P value | χ2 | P value | χ2 | P value | χ2 | P value | P value | Df | Var | ||
| All patients with heroin dependence | 250/612b | 13.0 | 0.0003 | 4.2 | 0.042 | 6.5 | 0.011 | 0.0004 | 4 | 0.02 | ||
| Patients with only heroin dependence | 43/120a | 5.8 | 0.016 | 3.7 | 0.054 | 4.8 | 0.028 | 7.9 | 0.005 | 0.0001 | 5 | 0.13 |
| All patients with alcohol dependence | 580/700c | 47.3* | <0.0001 | < 0.0001 | 1 | 0.04 | ||||||
| Patients with only alcohol dependence | 148/294a | 52.1* | <0.0001 | < 0.0001 | 1 | 0.10 | ||||||
| All patients with cocaine dependence | 660/620d | 115* | <0.0001 | < 0.0001 | 1 | 0.08 | ||||||
| Patients with only cocaine dependence | 77/120a | 3.3 | 0.072 | 17.8 | <0.0001 | 0.0002 | 4 | 0.09 | ||||
| Total group of patients | 433/120a | 4.3 | 0.037 | 53.1 | <0.0001 | < 0.0001 | 4 | 0.10 | ||||
The Table summarizes the results of 7 logistic regression models.
Results are for effect likelihood ratio (L-R) tests and are given for p < 0.1.
The Ns for some analyses are higher because genotypes or CTQ scores were not included in some models as detailed below.
Since these are haplotype analyses the N’s are the number of chromosomes (2 per individual) in patients/comparison group as follows:
The European factor effect was included when significant to correct for population stratification.
Childhood trauma effect is the effect of the continuous measure: total CTQ score.
The 4 haplotypes included in the model represent 79% of the total haplotype diversity.
There were no significant effects for the other two haplotypes. There were no significant gene-environment interactions.
The two yin yang haplotypes were not associated with substance dependence. There were no significant interaction effects in the logistic regression analyses. The seven logistic regression whole model tests were significant when corrected for multiple testing (p < 0.004).
Secondary Analyses in the Total Group of Patients
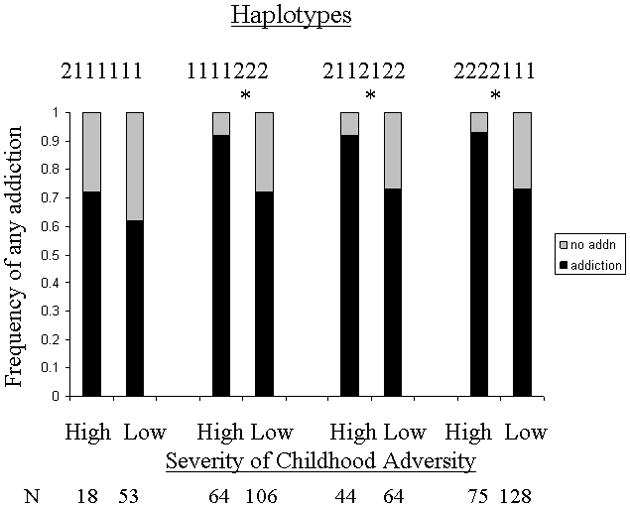
Within the logistic regression analysis for the total group of patients and controls, the comparison of haplotype 2111111 with the other three haplotypes showed a trend interactive effect with CTQ scores (p = 0.087). Therefore we asked whether the protective effect of haplotype 2111111 might differ with exposure to childhood adversity. In secondary analyses we used the dichotomous CTQ high/low variable (high childhood adversity designated as CTQ total score ≥ 1 S.D. above mean score of controls; low adversity being < 1 S.D. above mean CTQ score of controls). Figure 3 shows that carriers of the other three haplotypes who had been exposed to high childhood adversity were predictably more likely to have developed substance dependence than those exposed to low childhood adversity (χ2 = 6.7 – 11, p = 0.009 – 0.0009, 1df). In carriers of the 2111111 haplotype, high childhood adversity was associated with a numerically higher but non-significant (p = 0.44) frequency of addiction indicating that this haplotype may confer resilience to childhood adversity.
Incidental Analyses
For the sake of completeness, the following analyses were undertaken.
Block 2 SNP Analyses
None of the seven block 2 SNPs were associated with alcohol, cocaine or heroin dependence.
GABRA2 SNP rs11503014 Association with Addiction
Main Effects: Gene and Stressor
The intron 2 SNP, rs11503014, is not located within a haplotype block (Figure 1). The homozygote 11 frequency (0.33) and the heterozygote 12 frequency (0.36) in heroin addicted men were very similar and together differed significantly from the homozygote 22 frequency (0.23) (p = 0.003). The mean total CTQ score for the homozygote 11 and heterozygote 12 individuals were very similar (46.1, SD = 15.2 and 46.9, SD = 18.5 respectively) and together differed from that of the homozygous 22 individuals (43.6, SD = 15.5), p = 0.039. Thus these results provided the justification for combining the 11 and 12 genotypes in order to increase statistical power in the smaller data subsets.
Table 3 shows that there was a significant effect of rs11503014 in the total group of patients and there was a specific effect on heroin addiction. The effects of childhood trauma were greater on alcohol and cocaine dependence than on heroin dependence. The seven logistic regression whole model tests were significant when corrected for multiple testing (p < 0.004).
TABLE 3
The influence of GABRA2 rs11503014 and childhood trauma on heroin, alcohol and cocaine dependence
| Patient Groups | N’s Patients/Comparison Group | European Effect | Gene Effect | Childhood Trauma Effect | G × E Interaction | Whole | Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | P | χ2 | P | χ2 | P | χ2 | P | P value | Df | Var | ||
| All patients with heroin dependence | 157/385b | 6.5 | 0.011 | 8.7 | 0.003 | - | - | 0.0005 | 2 | 0.02 | ||
| Patients with only heroin dependence | 27/72a | 4.8 | 0.029 | 3.2 | 0.073 | 5.8 | 0.016 | - | 0.0012 | 3 | 0.14 | |
| All patients with alcohol dependence | 290/350c | - | - | 23.6* | < 0.0001 | - | < 0.0001 | 1 | 0.04 | |||
| Patients with only alcohol dependence | 74/145a | - | - | 26.0* | < 0.0001 | - | < 0.0001 | 1 | 0.11 | |||
| All patients with cocaine dependence | 184/166d | - | - | 23.7 | < 0.0001 | 4.3 | 0.039 | < 0.0001 | 3 | 0.06 | ||
| Patients with only cocaine dependence | 47/72a | - | - | 16.0 | < 0.0001 | 8.8 | 0.003 | 0.0002 | 3 | 0.13 | ||
| Total group of patients | 278/72a | - | 5.4 | 0.020 | 32.7 | < 0.0001 | 3.1 | 0.076 | < 0.0001 | 3 | 0.10 | |
The table summarizes the results of seven logistic regression models.
χ2 results are for effect likelihood ratio (L-R) tests and are given for p values < 0.1.
The Ns for some analyses are higher because genotypes or CTQ scores were not included in some models as detailed below.
N’s are the numbers of patients/comparison group as follows:
The European factor effect was included when significant to correct for population stratification.
Childhood trauma effect is the effect of the continuous measure: total CTQ score.
Gene × Environment Interaction
There was a significant gene × total CTQ score interaction effect on cocaine addiction for the group of patients with cocaine dependence only (p = 0.003) and for all patients with cocaine dependence (p = 0.039). There was also a trend effect in the total group of patients (p = 0.076) (Table 3).
Secondary Analyses
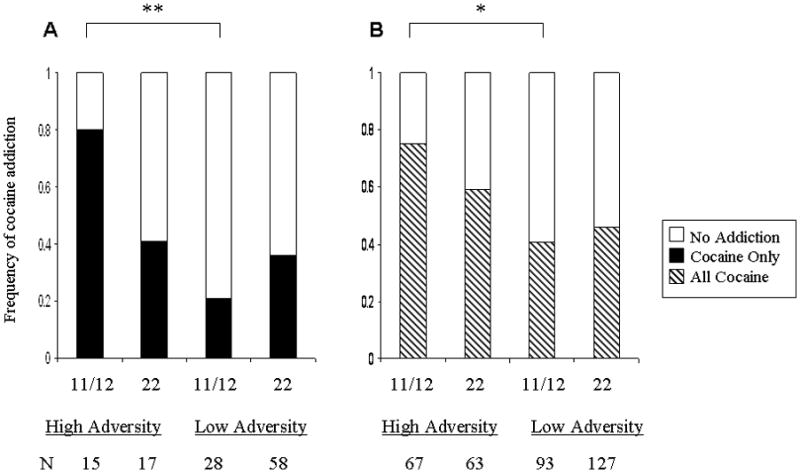
In order to illustrate the direction of the significant interaction for cocaine dependence we used the previously described CTQ high/low childhood adversity variable. Figure 4 Panel B shows that high childhood adversity was associated with increased cocaine dependence in both genotype groups, however, individuals with the rs11503014 11/12 genotype tended to have the greater risk compared with individuals with the 22 genotype (χ2 = 3.7, 1df, p = 0.054). The impact of high childhood adversity on cocaine addiction alone was only apparent in individuals with the 11/12 genotype (χ2 = 5.0, 1df, p = 0.026) (Figure 4, Panel A). The trend effect in the total group of patients was in the same direction as for the patients with cocaine dependence.
Gene-Environment Correlation
CTQ scores did not differ significantly (p = 0.072) between rs11503014 11/12 and 22 genotypes when ‘any addiction’ was included as a between factor variable. Thus there was no evidence of a gene-environment correlation.
DISCUSSION
In the present study we confirmed our hypothesis that GABRA2, childhood trauma and their interaction influence vulnerability to substance dependence, at least in African American men. Firstly, we found that the patients with heroin, alcohol and cocaine dependence had experienced significantly more childhood trauma than the controls. Furthermore, our results showed that the greater the severity of childhood trauma the greater the likelihood of polysubstance dependence. The latter finding is supported by previous studies (37). Secondly, our results showed that GABRA2 variation predicted addiction vulnerability, particularly for heroin dependence. Thirdly, an interaction between childhood trauma and GABRA2 variation was found to influence addiction risk, particularly for cocaine dependence.
Earlier studies, together with HapMap, have identified the same two GABRA2 haplotype blocks within Caucasians, Asians, Native Americans and African Americans. The previously reported significant association signals with alcoholism have been within the haplotype block that extends downstream from intron 3 (called block 2 in our study). In Caucasians, Asians and Native Americans there are two major yin-yang haplotypes within this block that account for nearly all of the haplotype diversity (24). Individuals of African origin also have these two yin-yang haplotypes however in addition they have two common haplotypes that are not present in other populations. In the present study these two unique haplotypes were associated with addiction vulnerability: one haplotype was associated with heroin dependence and the other haplotype was more common in controls and may be protective against addiction. In contrast, we found no association between the yin-yang haplotypes and alcohol dependence or drug dependence unlike the many earlier studies in Caucasians (12–21). The two previously reported studies in African Americans showed no association between the yin-yang haplotypes and alcohol dependence (22) or polysubstance abuse (23). Moreover, a recent, dense genomewide linkage scan for alcohol dependence in African Americans did not find a linkage peak at the GABAA receptor gene complex on chromosome 4 (45), unlike earlier studies in Caucasians (46) and Native Americans (47).
In this study we also found that an intron 2 SNP, rs11503014 that is not in LD with any SNPs in haplotype blocks 1 or 2, was associated with heroin dependence. Rs11503014 is not located within a haplotype block in the three HapMap populations or within Finnish Caucasian and Plains Indian samples for which we have genotyped the same SNPs as in the present study (data not shown). Rs11503014 is located nearby to an alternatively spliced exon 2 and within an exon (5′ UTR) of the alternative GABRA2 transcript NM_001114175. The DNA sequence within which rs11503014 is located is similar to the exonic splicing enhancers: srp55, srp40, sf2 and sc35. Thus it is theoretically possible that rs11503014 may be implicated in exon splicing. Our results therefore suggest that GABRA2 may have at least two independent loci that are implicated in the vulnerability to heroin dependence.
It has been shown that there is a common genetic factor for addiction to illicit drugs, including cocaine and opiates (4,5). However alcohol and drug dependence also have substantial disorder-specific genetic loading (6). In the present study we detected both a general and specific effect for GABRA2: one of the uniquely African haplotypes appeared to be protective against any addiction, however it was specifically protective for heroin dependence. In contrast, the other uniquely African haplotype predicted heroin dependence only. Likewise, rs11503014 variation was predictive for any addiction and specifically for heroin addiction.
Opiates and cocaine have different mechanisms of action in the CNS and may interact differently with GABRA2 variation and early life stress to influence the vulnerability to heroin or cocaine dependence. Our finding of a GABRA2-heroin dependence association is backed up by preclinical studies that indicate that GABAA receptors may influence the actions of opiates; for example, hyperpolarization of GABAergic neurons in the ventral tegmental area (where GABAA α2 receptors are highly expressed) by opiates results in increased firing of dopamine neurons within the dopamine reward pathway (8–10,48–52).
In our study, childhood trauma had the least impact on heroin dependence. In contrast, we found a strong effect of childhood trauma on cocaine dependence. Although there was no main effect of GABRA2, severe childhood trauma was associated with cocaine dependence only in individuals with the rs11503014 11/12 genotypes. Preclinical studies support our findings: rats subjected to early life stress are more sensitive to cocaine, demonstrate increased cocaine self-administration (27,53–55) and, perhaps in line with this, have an altered pattern of distribution of GABAA receptor α2 subunits (25) that have been implicated in cocaine sensitization (56). Therefore, bases on these preclinical findings, one speculative explanation for the gene × environment (G×E) interaction in our study might be that if rs11503014 (or a tightly linked SNP) is indeed implicated in exon splicing and thus might influence GABRA2 expression, carriers of the variant allele might be more sensitive to early life stress and subsequent vulnerability to cocaine addiction. However, one caveat should be discussed. Due to the limited sample size and loss of power stemming from categorization of variables we did not expect strong effects of G×E interactions and indeed the RSquare values (reflecting the proportion of the total uncertainty that is attributed to the model fit) of the whole model tests for rs111503014 (0.13 for cocaine dependence only, 0.06 for all cocaine dependence) are modest indicating that, if there were no a priori biological hypothesis, the likelihood of expected cross validation in other samples on a statistical basis alone might be low.
Strengths of the present study include the large sample size of African-American subjects, a group that has been under-represented in genetic studies of addiction. Moreover, since the dataset included individuals with polysubstance dependence as well as individuals addicted to a single substance we were able to parse out both specific and general influences of GABRA2 variation on addiction. Furthermore, it should be noted that we corrected for the effects of population stratification by using ethnic factor scores derived from 186 AIMS as covariates in our analyses. The European factor had a significant effect on heroin addiction in most analyses but no effect on cocaine addiction or alcoholism.
There are some limitations to the present study. Data on other Axis 1 diagnoses were not available for the patients. Since it is known that there is high comorbidity between substance dependence and other psychiatric disorders, particularly major depression, and the controls were free of all Axis 1 diagnoses, it is possible that the signals for association found in our study derived from hidden comorbidity. Nevertheless it should be noted that the extensive literature on association studies with GABRA2 (12–21) has largely focused on alcohol and drug dependence and no published study has yet shown a GABRA2 association with depression. Moreover, Covault et al’s 2004 (19) study showed that the association with alcoholism became stronger when alcoholics with major depression were removed.
Measures of childhood trauma were retrospectively derived from the CTQ. Longitudinal measures are preferable; for example a recent study (57)suggests that the GABRA2 × childhood trauma interaction might be moderated across development by other environmental factors. Nevertheless, the CTQ is widely used and has been shown to have high reliability and validity in both sexes, in different ethnic groups and in psychiatric patients (42,58–61). Although the overall dataset was large some of the analytical subsets were smaller hence the G×E interactions that we have detected may be an underestimate. Moreover, because of these power issues we had to combine the rs11503014 11/12 genotypes in all analyses. The controls were derived from two sources and although their mean age was appreciably lower than that of patients they had largely passed through the peak age of risk for onset of addictive disorders.
In conclusion, the present study has shown that childhood trauma is a strong predictor for alcohol, cocaine and heroin dependence in African American men. Together with childhood trauma, GABRA2 variation influences risk and resilience for all addiction but most strongly for heroin dependence. Our results suggest that GABRA2 may have at least two independent loci that are implicated in the vulnerability to heroin addiction. The two GABRA2 risk – resilience haplotypes are unique to African Americans. There were no findings with the yin-yang haplotypes that confer addiction risk in Caucasians. Moreover, since there is evidence for sexual dimorphism in the influence of GABRA2 on addiction vulnerability with previous results being significant only in men (16), the results of our study may not extend to African American women. Thus the findings of the present study may be unique to African-American men and await replication in other similar datasets.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, NIH and in part by grant RO1 DA 10336-02 to AR from the National Institute of Drug Abuse, NIH.
FINANCIAL DISCLOSURES
The authors declare that, except from income received from our primary employers, no financial support or compensation has been received from any individual or corporate entity over the past 2 years for research or professional services and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.biopsych.2009.08.019
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2964936?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.biopsych.2009.08.019
Article citations
PRKCB methylation: a potential biomarker of MDD with childhood chronic stress, a cross-sectional study in drug-naive, first-episode adolescent MDD.
Epigenetics, 19(1):2408159, 29 Sep 2024
Cited by: 0 articles | PMID: 39342638 | PMCID: PMC11444515
Lifetime adversity predicts depression, anxiety, and cognitive impairment in a nationally representative sample of older adults in the United States.
J Clin Psychol, 80(5):1031-1049, 31 Jan 2024
Cited by: 0 articles | PMID: 38294127 | PMCID: PMC11216061
Candidate gene-environment interactions in substance abuse: A systematic review.
PLoS One, 18(10):e0287446, 31 Oct 2023
Cited by: 1 article | PMID: 37906564 | PMCID: PMC10617739
Review Free full text in Europe PMC
Circadian rhythm disruptions associated with opioid use disorder in synaptic proteomes of human dorsolateral prefrontal cortex and nucleus accumbens.
Mol Psychiatry, 28(11):4777-4792, 06 Sep 2023
Cited by: 4 articles | PMID: 37674018 | PMCID: PMC10914630
Relationships between childhood trauma and mental health during the COVID-19 pandemic: a network analysis.
Front Psychiatry, 14:1251473, 08 Sep 2023
Cited by: 0 articles | PMID: 37743981 | PMCID: PMC10515217
Go to all (77) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
SNPs (2)
- (5 citations) dbSNP - rs11503014
- (1 citation) dbSNP - rs111503014
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide.
Neuropsychopharmacology, 35(8):1674-1683, 20 Jan 2010
Cited by: 163 articles | PMID: 20090668 | PMCID: PMC2962602
Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence.
Mol Psychiatry, 16(11):1139-1146, 14 Sep 2010
Cited by: 56 articles | PMID: 20838391 | PMCID: PMC3003772
GABRG1 and GABRA2 variation associated with alcohol dependence in African Americans.
Alcohol Clin Exp Res, 36(4):588-593, 15 Sep 2011
Cited by: 25 articles | PMID: 21919924 | PMCID: PMC3250564
New insights into the genetics of addiction.
Nat Rev Genet, 10(4):225-231, 01 Apr 2009
Cited by: 124 articles | PMID: 19238175 | PMCID: PMC2879628
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Intramural NIH HHS (2)
Grant ID: ZIA AA000306-04
Grant ID: ZIA AA000305-06
NIDA NIH HHS (2)
Grant ID: R01 DA 10336-02
Grant ID: R01 DA010336-02