Abstract
Summary
The regulation of lymphocyte homeostasis is critical for the development and formation of productive immune responses. Cell numbers must be maintained to allow sufficient numbers of lymphocytes to combat foreign pathogens but prevent the accumulation of excess lymphocytes that may increase the risk of developing autoimmunity or neoplasia. Cell extrinsic growth factors are essential to maintain homeostasis and cell survival, and it has become increasingly apparent that a key mechanism of this control is through regulation of cell metabolism. The metabolic state of T cells can have profound influences on cell growth and survival and even differentiation. In particular, resting T cells utilize an energy efficient oxidative metabolism but shift to a highly glycolytic metabolism when stimulated to grow and proliferate by pathogen encounter. After antigen clearance, T cells must return to a more quiescent oxidative metabolism to support T-cell memory. This review highlights how these metabolic changes may be intricately involved with both T-cell growth and death in the control of homeostasis and immunity.Free full text

The metabolic life and times of a T-cell
Summary
The regulation of lymphocyte homeostasis is critical for the development and formation of productive immune responses. Cell numbers must be maintained to allow sufficient numbers of lymphocytes to combat foreign pathogens but prevent the accumulation of excess lymphocytes that may increase the risk of developing autoimmunity or neoplasia. Cell extrinsic growth factors are essential to maintain homeostasis and cell survival, and it has become increasingly apparent that a key mechanism of this control is through regulation of cell metabolism. The metabolic state of T cells can have profound influences on cell growth and survival and even differentiation. In particular, resting T cells utilize an energy efficient oxidative metabolism but shift to a highly glycolytic metabolism when stimulated to grow and proliferate by pathogen encounter. After antigen clearance, T cells must return to a more quiescent oxidative metabolism to support T-cell memory. This review highlights how these metabolic changes may be intricately involved with both T-cell growth and death in the control of homeostasis and immunity.
Introduction
Just as architect Louis Sullivan described in the late 1800s that ‘form ever follows function’, the metabolic phenotype of a cell must be tailored to suit the function and demands of each cell’s particular circumstance. T lymphocytes are prime examples of how cell metabolism can be dramatically altered to support the specific needs and functions of each cell state. A central feature that allows this flexibility in metabolism is direct regulation of metabolic pathways by cell extrinsic signals that drive T-cell survival, growth, and proliferation. In each case, if metabolism fails to match the cell demands, cell function is impaired or cells can undergo apoptosis (1–3). Conversely, metabolic excess may prevent apoptosis, exacerbate cell function, and potentially lead to inflammatory diseases (1, 4). Thus, it is critical to appreciate how T-cell metabolism is regulated and how alterations in cell metabolism impact cell function and fate.
One key mechanism to control lymphocyte metabolism and function is through growth factors and other cell extrinsic signals. Through these diverse stimuli, T lymphocytes can respond to their surroundings to maintain survival and homeostasis (5) or to grow and proliferate in an adaptive immune response (6). Importantly, these cell-extrinsic signals are now known to be critical regulators of cell metabolism as well as T-cell survival and function. When appropriate extrinsic signals are provided, T-cell glucose metabolism can be dramatically elevated (1, 7–9), and when extrinsic signals are inadequate, T cells fail to maintain sufficient rates of metabolism to prevent cell death (10–12). This coordination between extrinsic stimuli that promote T-cell survival, migration, and activation along with regulation of cell metabolism ensures that T cells have appropriate energetic and biosynthetic precursors to maintain homeostasis and immunity.
Upon entry into the peripheral pool of lymphocytes, the initial primary function of conventional T cells is surveillance (13, 14). They migrate throughout the secondary lymphoid organs and peripheral tissues in search of specific pathogens and cognate antigen that may elicit activation and an immune response. The cellular demands of quiescent and migrating T cells in this state are relatively straight forward—migration and basic cell support. These physiologic processes have specific metabolic needs that are largely supplied by efficient generation of adenosine triphosphate (ATP) as an energy source and modest levels of biosynthetic precursors for cell maintenance (15). To provide necessary metabolites, resting T cells largely subsist using mitochondrial oxidative metabolism of glucose and glutamine with the bulk of metabolic precursors fully oxidized for maximal ATP generation (16, 17).
The activation of naive T cells in the periphery leads to an entirely different set of metabolic demands and commences when the T-cell receptor encounters cognate antigen presented by major histocompatibility complex (MHC) proteins on the surface of antigen-presenting cells (APCs). This recognition event orchestrates a signaling cascade marked by protein tyrosine phosphorylation, the production of reactive oxygen intermediates, and the translocation of transcription factors to the nucleus (18–20). These signals initiate a new program of gene expression and differentiation into effector cells that is accompanied by increased protein synthesis, cell growth, and effector functions such as cytokine production and cytolytic activity (21–23). In addition, alteration of adhesion molecules and chemokine receptors, such as CD62L and CCR7, allow egress from lymphoid organs and migration to the site of infection (24). Activated T cells also undergo massive clonal expansion beginning within a day of stimulation and continuing with doubling of cell mass and cell divisions as rapid as every 6–8 h.
As lymphocytes shift from quiescence to a highly active effector phenotype, cells must drastically alter their metabolism to support macromolecule synthesis and the bioenergetically demanding process of growth and effector function (25–27). In particular, glucose metabolism increases dramatically to stimulate increased glycolysis as an energy source (28, 29), provide fuel for the pentose phosphate pathways that produce pentose sugars required for nucleic acid synthesis and NADH for reducing power (30), and contribute to lipid synthesis (31). These metabolic changes are essential for lymphocyte activation and survival, as insufficient nutrients can strongly suppress T-cell proliferation and adaptive immunity. Indeed, it has long been appreciated that malnutrition is associated with atrophy of lymphoid organs and a consequent increase in susceptibility to opportunistic infections (3, 32, 33).
Upon pathogen clearance and termination of the immune response, the immune system returns to homeostasis with apoptosis of the majority of effector cells in the contraction phase, while many of the surviving cells further differentiate into long-lived memory cells (6, 34, 35). To accommodate these processes as cells cease to proliferate and re-enter quiescence, T-cell glucose metabolism decreases and reverts to an oxidative metabolism to provide efficient generation of ATP at the expense of biosynthetic precursors. Much as it is now recognized that T-cell activation and increased metabolism are dependent on extrinsic signals, pathogen clearance is followed by decreased antigen receptor stimulation and inflammatory cytokine levels (36). This results in an effective deprivation of many T cells from essential cell extrinsic signals. In the absence of these extrinsic signals, lymphocytes are unable to maintain increased glucose metabolism and exhibit increased susceptibility to apoptosis (7, 10, 37, 38).
Regulation of cell metabolism occurs hand-in-hand with changes in T-cell function and fate. In each phase of T-cell lifespan, metabolism must be matched to function to prevent apoptosis and to allow cell growth, proliferation, and effector function. This review focuses on the metabolic requirements of T cells for growth and survival and the critical role of cytokines in mediating this process in resting and proliferating T cells and how these pathways may influence the termination of the immune response and T-cell memory.
Naive lymphocyte survival and metabolism
T cells play an essential role in the control of tumors and viral or bacterial infections through control of the adaptive immune response (24, 35, 39). After surviving developmental selection in the thymus, naive lymphocytes enter the periphery and migrate through the peripheral lymphoid organs until they encounter their specific cognate antigen and become activated (14). Although quiescent naive T cells can have a long lifespan in vivo, the immune system relies on cell death to maintain homeostasis of lymphocyte numbers and avoid disease (5, 40). When these homeostatic mechanisms function properly, cell proliferation, differentiation, and death are balanced to maintain relatively constant lymphocyte numbers in adult animals. Metabolically, naive T cells consume glucose and other essential nutrients such as glutamine at a low rate, essentially supplying energy only to maintain normal house keeping functions (16). In particular, glucose is utilized for oligosaccharide synthesis, lactate production, and oxidation to CO2 for ATP generation (41) (Fig. 1). Importantly, naive cells rely on extrinsic signals to maintain this metabolic phenotype. Cells deprived of these necessary extrinsic signals are unable to maintain glucose uptake and metabolism sufficiently to prevent cell atrophy and apoptosis (7, 10). Atrophy results in increased proteolysis and decreased cell size that is evident when the anti-apoptotic proteins Bcl2 or Bcl-xl are expressed as transgenes in T cells to prevent apoptosis (10). In these cases, transgenic T cells accumulate in large numbers in vivo but do so as small cells with reduced levels of glucose metabolism. Importantly, the in vivo atrophic state of Bcl-xL transgenic T cells is not cell intrinsic and can be reversed by adoptive transfer of T cells into hosts with normal T-cell numbers and accessibility to extrinsic factors. Thus, the metabolic state of resting lymphocytes is limited by the microenvironment and availability of trophic signals rather than by the availability of nutrients.
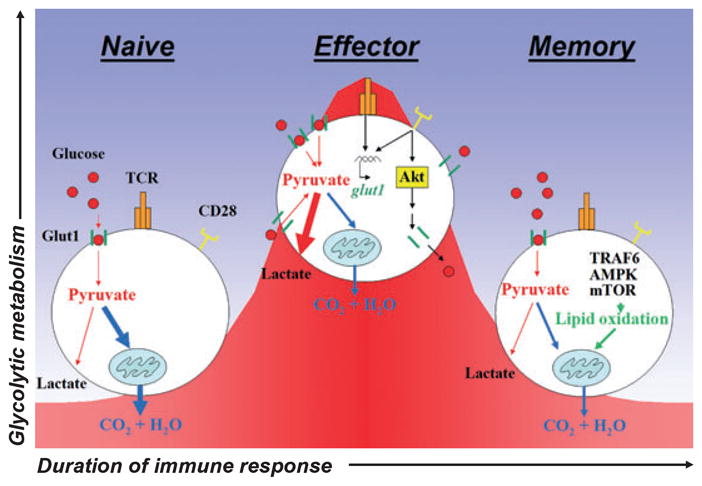
Naive quiescent T cells meet basal energy demands through the oxidation of glucose and glutamine in the mitochondria to yield maximal ATP production. Upon activation, naive lymphocytes shift from a resting to highly proliferative and functional effector state. This transition requires a massive increase in glycolytic metabolism characterized by T-cell receptor (TCR) and costimulatory receptor (CD28)-stimulated expression of the glucose transporter Glut1, Akt-dependent trafficking of Glut1 to the cell surface, and increased glucose uptake. Following pathogen clearance, surviving effector cells differentiate into long-lived memory cells and revert to an oxidative metabolic state that is characterized by lipid oxidation and promoted by TRAF6, AMPK, and mTOR.
T cells receive a myriad of signals in vivo that may provide essential extrinsic signals for the regulation of metabolism, trophic state, and survival. Most critically, T cells rely on chemokine signals to provide migratory direction (42) and T-cell receptor (TCR) and interleukin-7 (IL-7) signals for survival (5, 40). When T cells are treated with pertussis toxin to block chemokine receptor signaling, they fail to migrate into normal periarteriolar T-cell zones in secondary lymphoid organs and are deprived signals normally present in these areas (43, 44). This results in T-cell atrophy and shortened lifespan in vivo (44). Chemokines themselves may act to promote T-cell metabolism and survival through activation of specific signaling pathways, such as the phosphatidyl-inositol-3 kinase (PI3K)/Akt pathway (45–47), but our data suggest this is a minimal role for chemokines relative to providing directional cues towards essential extrinsic signals that can efficiently sustain T-cell metabolism and survival (44).
The TCR provides a critical cell survival signal to maintain T-cell homeostasis (5, 40). In addition, the TCR can play a key role in the regulation of expression of the glucose transporter, Glut1. In the absence of TCR signals, Glut1 expression decreases, thus reducing the glucose uptake ability of T cells and limiting both energetic and biosynthetic capacity that ultimately results in nutrient stress and apoptosis (1, 7, 10). It remains largely uncertain how TCR signals may regulate Glut1 expression, although TCR signals can efficiently stimulate mitogen-activated protein kinase (MAPK) pathways, and these signals have been shown in myotubes and adipocytes to play important roles in Glut1 transcriptional upregulation (48). TCR signals can also activate the adenosine monophosphate (AMP)-activated protein kinase (AMPK) (49), which can also promote glucose uptake and oxidation in a manner consistent with the metabolism of resting T cells (50, 51).
In addition to the TCR, IL-7 is also poised to serve as a homeostatic factor and has a better-defined role in metabolic regulation. IL-7 is produced by stromal cells, the IL-7 receptor (IL-7R) is present on most T cells, and IL-7 downregulates its own receptor, allowing IL-7 to signal large numbers of resting T cells within T-cell areas of secondary lymphoid zones and to be used efficiently when supplies are limiting (5). In normal hosts, IL-7 is required for survival of naive T-cell populations, and IL-7 contributes to homeostatic cycling of naive and memory cells (52). In development, thymocytes fail to differentiate in humans and mice deficient for IL-7 or IL-7 signals (53). Similarly, mature T cells require IL-7 for survival in the periphery, and generation of T-cell memory is impaired in IL-7-deficient hosts (54–56). Conversely, transgenic overexpression of IL-7 can increase T-cell numbers, demonstrating that it is a limiting component of homeostatic regulation of T-cell survival (57, 58). Although it is know that IL-7 is critical to promoting thymocyte development and peripheral T-cell homeostasis, the mechanism by which it functions is not fully understood.
Control of naive T-cell survival by IL-7 appears to occur through several pathways. One direct mechanism is by promoting a favorable balance of expression of anti-apoptotic Bcl-2 family members, including Bcl-2 and Mcl-1, and proapoptotic proteins Bax, Bad, Puma, Noxa, and Bim (59–61). In particular, Bcl-2 interacting mediator of cell death (Bim), which contains only a protein-interaction motif known as the BH3 domain, binds to and neutralizes Bcl-2 pro-survival function. Withdrawal of IL-7 induces the upregulation of Bim expression and apoptosis in lymphocytes (62). Furthermore, disruption of the bim gene provides resistance to apoptosis when T cells are deprived IL-7, suggesting that the regulation of the pro-apoptotic activity of Bim is critical to suppress a default apoptotic program (37, 63). In addition to transcriptional regulation of Bim, however, it is apparent that an additional function for IL-7 is required for efficient maintenance of cell survival. This is evidenced in particular by the failure of transgenic expression of Bcl-2 or deficiency of Bim or Bax to fully rescue T-cell numbers or function in IL-7 deficiency (64–66). IL-7 can also promote glucose uptake and glycolysis to support basal cell metabolism and naive T-cell survival (7, 8, 52). Furthermore, using a conditional mouse model for IL-7 deletion, Jacobs et al. have recently demonstrated that naive T cells are unable to maintain glycolysis in vivo in the absence of IL-7 (67). This homeostatic metabolic role for IL-7 may be critical for T-cell homeostasis and survival, as IL-7 fails to efficiently inhibit T-cell death when glucose is limiting despite maintaining a robust induction of Bcl-2 (7).
The IL-7R, composed of the IL-7Rα and common γ chain, initiates several signaling cascades that may mediate IL-7 regulation of glucose metabolism (5). On the receptor, residue Tyr499 in the cytoplasmic domain is required for T-cell development (68, 69) and is necessary to activate both the Jak/signal transducer and activator of transcription 5 (STAT5) and PI3K/Akt pathways (7, 70). In response to IL-7, both STAT5α and STAT5β can induce the expression of Pim kinases, which in turn can promote glycolysis (71). Similarly, IL-7 signaling promotes Akt1/2 kinase activation, known regulators of glucose uptake and metabolism (70). Unlike T-cell activation characterized by immediate and transient robust Akt activation, IL-7 stimulation of naive T cells results in delayed and sustained levels of Akt activation (7). A low-level activation of PI3K/Akt allows for its accumulation and is well-suited to the role of IL-7 as a survival and homeostatic factor that maintains basal metabolism rather than as a stimulatory factor. In support of a key role for activation of the PI3K/Akt pathway for control of glucose metabolism in T cells, constitutively active forms of Akt1 promote glucose consumption and trafficking of the glucose transporter Glut1 to the cell surface (72, 73), and inhibition of the PI3K pathway decreases surface Glut1 levels, glucose uptake, and glycolysis (72–74) (Fig. 2). Given the inability of IL-7 to promote cell survival when glucose becomes limiting, it is apparent that glucose uptake is a highly regulated aspect of IL-7 signaling that is required for IL-7 function and may play a pivotal role in maintenance of T-cell development and homeostasis.

T cells utilize common γ-chain cytokine signals (i.e. IL-7R, IL-2R) to promote Akt-dependent trafficking of Glut1 to the cell surface to promote glucose uptake and to stabilize expression of anti-apoptotic proteins (i.e. Bcl-2, Mcl-1). Furthermore, growth signals from the TCR also stimulate expression of Glut1 and contribute to increased glycolytic and mitochondrial metabolism. In the absence of these extrinsic signals, T cells fail to maintain glycolytic and mitochondrial metabolism as Glut1 becomes internalized and trafficked to lysosomes. Anti-apoptotic protein expression decreases while pro-apoptotic protein expression increases (i.e. Bim, Puma), sensitizing to programmed cell death.
T-cell activation and metabolism
The highly energetic process of T-cell activation commences upon recognition of foreign antigen presented by stimulated antigen-presenting cells (APCs) and leads to physiological and phenotypic changes to support the rapid cell growth, proliferation, and the generation of effector T cells (6, 23, 24, 35). Although most investigation has centered on the signal transduction pathways that promote transcriptional activation of effector and cell growth genes, it has become apparent that cell metabolism must also play a critical role in immunity to permit transcriptional programs of highly energetic processes of cell growth, proliferation, and effector function (16, 25). Thus, for lymphocytes to rapidly respond to the presence of pathogens and shift from a quiescent phenotype to a highly active state, they must drastically alter their metabolism to support the bioenergetically demanding process of growth and effector function.
Although T-cell activation induces a rapid elevation in mitochondrial metabolism and oxygen consumption (15, 41), the most dramatic metabolic change is due to a sharp rise in glycolysis that results in a significant increase in the production of lactate (1, 9, 75, 76). This glycolytic increase in the presence of molecular oxygen has been termed aerobic glycolysis, distinguishing it from the shift to glycolysis caused by oxygen limitation. Furthermore, this glycolytic program is reminiscent of the metabolism of many cancer cells (77) and allows T cells to rapidly supply their energy needs and increased intracellular glucose levels support the pentose phosphate pathways, which produce pentose sugars required for nucleic acid synthesis and NADPH for reducing power (30) and lipid synthesis (31). Glucose metabolism in particular has been shown regulated by cytokines (7), antigen receptor activation, and costimulation (1, 9). In the absence of such signals, glycolytic flux decreases to a level that can no longer sustain cell viability, and pro-apoptotic Bcl-2 family proteins become activated eliciting cell death (10, 78).
This shift in metabolic profile towards a highly glycolytic phenotype can have a major influence on T-cell function and fate. If T cells fail to sufficiently upregulate glucose metabolism, both lymphocyte function and survival can be severely impaired. Multiple studies demonstrate that despite the presence of alternative metabolic fuels, limiting glucose metabolism in activated T cells results in decreased T-cell proliferation, deficient production of the effector cytokine interferon-γ (IFNγ), and the induction and activation of proapoptotic Bcl-2 family proteins such as Noxa and Bax (1, 2, 11, 79). In addition, failure to obtain sufficient nutrients may lead to T-cell anergy and an inability to respond to mitogenic signals (80). In contrast, increased glucose metabolism can promote or enhance T-cell activation and function. Transgenic overexpression of Glut1 in T cells increased cell size, cytokine production, and proliferation upon activation (1). While this T-cell hyper-reactivity was likely due in part to the availability of additional metabolic fuel for energy and biosynthesis, the elevated glucose uptake also led to inhibitory phosphorylation of glycogen synthase kinase 3 (GSK3) (81), thus directly augmenting T-cell activation by potentially reducing export of nuclear factor of activated T cells (NFAT) (82) and protecting against apoptosis by stabilizing Bcl-2 family member Mcl-1 (81, 83). As a result of these metabolic and signaling changes, activated T cells accumulated in aged Glut1 transgenic mice and signs of autoimmunity and inflammation were evident (1).
T-cell activation requires three distinct signals that may regulate T-cell metabolism to support rapid cell growth and proliferation: the TCR, which provides antigen specificity; costimulatory receptors, provided by stimulated APCs; and cytokines that promote the growth and differentiation of lymphocytes. TCR stimulation initiates the T-cell activation program, but alone does not cause increases in glucose metabolism consistent with fully activated T cells. Instead, ligation of the costimulatory receptor CD28 is necessary for maximal upregulation of glucose uptake and glycolysis (1, 9). While cytokines, such as IL-2, play an important role to sustain T-cell metabolism in activated T cells (7), they appear to play minimal role in the initial upregulation of glycolysis early upon T-cell activation. Thus, a major feature of T-cell costimulation is the coordinated regulation of transcriptional growth and differentiation responses with upregulation of T-cell metabolism to support these new metabolic demands.
The molecular mechanism to drive enhanced glycolytic metabolism in activated T cells is not certain, but regulation of Glut1 and glucose uptake are important. The central role for Glut1 is apparent, as T cells are highly glucose dependent for growth and proliferation, strongly upregulate Glut1 after activation, and can have increased proliferation and function when Glut1 is transgenically overexpressed (1, 2). Glut1 knockout animals are early embryonic lethal (84), and conditional Glut1 knockouts have not yet been described. RNAi of Glut1 in lymphoid cell lines can decrease proliferation and glucose limitation can cause cell cycle arrest or apoptosis (2, 85, 86). Thus, Glut1 appears to be a limiting component to support proliferation and survival of activated T cells.
Glut1 is regulated at multiple levels, with both transcriptional induction and protein trafficking playing key roles (7, 73). Glut1 is expressed at low levels in resting T cells, but T-cell activation can cause a rapid and robust transcriptional induction. Of the TCR and CD28 signals that are required for maximal glucose uptake and metabolism in activated T cells, it appears as though TCR-derived signals are account for the majority of this transcriptional induction. Stimulation of T cells with a strong TCR agonist or cross-linking of the TCR-associated CD3 proteins (1) can lead to a rapid and maximal induction of Glut1 protein. Lower levels of TCR stimulation can still upregulate Glut1 but are augmented by addition of CD28-mediated costimulation (1, 9). As a key function of CD28 is to enhance TCR-derived signals and strong TCR signals alone are sufficient, these data suggest that the TCR and its associated signaling pathways may be the critical elements for Glut1 induction. Consistently, the MAPK pathway and Myc activation have been shown to play a role in Glut1 expression (48, 87) and may mediate the TCR-dependent control of Glut1. In addition, however, TCR signals have been recently shown to activate AMPK (49), which is well established to upregulate glucose uptake to promote energy generation (50, 51) and may due so in part through upregulation of Glut1 expression.
In addition to expression, Glut1 intracellular trafficking is highly regulated. Like the related family member Glut4, Glut1 can exist in intracellular pools and be shuttled to the cell surface upon growth factor stimulation (73, 88). In the case of T-cell activation, upregulation of Glut1 protein does not necessitate that Glut1 travels to the cell surface to support glucose uptake. Costimulation through CD28, however, can provide a strong signal to direct Glut1 cell surface trafficking (1). It is likely this occurs through activation of the PI3K/Akt signaling pathway, which the TCR only poorly activates yet can directly affect Glut1 localization (9). In particular, Akt activation can promote Glut1 cell surface trafficking and prevent internalization of Glut1 once it is on the cell surface, thus further enhancing total cell surface Glut1 and glucose uptake. Indeed, T cells expressing constitutively active Akt have similar levels of total Glut1 protein, but a higher proportion appears to reside on the cell surface and glucose uptake and glycolysis are elevated relative to controls (4). Conversely, inhibitory receptors, such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) can inhibit PI3K activation and can reduce cell surface Glut1 levels (1, 9). Trafficking of Glut1 can play a key role in overall glucose uptake, as inhibition of the PI3K pathway prevents the upregulation of glucose metabolism in activated T cells, as well as activated B cells, and leads to Glut1 internalization where it is targeted to lysosomes for degradation (88, 89).
Despite the central role of Glut1 to support T-cell glucose metabolism, it is not sufficient to fully drive aerobic glycolysis, and metabolism must be regulated at additional steps downstream of the initial glucose uptake. Akt has been shown to increase hexokinase activity, resulting in glucose phosphorylation to prevent its transport out of the cell and to instead support flux through both glycolysis and the pentose phosphate pathway (72, 90, 91). In addition, Akt can also promote activity of the key glycolytic enzyme, phosphofructokinase, by phosphorylation of phosphofructokinase-2 and generation of the allosteric activator, 2,6-phospho-fructose (92). This enzyme is often a rate-limiting step in glycolysis and likely plays a critical role to support T-cell metabolism for growth and proliferation.
In addition to glucose metabolism, activated T cells also become highly dependent on availability of amino acids. These are used both for protein synthesis and as metabolic fuels for mitochondrial oxidation and ATP generation. In particular, T cells cannot uptake cystine and require the reductive activity of APCs to provide the oxidized form of cystine, cysteine, to allow intracellular transport of this essential amino acid (16, 93, 94). Also, T cells are highly dependent on arginine, and expression of arginase by surrounding cells can degrade arginine and suppress T-cell activation, an immunosuppressive strategy that is employed by myeloid derived suppressor cells and can also contribute to the immunosuppressive environment within tumors (95–97). The amino acid glutamine is also critical for T-cell metabolism and function, although as a mitochondrial fuel in addition to its role as an essential protein building block (98, 99). This dependence on glutamine may be mediated by cMyc, which is induced in T-cell activation and promotes expression of enzymes involved in glutamine metabolism. Importantly, cells with cMyc expression may be particularly sensitive to inhibition of glutamine metabolism (100, 101), resulting in cell cycle arrest or death—an addiction to glutamine that may be potentially exploited as a metabolic means to immunosuppression.
Chronic stimulation of lymphocytes during inflammatory disease, chronic infection, or transplantation may lead to an alternate metabolic profile in comparison to highly glycolytic T cells generated during acute stimulation. For example, inhibitory costimulatory receptors, such as programmed death-1 (PD-1), have been shown to suppress T-cell growth and proliferation when chronically stimulated (102, 103). The ability of these suppressive receptors to inhibit PI3K activation would lead to decreased PI3K/Akt function and may result in reduced cell surface Glut1 and glycolytic rates. Consistent with an alternate mode of metabolism for chronically stimulated T cells, lymphocytes isolated from patients with systemic lupus erythematosus have been shown to have dysfunctional mitochondria and to be dependent on mitochondrial metabolism (104, 105). Indeed, an inhibitor of the mitochondrial F1/F0 ATPase, which can reduce mitochondrial generation of ATP and lead to generation of reactive oxygen species (ROS), has been found to selective target chronically stimulated T cells for apoptosis and spare resting and acutely activated T cells (106, 107).
In addition to the types of T-cell stimulation, the metabolic program of activated T cells must match the metabolic demands of each cell functional response, and given the diverse array of differentiated phenotypes for activated T cells, it is likely that T-cell metabolism is not uniformly shared. In particular, regulatory T cells (Tregs) are demonstrated to be poorly proliferative, whereas inflammatory T-cell fates can be highly proliferative. Consistent with this possibility, inhibition or genetic loss of mammalian target of rapamycin (mTOR) leads to diminished nutrient uptake (74) and can also favor development of Tregs over inflammatory T-cell fates (108–110). Connections between metabolism and T-cell differentiation and the role of metabolism in specific T-cell subsets, however, remain largely uncertain. It is intriguing, however, that Treg cells have recently been identified as enriched in lipid-rich adipose tissues (111), suggesting that alterations in cell metabolism and availability of alternate fuels may affect Treg generation and provide a possible metabolic means to suppress or modify immunity.
Metabolism in clonal contraction and memory
Following pathogen clearance, excess numbers of pathogenspecific T-cell clones occupy the lymphoid compartment and dominate the T-cell repertoire. To restore immune homeostasis, approximately 90% of these effector cells undergo a process of apoptosis or programmed cell death (6, 34), which ensures that the lymphocyte pool remains diverse (112). Surviving T cells from the initial clones differentiate into a small population of memory cells that persist to protect against subsequent infection. This secondary response, initiated by re-exposure to pathogen, is faster and of a greater magnitude then the primary infection and is the basis for efficacy of T-cell-specific vaccines (24, 35). A chief component of clonal contraction and generation of memory is the loss of antigenic and inflammatory signals after antigen or pathogen clearance (36). Much like how inadequate growth factors lead to decreased metabolism of resting T cells, this loss of antigen or inflammatory cytokine signals leads to a state of growth factor withdrawal.
Loss of growth factor signals following an immune response can lead to a sharp decline in glucose metabolism and result in cellular atrophy and apoptosis of many cells. Activated T cells rely on continued presence of cytokines, such as IL-2, to maintain surface levels of Glut1 (7). When IL-2 and other inflammatory cytokines become limiting, the PI3K/Akt pathway inactivates, resulting in Glut1 internalization and targeting to lysosomes for degradation, thus preventing T cells from utilizing extracellular glucose as a fuel (73, 88, 89). Likewise, decreased activity of the PI3K/Akt pathway also results in internalization of amino acid transporters, further reducing accessibility to extracellular building blocks and fuel (74). The combined loss of nutrient uptake and decreased PI3K/Akt signaling lead T cells to cease growth and instead activate alternate pathways for metabolic fuel such as autophagy, a process of self-digestion that can provide alternate fuels through lysosomal degradation of intracellular components and membranes. Ultimately, autophagy is a self-limited survival strategy, and the majority of cells undergo apoptosis (113).
It remains unclear precisely how some cells survive to become long-lived memory T cells, but it is now apparent that a distinct metabolic phenotype is favored and may promote survival as memory T cells. One proposed mechanism is the selective upregulation of IL-7Rα on a subset of T cells, which may allow these cells to maintain some homeostatic regulation of nutrient uptake and metabolism (55, 114, 115). Alternatively, asymmetric cell division may predispose specific responding T cells to memory very early on in the immune response (116). In either case, it is clear that memory T cells must adopt an alternative metabolic profile from their effector counterparts. In particular, as memory T cells cycle slowly and persist for prolonged periods, they require a metabolic phenotype more similar to that of resting naive T cells, with minimal biosynthetic demands and efficiency for ATP production at a premium. Consistent with a transition to an efficient oxidative metabolism, tumor necrosis factor receptor activating factor 6 (TRAF6) was recently defined as important to promote a transition to a lipid oxidative metabolism and generation of memory (117). It is not certain precisely how TRAF6 may promote lipid β-oxidation, but the alternate approach to promote lipid oxidation by treatment of animals with the AMPK activator metformin was shown to enhance generation of memory T cells in a viral response model (117). Likewise, inhibition of mTOR also enhanced CD8+ T-cell memory (118).
The long-term metabolic phenotype of memory T cells remains unknown. Given the dependence of these cells on the cytokines IL-7 and IL-15 for survival (54, 55, 119–121), it is likely that memory T cells utilize an oxidative metabolism similar to that of naive T cells. The ability of memory T cells to rapidly grow and proliferate upon restimulation, however, suggests that memory T cells may have robust metabolic response to stimulation and likely the capacity to shift metabolic programs quickly to a pro-growth glycolytic state. However, detailed and direct analyses of memory T-cell metabolism and metabolic plasticity have yet to be described.
Metabolism and cell death
At each phase of the T-cell lifespan, cell metabolism is regulated by growth factors to supply the cell with appropriate energy and biosynthetic precursors. If cells do not receive sufficient growth factor signals, this loss of metabolism correlates closely with activation of nutrient stress response pathways and apoptosis (5, 7, 10, 78, 122). Nonetheless, it is important to note that resistance to apoptosis, such as provided by deficiency in the pro-apoptotic proteins Bax and Bak (113, 123) or Bim and Puma (86) or by expression of the anti-apoptotic proteins Bcl-2 or Bcl-xL (10), can allow cells to persist for prolonged periods of time even in the absence of glucose. Thus, loss of nutrients does not lead inexorably to cell death. Rather, the inability of cells to sustain cell metabolism sufficient to meet cellular demands leads to a cell stress capable of activating pro-apoptotic members of the Bcl-2 family of proteins. It is likely that such a nutrient stress may contribute to apoptosis of many T cells that fail to compete for homeostatic factors, such as IL-7, or after the clearance of antigen and reduction of inflammatory cytokines in the contraction phase of a T-cell immune response.
The Bcl-2 family consists of both pro-apoptotic and anti-apoptotic members and the balance of these two classes of proteins largely dictates sensitivity of T cells to cell stress and the likelihood of apoptosis (124). For T cells, the pro-apoptotic protein Bim has been shown to play an essential role in apoptosis of growth factor deprived resting and activated T cells and is essential for T-cell homeostasis, deletion of auto-reactive thymocytes and B cells, and death of antigen activated T cells during shutdown of an immune response. Indeed, resting T cells accumulate in Bim−/− animals (62, 63) and Bim−/− T cells can persist long-term following an immune response and accumulate as memory T cells (37, 125, 126). Although the kinetics of T-cell responses to herpes simplex virus-1 (HSV-1) in the lymph node of wildtype and Bim−/− mice are similar, infection induced a rapid increase in the number of HSV-specific T cells in the Bim-deficient mice compared to wildtype mice. This accumulation of cells did not result from trafficking or proliferative differences but was due to these cells being resistant to apoptosis induced by cytokine limitation. In wildtype activated T cells that are deprived of growth factors, Bim expression is driven at least in part by the transcription factor FOXO3 (127). Importantly, ectopic expression of Glut1 can sustain glucose metabolism even after growth factor withdrawal (72), but Bim is induced normally, suggesting that the loss of cell signaling rather than decreased glucose uptake and metabolism is critical to upregulate Bim. Despite this induction of Bim, Glut1 expression can nevertheless protect cells from apoptosis upon growth factor withdrawal. Thus, cell metabolic state can influence apoptosis but does not appear to do so through direct regulation of Bim.
The related pro-apoptotic Bcl-2 family protein Puma, in contrast, does appear sensitive to changes in cellular metabolic state and may be induced directly in response to a loss of glucose metabolism and bioenergetics that occurs after deprivation from growth factor signals. Like Bim, Puma contributes to T-cell apoptosis after growth factor withdrawal or other stresses (128, 129). Puma also plays significant role in the death of antigen-specific CD8+ T cells during an acute immune response and plays a physiological role during the shutdown of an immune response (130). Puma−/−Bim−/− T cells have synergistic resistance to cell death stimuli, indicating a non-redundant role for Puma in contributing to programmed cell death (131). Puma is regulated largely at a transcriptional level, with the tumor suppressor p53 playing a major role (132, 133). It has been shown that glucose limitations can lead to activation of AMPK (51) and AMPK-mediated phosphorylation of p53 to activate its transcriptional response (134). Glucose withdrawal does indeed lead to a robust induction of Puma, and Puma-deficient cells are particularly resistant to apoptosis following glucose withdrawal (86). Conversely, maintenance of glucose uptake prevents p53 activation and Puma induction even after growth factor withdrawal. Thus, while both Bim and Puma contribute to promote apoptosis of growth factor-deprived T cells, only Puma is regulated by the metabolic stress that occurs with loss of growth factor signaling.
Noxa and Mcl-1 have also been implicated as important as Bcl-2 family proteins involved in apoptosis of activated T cells. Like Puma, Noxa is a p53 transcriptional target and can efficiently induce apoptosis (135). It is upregulated upon T-cell activation and associates with the anti-apoptotic Bcl-2 family protein Mcl-1, an anti-apoptotic protein. When growth factor signals and glucose become limiting for T cells, Noxa levels do not increase but can sensitize T cells to apoptosis through sequestration of Mcl-1 and can indirectly promote activation of pro-apoptotic Bcl-2 family proteins (11). Mcl-1 is upregulated by IL-7 and growth factors and is downregulated upon loss of these signals. In addition, Mcl-1 is sensitive to changes in cell metabolism both at a level of protein translation as well as protein degradation (81, 136, 137). Inhibition of cell metabolism through glucose limitation or oxygen deprivation can lead to degradation of Mcl-1 protein. In addition, given the short half-life of Mcl-1 protein, inhibition of mTOR activity and mTOR-driven protein translation by glucose deprivation and activation of the AMPK pathway can lead to insufficient production of new Mcl-1 molecules to sustain total Mcl-1 levels and a rapid overall reduction in Mcl-1 expression (136). Loss of Mcl-1 can have dramatic affects on T cells, as conditional knockouts have shown an essential role for this anti-apoptotic protein in T-cell survival (61). Thus, in addition to direct regulation of Bim by growth factor signals, the combined decrease in Mcl-1 expression, high expression of Noxa, and induction of Puma strongly sensitizes T cells to apoptosis when nutrients become limiting.
In addition to the Bcl-2 family-regulated intrinsic pathway of cell death, the cell-extrinsic pathway of apoptosis may also play an important role in the termination of immune responses. Defects in Fas or its cognate ligand FasL in mice or humans cause T-cell hyperplasia and auto-antibody production, which resemble systemic lupus erythematosus and can lead to significant morbidity and mortality (138, 139). Although T-cell responses to peptide or Staphylococcus enterotoxin B (SEB) are abnormally enhanced and prolonged in Fas-deficient mice (140), the loss of Fas, tumor necrosis factor receptor 1 (TNFR1), or even both does not impede the death of T cells during shutdown of immune responses to acute HSV-1 infection (37). However, recent studies have demonstrated that Fas can support pro-apoptotic Bcl-2 family proteins to eliminate lymphocytes during T-cell contraction. Weant et al. (141) found that compound mutants of Bim and Fas dramatically increased numbers of memory T-cell in the lymph node following an acute LCMV infection. Similarly, Hughes et al. (142) found a role for Fas and Bim following chronic MHV infection. In the case of chronic infection, antigen persistence can lead to expression of FasL, which in turn can interact with Fas expressed on both T cells and dendritic cells (DCs). Death of DCs and removal of stimulation signals to T cells may account for increased Bcl-2 family-dependent death after antigen has been cleared. In addition, to death receptor-mediated influence over DCs and antigen presentation, decreased glucose metabolism in T cells after antigen clearance may also enhance T-cell sensitivity to death receptor signaling, as inhibition of glucose metabolism can lead to increased apoptosis in response to Fas (136, 143).
Concluding remarks
For T-cell homeostasis and adaptive immunity, it is critical that T-cell metabolism adequately meets and is suited to the needs of specific T-cell functions. If metabolism fails to support T-cell demands, autophagy, cell cycle arrest, and apoptosis via Bcl-2 family proteins may ensue. Conversely, excess metabolism may promote T-cell hyper-reactivity and autoimmunity. This metabolic control requires the integration of input from multiple signaling pathways, including T-cell receptor stimulation, costimulatory signaling, and cytokine receptors, and occurs in parallel with the specific transcriptional and signaling pathways activated by each signal. Such coordinated control ensures that energetic and biosynthetic supplies appropriately support T-cell functional demands. A greater understanding of how these pathways are regulated and function in lymphocytes becomes increasingly important with the availability and interest in the therapeutic use of drugs that alter metabolism. Through metabolic manipulation, it may be possible to extend survival of lymphocytes and ultimately alter their differentiation. Conversely, it may be possible to target metabolic pathways and mediators to control the formation of T-cell lineages or to suppress T-cell responses by blocking specific metabolic pathways essential for T-cell growth and proliferation. This approach has proved promising in cancer therapy, and given the similarities of cancer cell metabolism with that of activated lymphocytes, it is likely that a similar strategy may provide a new approach to modulation of the immune system.
Acknowledgments
We thank members of the Rathmell lab for constructive comments and suggestions. This work was supported by NIH R01 AI063345 (J.C.R.), J.C.R. is a Bernard Osher Fellow of the American Asthma Foundation, and the Irvington Institute Postdoctoral Fellowship from the Cancer Research Institute (R.D.M.).
References
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1600-065x.2010.00911.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2983473?pdf=render
Citations & impact
Impact metrics
Article citations
Effect on the splenocyte function of weaned piglets induced by continuous lipopolysaccharide injections.
J Vet Res, 68(2):295-302, 09 May 2024
Cited by: 0 articles | PMID: 38947147
Metabolic imbalance driving immune cell phenotype switching in autoimmune disorders: Tipping the balance of T- and B-cell interactions.
Clin Transl Med, 14(3):e1626, 01 Mar 2024
Cited by: 2 articles | PMID: 38500390 | PMCID: PMC10948951
Virus infection pattern imprinted and diversified the differentiation of T-cell memory in transcription and function.
Front Immunol, 14:1334597, 09 Jan 2024
Cited by: 0 articles | PMID: 38264657 | PMCID: PMC10803622
Proteostasis in T cell aging.
Semin Immunol, 70:101838, 12 Sep 2023
Cited by: 2 articles | PMID: 37708826 | PMCID: PMC10804938
Review Free full text in Europe PMC
Crosstalk between autophagy and metabolic regulation of (CAR) T cells: therapeutic implications.
Front Immunol, 14:1212695, 22 Aug 2023
Cited by: 1 article | PMID: 37675121 | PMCID: PMC10477670
Review Free full text in Europe PMC
Go to all (107) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Homeostatic proliferation and survival of naïve and memory T cells.
Eur J Immunol, 39(8):2088-2094, 01 Aug 2009
Cited by: 156 articles | PMID: 19637200
Review
Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival.
J Leukoc Biol, 84(4):949-957, 24 Jun 2008
Cited by: 278 articles | PMID: 18577716 | PMCID: PMC2638731
Review Free full text in Europe PMC
The Ups and Downs of Metabolism during the Lifespan of a T Cell.
Int J Mol Sci, 21(21):E7972, 27 Oct 2020
Cited by: 17 articles | PMID: 33120978 | PMCID: PMC7663011
Review Free full text in Europe PMC
Molecular regulation of T lymphocyte homeostasis in the healthy and diseased immune system.
Immunol Res, 27(2-3):387-398, 01 Jan 2003
Cited by: 26 articles | PMID: 12857983
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: R01 CA123350-04
Grant ID: R01 CA123350
NIAID NIH HHS (1)
Grant ID: R01 AI063345





