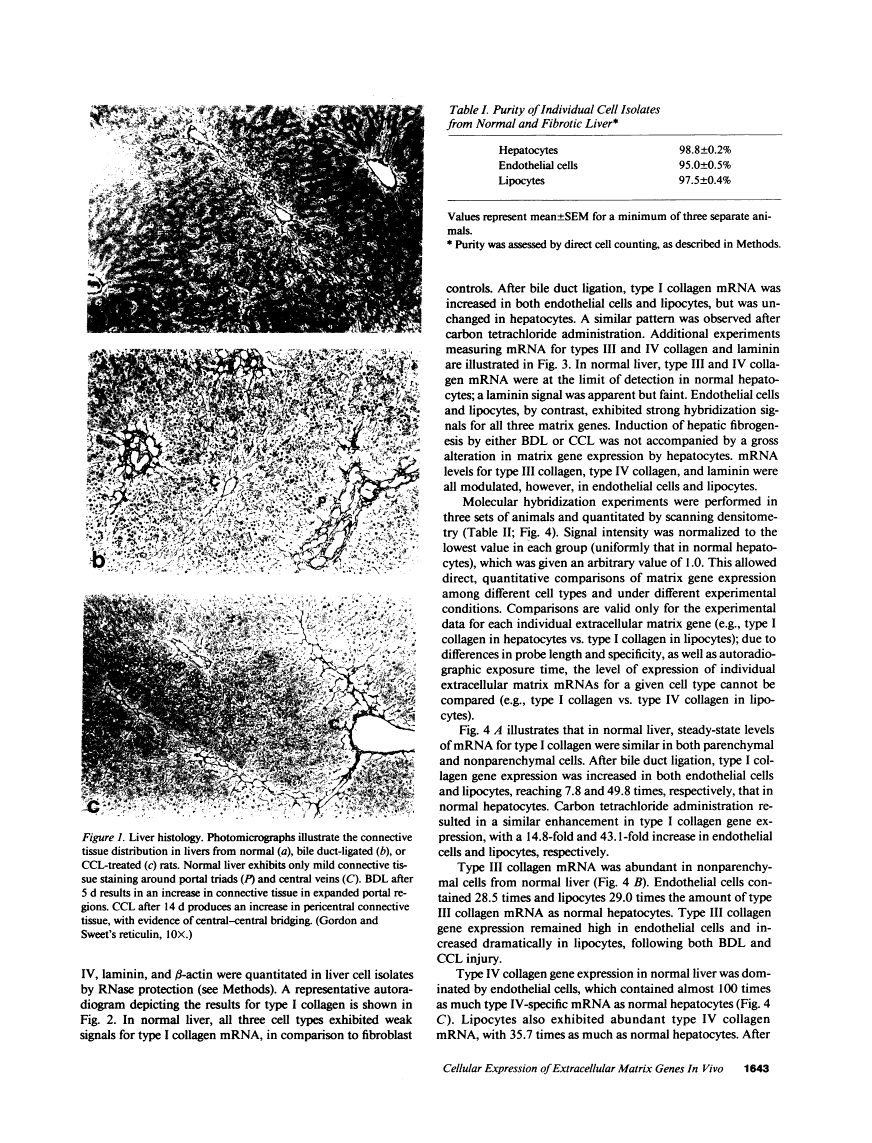
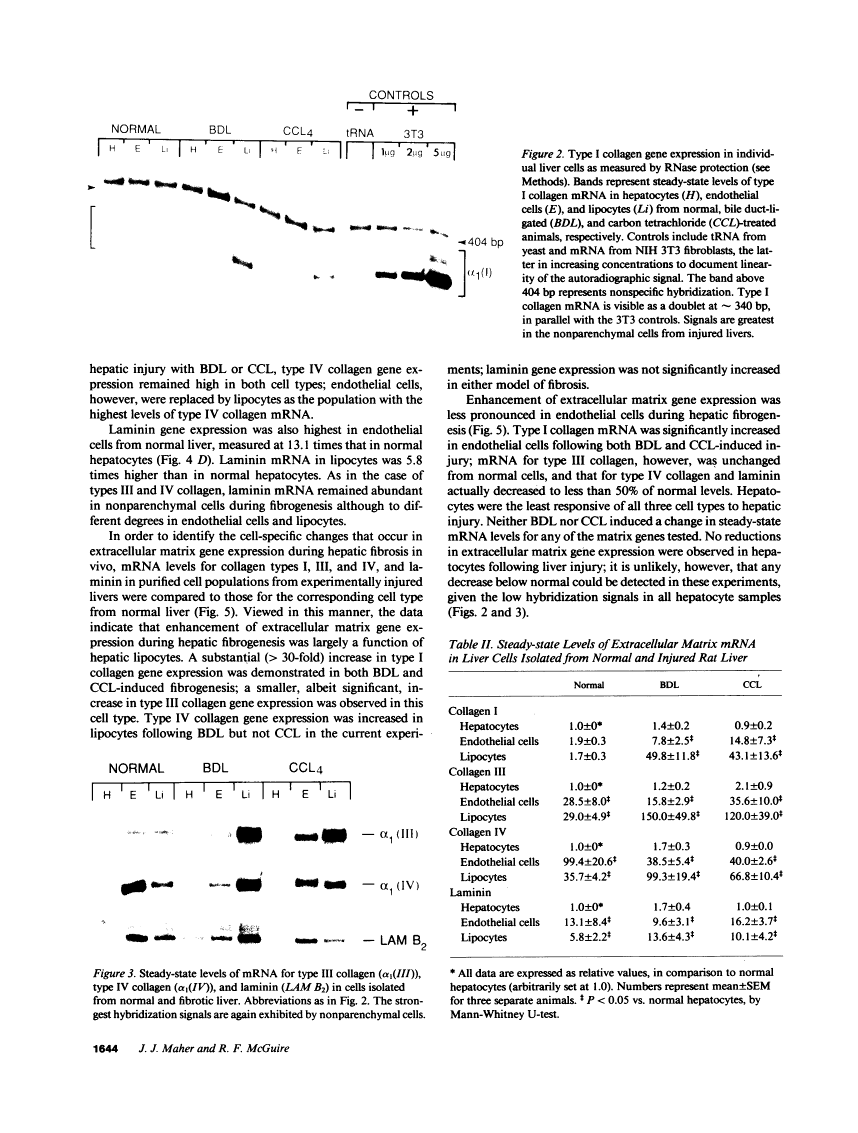
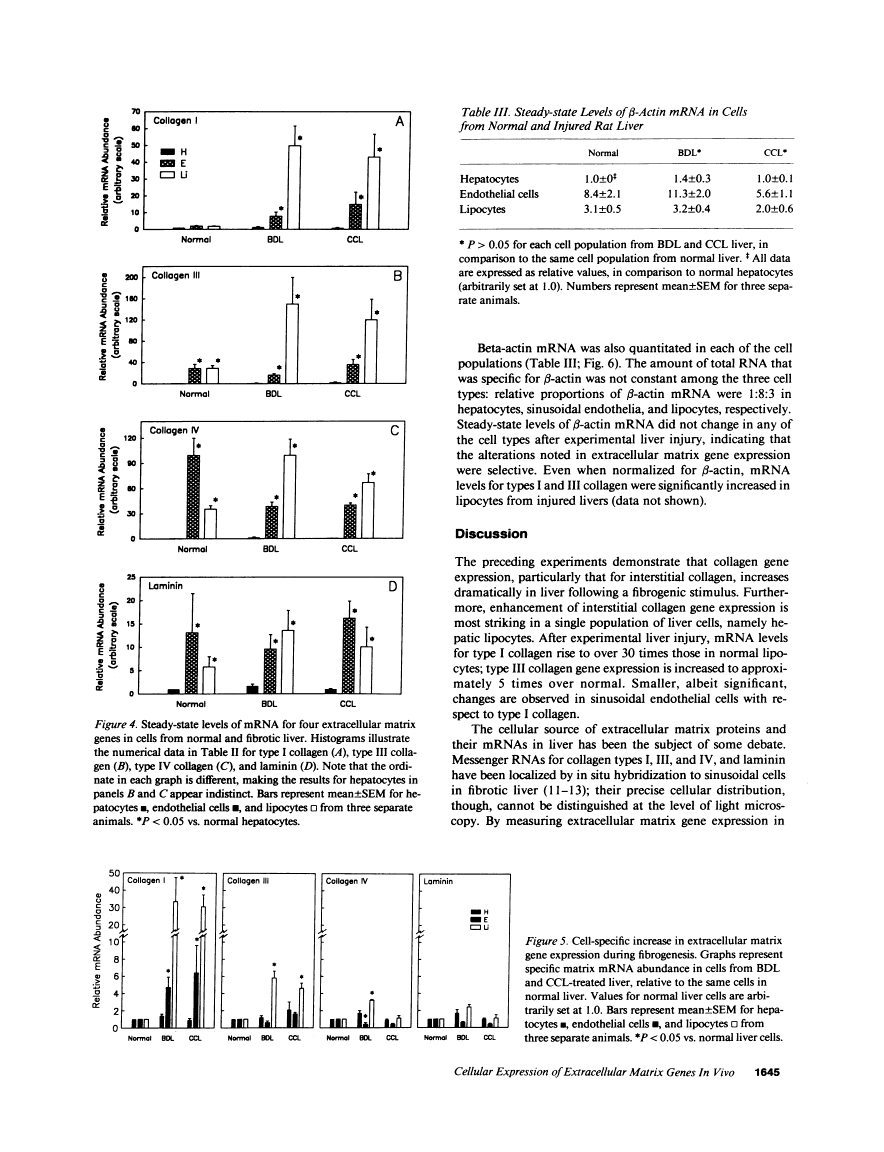
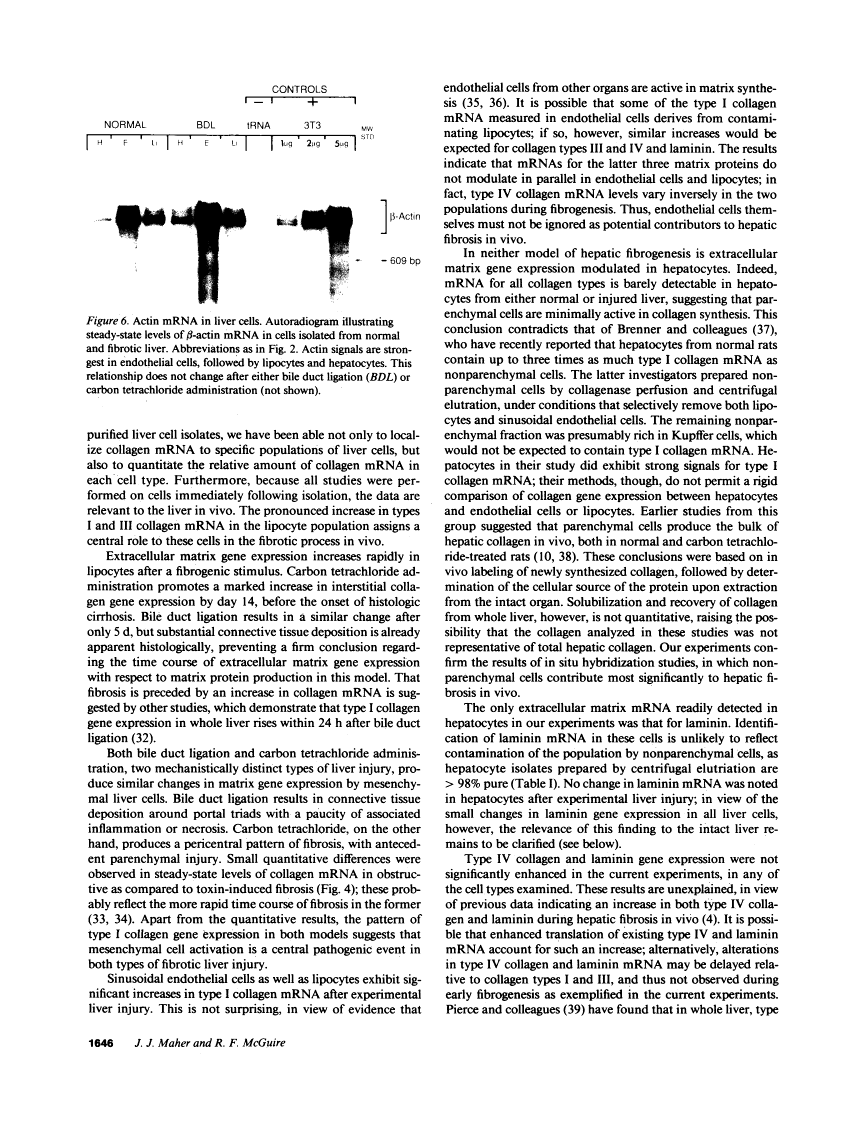
Abstract
Free full text

Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo.
Abstract
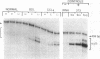
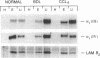
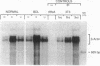
Whether parenchymal or nonparenchymal liver cells play a predominant role in the pathophysiology of hepatic fibrosis has not been firmly established in vivo. We have addressed this question by quantitating the relative abundance of specific mRNAs for collagen types I, III, and IV, and laminin in purified populations of hepatocytes, sinusoidal endothelial cells, and lipocytes from normal and fibrotic rat liver. In normal liver, type I collagen gene expression was minimal in all cell types; mRNA for types III and IV collagen were apparent in endothelial cells and lipocytes, but not in hepatocytes. Laminin mRNA was present in all cell types. Induction of fibrogenesis by either bile duct ligation or carbon tetrachloride administration was associated with a substantial increase in mRNA for types I and III collagen in nonparenchymal cells. Lipocytes from fibrotic animals exhibited a greater than 30-fold increase in type I collagen mRNA relative to normal lipocytes, and greater than 40-fold relative to hepatocytes. Type III collagen mRNA reached 5 times that in normal lipocytes and greater than 120 times that in hepatocytes. Endothelial cells exhibited an isolated increase in type I collagen mRNA, reaching five times that in normal liver. Type IV collagen and laminin gene expression were not significantly increased in nonparenchymal cells during fibrogenesis; in fact, mRNA for type IV collagen and laminin decreased by up to 50% in endothelial cells. Despite the pronounced changes that occurred in matrix gene expression in nonparenchymal cells during fibrogenesis, no change was noted in hepatocytes. We conclude that nonparenchymal liver cells, particularly lipocytes, are important effectors of hepatic fibrosis in vivo.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979 Apr;76(4):710–719. [Abstract] [Google Scholar]
- Murata K, Kudo M, Onuma F, Motoyama T. Changes of collagen types at various stages of human liver cirrhosis. Hepatogastroenterology. 1984 Aug;31(4):158–161. [Abstract] [Google Scholar]
- Seyer JM, Hutcheson ET, Kang AH. Collagen polymorphism in normal and cirrhotic human liver. J Clin Invest. 1977 Feb;59(2):241–248. [Europe PMC free article] [Abstract] [Google Scholar]
- Hahn E, Wick G, Pencev D, Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980 Jan;21(1):63–71. [Europe PMC free article] [Abstract] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984 Jul;51(1):57–74. [Abstract] [Google Scholar]
- Clément B, Emonard H, Rissel M, Druguet M, Grimaud JA, Herbage D, Bourel M, Guillouzo A. Cellular origin of collagen and fibronectin in the liver. Cell Mol Biol. 1984;30(5):489–496. [Abstract] [Google Scholar]
- Clement B, Rissel M, Peyrol S, Mazurier Y, Grimaud JA, Guillouzo A. A procedure for light and electron microscopic intracellular immunolocalization of collagen and fibronectin in rat liver. J Histochem Cytochem. 1985 May;33(5):407–414. [Abstract] [Google Scholar]
- Sakakibara K, Igarashi S, Hatahara T. Localization of type III procollagen aminopeptide antigenicity in hepatocytes from cirrhotic human liver. Virchows Arch A Pathol Anat Histopathol. 1985;408(2-3):219–228. [Abstract] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [Abstract] [Google Scholar]
- Chojkier M, Lyche KD, Filip M. Increased production of collagen in vivo by hepatocytes and nonparenchymal cells in rats with carbon tetrachloride-induced hepatic fibrosis. Hepatology. 1988 Jul-Aug;8(4):808–814. [Abstract] [Google Scholar]
- Milani S, Herbst H, Schuppan D, Hahn EG, Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. [Abstract] [Google Scholar]
- Milani S, Herbst H, Schuppan D, Riecken EO, Stein H. Cellular localization of laminin gene transcripts in normal and fibrotic human liver. Am J Pathol. 1989 Jun;134(6):1175–1182. [Europe PMC free article] [Abstract] [Google Scholar]
- Milani S, Herbst H, Schuppan D, Kim KY, Riecken EO, Stein H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology. 1990 Jan;98(1):175–184. [Abstract] [Google Scholar]
- Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. [Europe PMC free article] [Abstract] [Google Scholar]
- Maher JJ, Bissell DM, Friedman SL, Roll FJ. Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest. 1988 Aug;82(2):450–459. [Europe PMC free article] [Abstract] [Google Scholar]
- Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989 Jun 25;264(18):10756–10762. [Abstract] [Google Scholar]
- Davis BH, Pratt BM, Madri JA. Retinol and extracellular collagen matrices modulate hepatic Ito cell collagen phenotype and cellular retinol binding protein levels. J Biol Chem. 1987 Jul 25;262(21):10280–10286. [Abstract] [Google Scholar]
- Weiner FR, Giambrone MA, Czaja MJ, Shah A, Annoni G, Takahashi S, Eghbali M, Zern MA. Ito-cell gene expression and collagen regulation. Hepatology. 1990 Jan;11(1):111–117. [Abstract] [Google Scholar]
- Clayton DF, Darnell JE., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. [Europe PMC free article] [Abstract] [Google Scholar]
- Irving MG, Roll FJ, Huang S, Bissell DM. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984 Dec;87(6):1233–1247. [Abstract] [Google Scholar]
- Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987 Feb 15;161(1):207–218. [Abstract] [Google Scholar]
- Maher JJ, Friedman SL, Roll FJ, Bissell DM. Immunolocalization of laminin in normal rat liver and biosynthesis of laminin by hepatic lipocytes in primary culture. Gastroenterology. 1988 Apr;94(4):1053–1062. [Abstract] [Google Scholar]
- Genovese C, Rowe D, Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984 Dec 4;23(25):6210–6216. [Abstract] [Google Scholar]
- Kurkinen M, Condon MR, Blumberg B, Barlow DP, Quinones S, Saus J, Pihlajaniemi T. Extensive homology between the carboxyl-terminal peptides of mouse alpha 1(IV) and alpha 2(IV) collagen. J Biol Chem. 1987 Jun 25;262(18):8496–8499. [Abstract] [Google Scholar]
- Barlow DP, Green NM, Kurkinen M, Hogan BL. Sequencing of laminin B chain cDNAs reveals C-terminal regions of coiled-coil alpha-helix. EMBO J. 1984 Oct;3(10):2355–2362. [Europe PMC free article] [Abstract] [Google Scholar]
- Gunning P, Ponte P, Okayama H, Engel J, Blau H, Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. [Europe PMC free article] [Abstract] [Google Scholar]
- Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. [Europe PMC free article] [Abstract] [Google Scholar]
- Berger SL. Preparation and characterization of RNA: overview. Methods Enzymol. 1987;152:215–219. [Abstract] [Google Scholar]
- Mak KM, Lieber CS. Lipocytes and transitional cells in alcoholic liver disease: a morphometric study. Hepatology. 1988 Sep-Oct;8(5):1027–1033. [Abstract] [Google Scholar]
- Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984 Jun;65(3):305–311. [Europe PMC free article] [Abstract] [Google Scholar]
- Pérez-Tamayo R. Cirrhosis of the liver: a reversible disease? Pathol Annu. 1979;14(Pt 2):183–213. [Abstract] [Google Scholar]
- Madri JA, Williams SK. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. [Europe PMC free article] [Abstract] [Google Scholar]
- Tseng SC, Savion N, Gospodarowicz D, Stern R. Modulation of collagen synthesis by a growth factor and by the extracellular matrix: comparison of cellular response to two different stimuli. J Cell Biol. 1983 Sep;97(3):803–809. [Europe PMC free article] [Abstract] [Google Scholar]
- Brenner DA, Alcorn JM, Feitelberg SP, Leffert HL, Chojkier M. Expression of collagen genes in the liver. Mol Biol Med. 1990 Apr;7(2):105–115. [Abstract] [Google Scholar]
- Chojkier M. Hepatocyte collagen production in vivo in normal rats. J Clin Invest. 1986 Aug;78(2):333–339. [Europe PMC free article] [Abstract] [Google Scholar]
- Pierce RA, Glaug MR, Greco RS, Mackenzie JW, Boyd CD, Deak SB. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987 Feb 5;262(4):1652–1658. [Abstract] [Google Scholar]
- Flaim KE, Hutson SM, Lloyd CE, Taylor JM, Shiman R, Jefferson LS. Direct effect of insulin on albumin gene expression in primary cultures of rat hepatocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):E447–E453. [Abstract] [Google Scholar]
- Tolstoshev P, Haber R, Trapnell BC, Crystal RG. Procollagen messenger RNA levels and activity and collagen synthesis during the fetal development of sheep lung, tendon, and skin. J Biol Chem. 1981 Sep 25;256(18):9672–9679. [Abstract] [Google Scholar]
- Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989 Dec;84(6):1780–1785. [Europe PMC free article] [Abstract] [Google Scholar]
- Czaja MJ, Weiner FR, Flanders KC, Giambrone MA, Wind R, Biempica L, Zern MA. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989 Jun;108(6):2477–2482. [Europe PMC free article] [Abstract] [Google Scholar]
- Arthur MJ, Friedman SL, Roll FJ, Bissell DM. Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J Clin Invest. 1989 Oct;84(4):1076–1085. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci114886
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/114886/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1172/jci114886
Article citations
Liver fibrosis: Our evolving understanding.
Clin Liver Dis (Hoboken), 23(1):e0243, 01 Jan 2024
Cited by: 0 articles | PMID: 38961878 | PMCID: PMC11221862
Review Free full text in Europe PMC
Targeting necroptosis in fibrosis.
Mol Biol Rep, 50(12):10471-10484, 01 Nov 2023
Cited by: 0 articles | PMID: 37910384 | PMCID: PMC10676318
Review Free full text in Europe PMC
Patterns of IgA Autoantibody Generation, Inflammatory Responses and Extracellular Matrix Metabolism in Patients with Alcohol Use Disorder.
Int J Mol Sci, 24(17):13124, 23 Aug 2023
Cited by: 0 articles | PMID: 37685930 | PMCID: PMC10487441
Exploring the Regulatory Role of ncRNA in NAFLD: A Particular Focus on PPARs.
Cells, 11(24):3959, 07 Dec 2022
Cited by: 3 articles | PMID: 36552725 | PMCID: PMC9777112
Review Free full text in Europe PMC
Anti-fibrotic effect of extracellular vesicles derived from tea leaves in hepatic stellate cells and liver fibrosis mice.
Front Nutr, 9:1009139, 06 Oct 2022
Cited by: 3 articles | PMID: 36276815 | PMCID: PMC9582986
Go to all (268) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Interferon gamma inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis.
J Investig Med, 42(4):660-670, 01 Dec 1994
Cited by: 58 articles | PMID: 8521029
Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat.
Am J Pathol, 137(6):1333-1342, 01 Dec 1990
Cited by: 68 articles | PMID: 2260623 | PMCID: PMC1877723
Entactin gene expression in normal and fibrotic rat liver and in rat liver cells.
Lab Invest, 70(4):525-536, 01 Apr 1994
Cited by: 17 articles | PMID: 8176891
The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis.
Lab Invest, 53(2):166-186, 01 Aug 1985
Cited by: 177 articles | PMID: 3894794
Review
Funding
Funders who supported this work.
NIAAA NIH HHS (1)
Grant ID: AA-07810
NIDDK NIH HHS (3)
Grant ID: DK-31198
Grant ID: 1P-30 DK-26743
Grant ID: P30 DK026743