Abstract
Free full text

Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor.
Abstract
Experimental cerebral malaria (ECM), a lethal hyperacute neurological syndrome associated with high blood levels of tumor necrosis factor, develops in genetically susceptible (CBA/Ca) mice 7 days after infection with Plasmodium berghei ANKA strain. Injections of neutralizing monoclonal antibody against recombinant murine interferon gamma, not later than 4 days after infection, markedly reduced the incidence of ECM and the elevation in serum levels of tumor necrosis factor. This treatment prevented the cerebral lesions (plugging of brain vessels by monocytes, lymphocytes, and parasitized erythrocytes). In contrast, the extent of macrophage infiltration in lymphoid organs (which is a characteristic feature of mice developing ECM), as well as the course of infection, remained unaffected by the antibody treatment. Protected mice died at a later time of severe anemia and overwhelming parasitemia, the usual outcome of P. berghei infection in mice that are not susceptible to ECM. The present data indicate that interferon gamma constitutes an important link in the cytokine network that leads to brain vessel inflammation in experimental malaria. It is proposed that interferon gamma released by activated CD4+ T cells acts by augmenting both production and action of tumor necrosis factor.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (698K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Wyler DJ. Steroids are out in the treatment of cerebral malaria: what's next? J Infect Dis. 1988 Aug;158(2):320–324. [Abstract] [Google Scholar]
- Grau GE, Piguet PF, Engers HD, Louis JA, Vassalli P, Lambert PH. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [Abstract] [Google Scholar]
- Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. [Abstract] [Google Scholar]
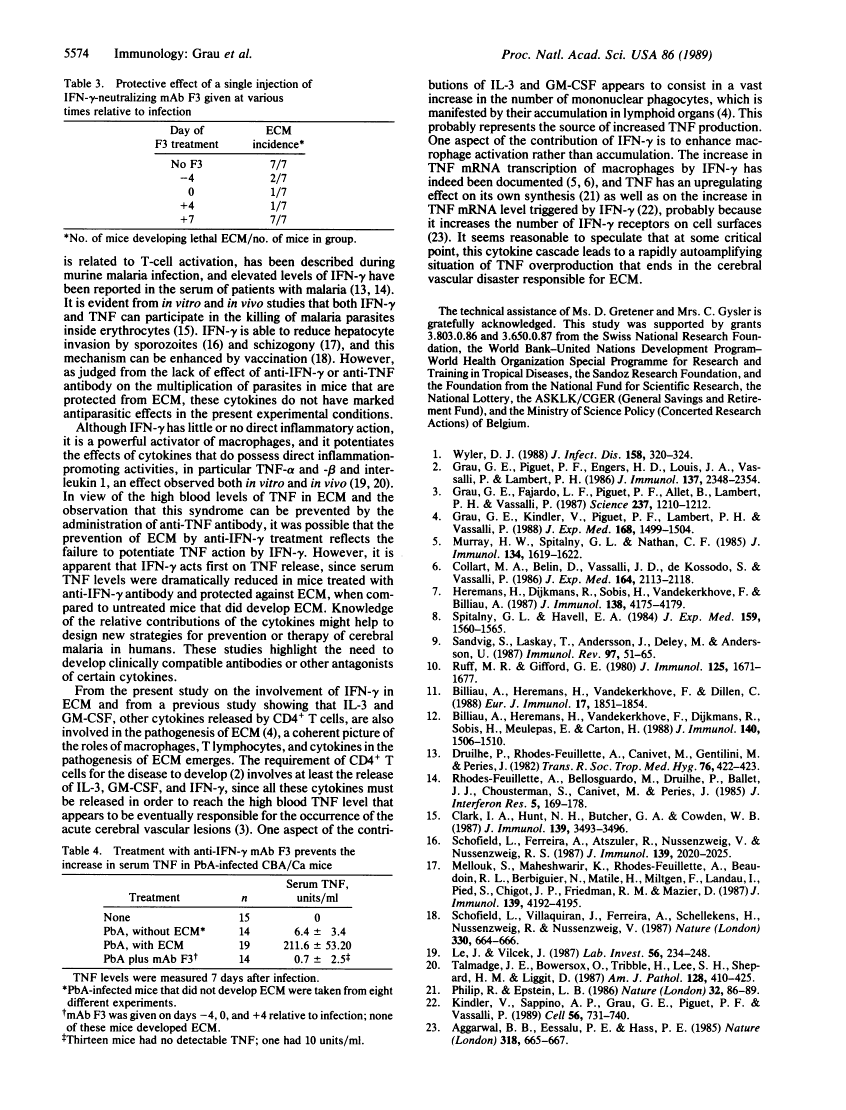
- Grau GE, Kindler V, Piguet PF, Lambert PH, Vassalli P. Prevention of experimental cerebral malaria by anticytokine antibodies. Interleukin 3 and granulocyte macrophage colony-stimulating factor are intermediates in increased tumor necrosis factor production and macrophage accumulation. J Exp Med. 1988 Oct 1;168(4):1499–1504. [Europe PMC free article] [Abstract] [Google Scholar]
- Murray HW, Spitalny GL, Nathan CF. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [Abstract] [Google Scholar]
- Collart MA, Belin D, Vassalli JD, de Kossodo S, Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. [Europe PMC free article] [Abstract] [Google Scholar]
- Heremans H, Dijkmans R, Sobis H, Vandekerckhove F, Billiau A. Regulation by interferons of the local inflammatory response to bacterial lipopolysaccharide. J Immunol. 1987 Jun 15;138(12):4175–4179. [Abstract] [Google Scholar]
- Spitalny GL, Havell EA. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. [Europe PMC free article] [Abstract] [Google Scholar]
- Sandvig S, Laskay T, Andersson J, De Ley M, Andersson U. Gamma-interferon is produced by CD3+ and CD3- lymphocytes. Immunol Rev. 1987 Jun;97:51–65. [Abstract] [Google Scholar]
- Ruff MR, Gifford GE. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [Abstract] [Google Scholar]
- Billiau A, Heremans H, Vandekerckhove F, Dillen C. Anti-interferon-gamma antibody protects mice against the generalized Shwartzman reaction. Eur J Immunol. 1987 Dec;17(12):1851–1854. [Abstract] [Google Scholar]
- Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988 Mar 1;140(5):1506–1510. [Abstract] [Google Scholar]
- Druilhe P, Rhodes-Feuillette A, Canivet M, Gentilini M, Periês J. Circulating interferon in patients with Plasmodium falciparum, P. ovale and P. vivax malaria. Trans R Soc Trop Med Hyg. 1982;76(3):422–423. [Abstract] [Google Scholar]
- Rhodes-Feuillette A, Bellosguardo M, Druilhe P, Ballet JJ, Chousterman S, Canivet M, Périès J. The interferon compartment of the immune response in human malaria: II. Presence of serum-interferon gamma following the acute attack. J Interferon Res. 1985 Winter;5(1):169–178. [Abstract] [Google Scholar]
- Clark IA, Hunt NH, Butcher GA, Cowden WB. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987 Nov 15;139(10):3493–3496. [Abstract] [Google Scholar]
- Schofield L, Ferreira A, Altszuler R, Nussenzweig V, Nussenzweig RS. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987 Sep 15;139(6):2020–2025. [Abstract] [Google Scholar]
- Mellouk S, Maheshwari RK, Rhodes-Feuillette A, Beaudoin RL, Berbiguier N, Matile H, Miltgen F, Landau I, Pied S, Chigot JP, et al. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J Immunol. 1987 Dec 15;139(12):4192–4195. [Abstract] [Google Scholar]
- Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. [Abstract] [Google Scholar]
- Le J, Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [Abstract] [Google Scholar]
- Talmadge JE, Bowersox O, Tribble H, Lee SH, Shepard HM, Liggitt D. Toxicity of tumor necrosis factor is synergistic with gamma-interferon and can be reduced with cyclooxygenase inhibitors. Am J Pathol. 1987 Sep;128(3):410–425. [Europe PMC free article] [Abstract] [Google Scholar]
- Philip R, Epstein LB. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. [Abstract] [Google Scholar]
- Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. [Abstract] [Google Scholar]
- Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.86.14.5572
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/86/14/5572.full.pdf
Citations & impact
Impact metrics
Article citations
Pathogenetic mechanisms and treatment targets in cerebral malaria.
Nat Rev Neurol, 19(11):688-709, 19 Oct 2023
Cited by: 5 articles | PMID: 37857843
Review
Regulatory Functions of Hypoxia in Host-Parasite Interactions: A Focus on Enteric, Tissue, and Blood Protozoa.
Microorganisms, 11(6):1598, 16 Jun 2023
Cited by: 3 articles | PMID: 37375100 | PMCID: PMC10303274
Review Free full text in Europe PMC
The Complexity of Interferon Signaling in Host Defense against Protozoan Parasite Infection.
Pathogens, 12(2):319, 15 Feb 2023
Cited by: 3 articles | PMID: 36839591 | PMCID: PMC9962834
Review Free full text in Europe PMC
Pathophysiology of Cerebral Malaria: Implications of MSCs as A Regenerative Medicinal Tool.
Bioengineering (Basel), 9(6):263, 20 Jun 2022
Cited by: 3 articles | PMID: 35735506 | PMCID: PMC9219920
Review Free full text in Europe PMC
Live Vaccination with Blood-Stage Plasmodium yoelii 17XNL Prevents the Development of Experimental Cerebral Malaria.
Vaccines (Basel), 10(5):762, 11 May 2022
Cited by: 0 articles | PMID: 35632518 | PMCID: PMC9145751
Go to all (208) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Essential role of tumor necrosis factor and other cytokines in the pathogenesis of cerebral malaria: experimental and clinical studies.
Verh K Acad Geneeskd Belg, 54(2):155-175, 01 Jan 1992
Cited by: 6 articles | PMID: 1357836
Plasmodium berghei: recombinant interferon-gamma and the development of parasitemia and cerebral lesions in malaria-infected mice.
Exp Parasitol, 77(2):212-223, 01 Sep 1993
Cited by: 23 articles | PMID: 8375490
Prevention of experimental cerebral malaria by anticytokine antibodies. Interleukin 3 and granulocyte macrophage colony-stimulating factor are intermediates in increased tumor necrosis factor production and macrophage accumulation.
J Exp Med, 168(4):1499-1504, 01 Oct 1988
Cited by: 57 articles | PMID: 3049913 | PMCID: PMC2189068
Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data.
Immunol Rev, 112:49-70, 01 Dec 1989
Cited by: 166 articles | PMID: 2575074
Review