Abstract
Free full text

Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum.
Abstract
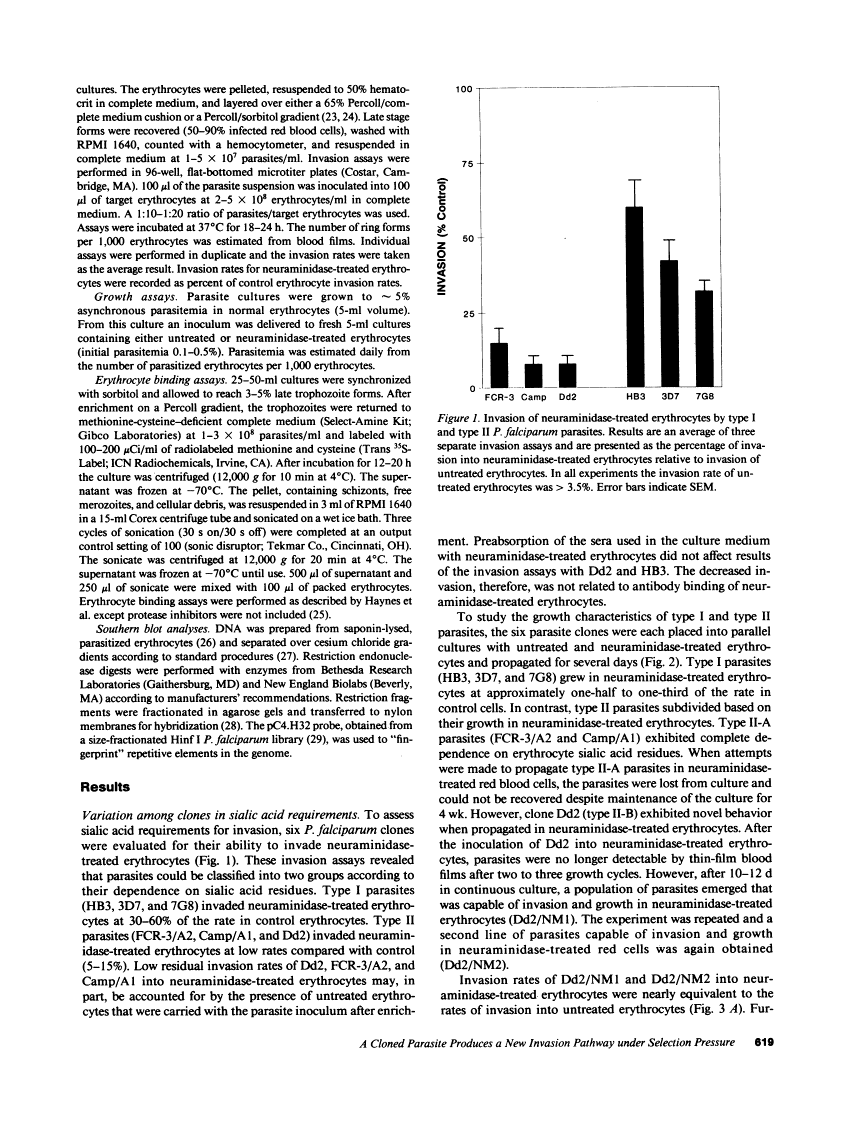
The human malaria parasite Plasmodium falciparum demonstrates variability in its dependence upon erythrocyte sialic acid residues for invasion. Some lines of P. falciparum invade neuraminidase-treated or glycophorin-deficient red blood cells poorly, or not at all, while other lines invade such cells at substantial rates. To explore the molecular basis of non-sialic acid dependent invasion, we selected parasite lines from a clone (Dd2) that initially exhibited low invasion of neuraminidase-treated erythrocytes. After maintaining Dd2 for several cycles in neuraminidase-treated erythrocytes, parasite lines were recovered that invaded both untreated and neuraminidase-treated erythrocytes at equivalently high rates (Dd2/NM). The change in phenotype was maintained after removal of selection pressure. Four subclones of Dd2 were isolated and each readily converted from sialic acid dependence to non-sialic acid dependence during continuous propagation in neuraminidase-treated erythrocytes. The neuraminidase-selected lines and the Dd2 clone demonstrated identical restriction fragment length polymorphism markers indicating that the Dd2 clone was not contaminated during the selection process. Parasite proteins that bound to neuraminidase-treated and untreated erythrocytes were indistinguishable among the parent Dd2 clone and the neuraminidase-selected lines. The ability of the Dd2 parasite to change its invasion requirements for erythrocyte sialic acid suggests a switch mechanism permitting invasion by alternative pathways.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
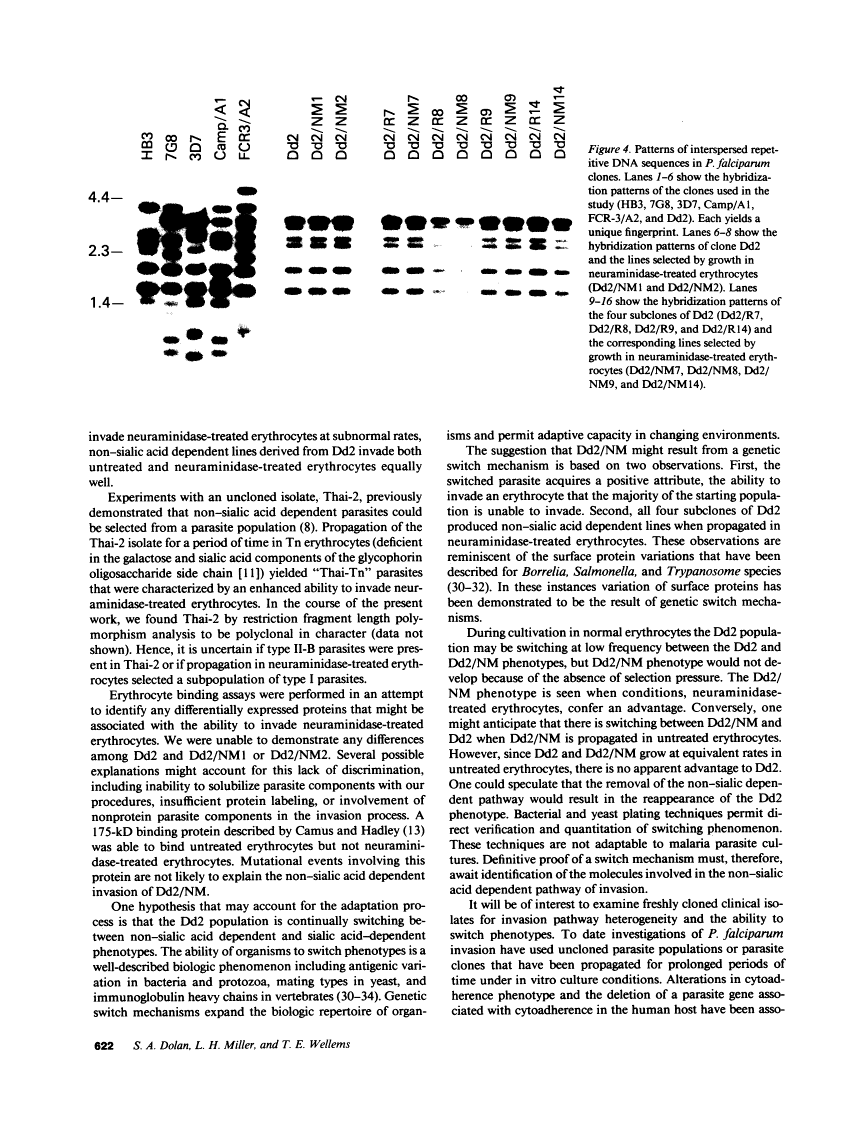
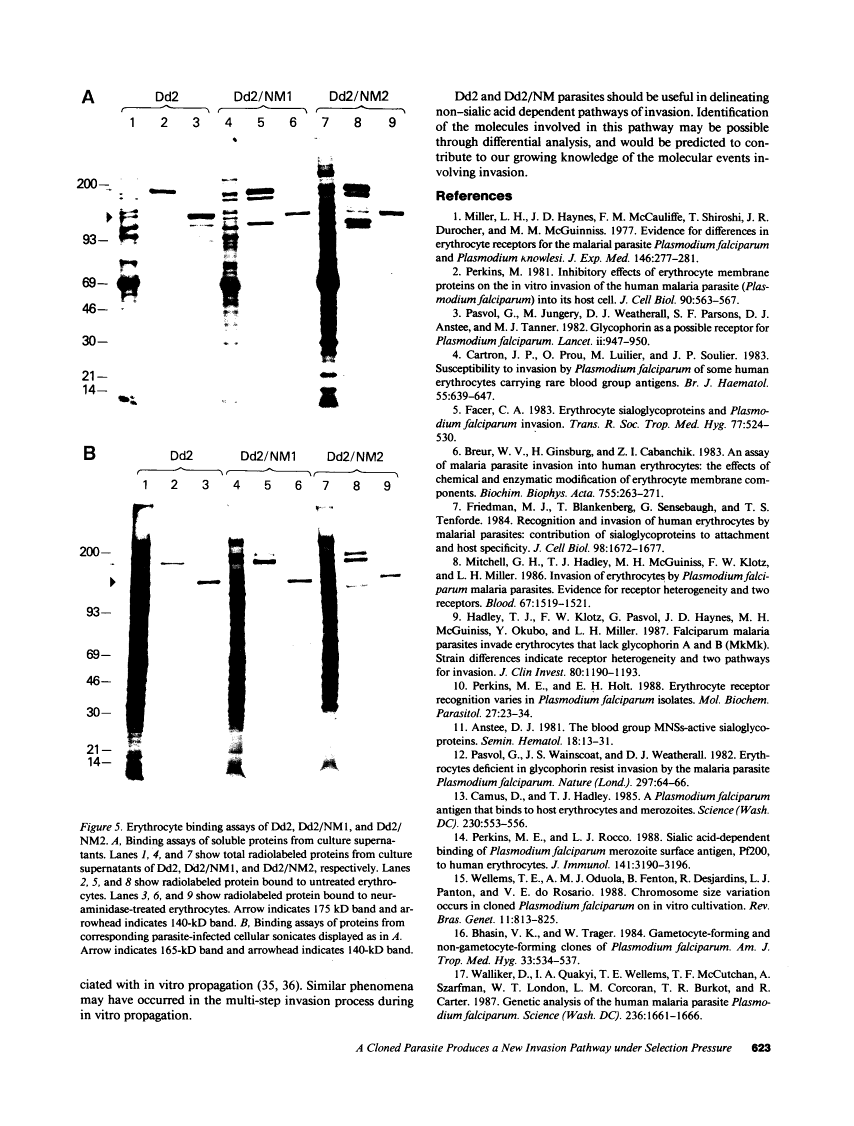
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Miller LH, Haynes JD, McAuliffe FM, Shiroishi T, Durocher JR, McGinniss MH. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977 Jul 1;146(1):277–281. [Europe PMC free article] [Abstract] [Google Scholar]
- Perkins M. Inhibitory effects of erythrocyte membrane proteins on the in vitro invasion of the human malarial parasite (Plasmodium falciparum) into its host cell. J Cell Biol. 1981 Sep;90(3):563–567. [Europe PMC free article] [Abstract] [Google Scholar]
- Pasvol G, Jungery M, Weatherall DJ, Parsons SF, Anstee DJ, Tanner MJ. Glycophorin as a possible receptor for Plasmodium falciparum. Lancet. 1982 Oct 30;2(8305):947–950. [Abstract] [Google Scholar]
- Cartron JP, Prou O, Luilier M, Soulier JP. Susceptibility to invasion by Plasmodium falciparum of some human erythrocytes carrying rare blood group antigens. Br J Haematol. 1983 Dec;55(4):639–647. [Abstract] [Google Scholar]
- Facer CA. Erythrocyte sialoglycoproteins and Plasmodium falciparum invasion. Trans R Soc Trop Med Hyg. 1983;77(4):524–530. [Abstract] [Google Scholar]
- Breuer WV, Ginsburg H, Cabantchik ZI. An assay of malaria parasite invasion into human erythrocytes. The effects of chemical and enzymatic modification of erythrocyte membrane components. Biochim Biophys Acta. 1983 Jan 25;755(2):263–271. [Abstract] [Google Scholar]
- Friedman MJ, Blankenberg T, Sensabaugh G, Tenforde TS. Recognition and invasion of human erythrocytes by malarial parasites: contribution of sialoglycoproteins to attachment and host specificity. J Cell Biol. 1984 May;98(5):1672–1677. [Europe PMC free article] [Abstract] [Google Scholar]
- Mitchell GH, Hadley TJ, McGinniss MH, Klotz FW, Miller LH. Invasion of erythrocytes by Plasmodium falciparum malaria parasites: evidence for receptor heterogeneity and two receptors. Blood. 1986 May;67(5):1519–1521. [Abstract] [Google Scholar]
- Hadley TJ, Klotz FW, Pasvol G, Haynes JD, McGinniss MH, Okubo Y, Miller LH. Falciparum malaria parasites invade erythrocytes that lack glycophorin A and B (MkMk). Strain differences indicate receptor heterogeneity and two pathways for invasion. J Clin Invest. 1987 Oct;80(4):1190–1193. [Europe PMC free article] [Abstract] [Google Scholar]
- Perkins ME, Holt EH. Erythrocyte receptor recognition varies in Plasmodium falciparum isolates. Mol Biochem Parasitol. 1988 Jan 1;27(1):23–34. [Abstract] [Google Scholar]
- Anstee DJ. The blood group MNSs-active sialoglycoproteins. Semin Hematol. 1981 Jan;18(1):13–31. [Abstract] [Google Scholar]
- Pasvol G, Wainscoat JS, Weatherall DJ. Erythrocytes deficiency in glycophorin resist invasion by the malarial parasite Plasmodium falciparum. Nature. 1982 May 6;297(5861):64–66. [Abstract] [Google Scholar]
- Camus D, Hadley TJ. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985 Nov 1;230(4725):553–556. [Abstract] [Google Scholar]
- Perkins ME, Rocco LJ. Sialic acid-dependent binding of Plasmodium falciparum merozoite surface antigen, Pf200, to human erythrocytes. J Immunol. 1988 Nov 1;141(9):3190–3196. [Abstract] [Google Scholar]
- Bhasin VK, Trager W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am J Trop Med Hyg. 1984 Jul;33(4):534–537. [Abstract] [Google Scholar]
- Walliker D, Quakyi IA, Wellems TE, McCutchan TF, Szarfman A, London WT, Corcoran LM, Burkot TR, Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. [Abstract] [Google Scholar]
- Burkot TR, Williams JL, Schneider I. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the same isolate. Trans R Soc Trop Med Hyg. 1984;78(3):339–341. [Abstract] [Google Scholar]
- Trager W, Tershakovec M, Lyandvert L, Stanley H, Lanners N, Gubert E. Clones of the malaria parasite Plasmodium falciparum obtained by microscopic selection: their characterization with regard to knobs, chloroquine sensitivity, and formation of gametocytes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6527–6530. [Europe PMC free article] [Abstract] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. [Abstract] [Google Scholar]
- Rosario V. Cloning of naturally occurring mixed infections of malaria parasites. Science. 1981 May 29;212(4498):1037–1038. [Abstract] [Google Scholar]
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [Abstract] [Google Scholar]
- Dluzewski AR, Ling IT, Rangachari K, Bates PA, Wilson RJ. A simple method for isolating viable mature parasites of Plasmodium falciparum from cultures. Trans R Soc Trop Med Hyg. 1984;78(5):622–624. [Abstract] [Google Scholar]
- Aley SB, Sherwood JA, Howard RJ. Knob-positive and knob-negative Plasmodium falciparum differ in expression of a strain-specific malarial antigen on the surface of infected erythrocytes. J Exp Med. 1984 Nov 1;160(5):1585–1590. [Europe PMC free article] [Abstract] [Google Scholar]
- Haynes JD, Dalton JP, Klotz FW, McGinniss MH, Hadley TJ, Hudson DE, Miller LH. Receptor-like specificity of a Plasmodium knowlesi malarial protein that binds to Duffy antigen ligands on erythrocytes. J Exp Med. 1988 Jun 1;167(6):1873–1881. [Europe PMC free article] [Abstract] [Google Scholar]
- Siddiqui WA, Kan SC, Kramer K, Richmond-Crum SM. In vitro production and partial purification of Plasmodium falciparum antigen. Bull World Health Organ. 1979;57 (Suppl 1):75–82. [Europe PMC free article] [Abstract] [Google Scholar]
- Rigaud G, Grange T, Pictet R. The use of NaOH as transfer solution of DNA onto nylon membrane decreases the hybridization efficiency. Nucleic Acids Res. 1987 Jan 26;15(2):857–857. [Europe PMC free article] [Abstract] [Google Scholar]
- Sinnis P, Wellems TE. Long-range restriction maps of Plasmodium falciparum chromosomes: crossingover and size variation among geographically distant isolates. Genomics. 1988 Nov;3(4):287–295. [Abstract] [Google Scholar]
- Magowan C, Wollish W, Anderson L, Leech J. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J Exp Med. 1988 Oct 1;168(4):1307–1320. [Europe PMC free article] [Abstract] [Google Scholar]
- Pologe LG, Ravetch JV. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. Nature. 322(6078):474–477. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci114753
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/114753/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Application of optical tweezer technology reveals that PfEBA and PfRH ligands, not PfMSP1, play a central role in Plasmodium falciparum merozoite-erythrocyte attachment.
PLoS Pathog, 20(9):e1012041, 23 Sep 2024
Cited by: 0 articles | PMID: 39312588 | PMCID: PMC11449297
Recent increase in low complexity polygenomic infections and sialic acid-independent invasion pathways in Plasmodium falciparum from Western Gambia.
Parasit Vectors, 16(1):309, 31 Aug 2023
Cited by: 0 articles | PMID: 37653544 | PMCID: PMC10472613
Identification of Potential Drug Targets in Erythrocyte Invasion Pathway of Plasmodium falciparum.
Curr Microbiol, 80(5):165, 05 Apr 2023
Cited by: 0 articles | PMID: 37020052
Dramatic Consequences of Reducing Erythrocyte Membrane Cholesterol on Plasmodium falciparum.
Microbiol Spectr, 10(1):e0015822, 23 Feb 2022
Cited by: 6 articles | PMID: 35196803 | PMCID: PMC8865471
Plasmodium vivax Strains Use Alternative Pathways for Invasion.
J Infect Dis, 223(10):1817-1821, 01 May 2021
Cited by: 17 articles | PMID: 32941614 | PMCID: PMC8161644
Go to all (144) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Upregulation of expression of the reticulocyte homology gene 4 in the Plasmodium falciparum clone Dd2 is associated with a switch in the erythrocyte invasion pathway.
Mol Biochem Parasitol, 145(2):205-215, 25 Oct 2005
Cited by: 56 articles | PMID: 16289357
Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum.
Mol Microbiol, 55(1):162-174, 01 Jan 2005
Cited by: 113 articles | PMID: 15612925
Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes.
Science, 309(5739):1384-1387, 01 Aug 2005
Cited by: 191 articles | PMID: 16123303
Selection of genetic variants from Plasmodium clones.
Acta Leiden, 60(1):93-99, 01 Jan 1991
Cited by: 3 articles | PMID: 1820719
Review