Abstract
Free full text

Colloquium PaperRegenerative Medicine
Normal and leukemic hematopoiesis: Are leukemias a stem cell disorder or a reacquisition of stem cell characteristics?
Abstract
Leukemia can be viewed as a newly formed, abnormal hematopoietic tissue initiated by a few leukemic stem cells (LSCs) that undergo an aberrant and poorly regulated process of organogenesis analogous to that of normal hematopoietic stem cells. A hallmark of all cancers is the capacity for unlimited self-renewal, which is also a defining characteristic of normal stem cells. Given this shared attribute, it has been proposed that leukemias may be initiated by transforming events that take place in hematopoietic stem cells. Alternatively, leukemias may also arise from more committed progenitors caused by mutations and/or selective expression of genes that enhance their otherwise limited self-renewal capabilities. Identifying the LSCs for each type of leukemia is a current challenge and a critical step in understanding their respective biologies and may provide key insights into more effective treatments. Moreover, LSC identification and purification will provide a powerful diagnostic, prognostic, and therapeutic tool in the clinic.
In many respects a leukemic cell resembles a stem cell. Stem cells are defined as clonogenic cells capable of both self-renewal and multilineage differentiation. The cells from the hematopoietic system are continually generated from self-renewing progenitors in the bone marrow called hematopoietic stem cells (HSCs), or blood-forming stem cells, which have been isolated in both humans and mice (1-4). HSCs can be divided into a long-term subset (LT-HSC), capable of indefinite self-renewal, and a short-term subset (ST-HSC) that self-renew for a defined interval. HSCs give rise to nonself-renewing oligolineage progenitors, which in turn give rise to progeny that are more restricted in their differentiation potential, and finally to functionally mature cells. The phenotypic and functional properties of normal HSCs have been extensively reviewed (5-7). Recent studies have demonstrated that normal stem cells and cancer cells share the ability to self-renew and that many pathways classically associated with cancer also regulate normal stem cell development. For most cancers, the target cell of the transformation events is unknown, but evidence indicates that certain types of leukemias arise from mutations that accumulate in HSCs. Conversely, analyses performed on mouse models of certain types of human leukemias have demonstrated that restricted progenitors or even differentiated cells may also become transformed. In this review, we will discuss the notion of the leukemic stem cell (LSC), with emphasis on myeloid leukemias, and the potential origin of these cells: HSCs or committed progenitor cells that have reacquired stem cell characteristics, mainly the ability to self-renew. Finally, we will describe the research under way to elucidate the molecular pathways leading to leukemia.
HSCs
The first experimental evidence to indicate the existence of HSCs was the discovery in 1961 by Till and McCulloch (8) of a population of clonogenic bone marrow cells capable of generating myelo-erythroid colonies in the spleen of lethally irradiated hosts. Occasionally these colonies contained clonogenic cells that could be further retransplanted into secondary lethally irradiated hosts and reconstitute the immune system. These were proposed to be HSCs, i.e., progenitor cells with the essential characteristic of self-renewal and differentiation potential for all types of blood cells (1-4). The development of clonal assays for all major hematopoietic lineages together with the availability of multiparameter fluorescence-activated cell sorting (FACS) has enabled the prospective purification of HSCs from mice and to highly enrich for HSCs from humans according to the cell-surface expression of specific molecules and their functional read-out in vivo and in vitro in stromal long-term colony-initiating assays (5). After the identification and prospective isolation of murine HSCs, considerable progress has been made toward the characterization of the mechanisms controlling their fates. During or after cell division, the two daughter cells of a stem cell each have to decide their fate. They can either choose to remain as HSCs, commit to differentiation, or die by apoptosis and also to stay in the bone marrow or migrate to the periphery. These processes of cell-fate decisions must be finely tuned to maintain a steady-state level of functional HSCs in the bone marrow and to constantly provide progenitors for the various hematopoietic lineages.
Surface Markers. Several marker combinations, such as [Linneg/low, Thy1.1low, c-Kithigh, Sca-1+], [Lin-, Thy1.1low, Sca-1+, rhodamine 123low] (9) or [Lin-, CD34-/int, c-Kit+, Sca-1+] (4), have been used to isolate nearly pure mouse HSC populations. Similar marker combinations [Lin-, Thy1+, CD34+, CD38neg/low] (9) are used to highly enrich human HSC populations. Although there are some variations in the exact frequencies found, the different methods of isolation all indicate that HSCs are rare cells. Using the [Linneg/low, Thy1.1low, c-Kithigh, Sca-1+] markers, ≈1:5,000 mouse bone marrow cells have long-term, multilineage, repopulating capability, i.e., is a LT-HSC, whereas ≈1:1,000 has a more limited, short-term, multilineage repopulating capability, i.e., is a ST-HSC or a multipotent progenitor (MPP). Recently, the expression of the receptor tyrosine kinase Flk-2 has been identified as a reliable marker to discriminate between LT-HSC [Thy1.1low, Flk-2neg], ST-HSC [Thy1.1low, Flk-2+], and MPP [Thy1.1-, Flk-2+] in combination with the [Linneg/low, c-Kithigh, and Sca-1+] markers (10). Morphologically, HSCs and MPPs resemble lymphocytes.
Differentiation Potential. Whereas LT-HSCs self-renew for the life of the host, the derivative ST-HSCs retain self-renewal capacity for ≈8 weeks (2) and give rise to the briefly self-renewing MPPs (11), which then differentiate into oligolineage-restricted progenitors through functionally irreversible maturation steps (see Fig. 1). Two kinds of oligolineage-restricted progenitors have been identified so far in the mouse: the common lymphoid progenitors (CLPs), which at a clonal level are restricted to give rise to T lymphocytes, B lymphocytes, and natural killer cells (12), and the common myeloid progenitors (CMPs), which are progenitors for the myelo-erythroid lineages (13). CMPs give rise to myelomonocytic progenitors (GMPs), which in turn produce monocytes/macrophages and granulocytes, and to megakaryotic/erythroid progenitors, which differentiate into megakaryocytes/platelets and erythrocyte, but still maintain the potential for B cell lineage differentiation at an extremely low frequency (13). Interestingly, both CMPs and CLPs can give rise to dendritic cells (14, 15), suggesting the existence of alternative commitment pathways to the mutually exclusive developmental pathways for myeloid and lymphoid lineages. All of these progenitor populations are separable as pure populations by using cell surface markers and have been shown to be devoid of detectable self-renewal activity after transplantation (16).
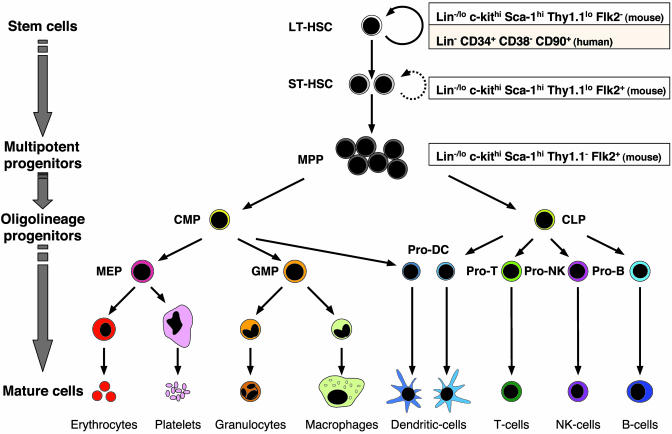
Hematopoietic and progenitor cell lineages. HSCs can be divided into LT-HSCs, highly self-renewing cells that reconstitute an animal for its entire life span, or ST-HSCs, which reconstitute the animal for a limited period. ST-HSCs differentiate into MPPs, which do not or briefly self-renew, and have the ability to differentiate into oligolineage-restricted progenitors that ultimately give rise to differentiated progeny through functionally irreversible maturation steps. The CLPs give rise to T lymphocytes, B lymphocytes, and natural killer (NK) cells. The CMPs give rise to GMPs, which then differentiate into monocytes/macrophages and granulocytes, and to megakaryotic/erythroid progenitors (MEP), which produce megakaryocytes/platelets and erythrocytes. Both CMPs and CLPs can give rise to dendritic cells. All of these stem and progenitor populations are separable as pure populations by using cell surface markers.
In parallel to the clarification of the developmental hierarchy between HSCs and committed progenitors, considerable progress has been made toward the identification of molecular mechanisms regulating lineage commitment within the hematopoietic system. Although it is largely beyond the scope of this review to describe these mechanisms in detail, they appear to represent a stepwise process characterized by the alternate expression of specific transcriptional regulators, growth factors, and growth factor receptors, whose combination determines lineage commitment and maturation (17, 18). With the recent use of DNA microarrays to investigate the gene expression profile of HSCs, progress has also been made toward the identification of the downstream effectors genes of the transcription factors (19, 20). Future gene expression profiling of defined HSCs and progenitor populations should rapidly advance our understanding of the molecular regulatory networks that control the development of all blood cells.
Proliferation, Apoptosis, and Self-Renewal. As HSCs mature from the long-term self-renewing pool to MPPs, they progressively lose their potential to self-renew but become more mitotically active. In young mice, the frequency of HSCs in hematopoietic tissues is relatively constant (21-23) and HSCs have long been considered to be a resting cell population, with only a few stem cells contributing to steady-state hematopoiesis. In fact, recent studies have shown that in young adult mice ≈8-10% of LT-HSCs randomly enter the cell cycle per day, with all HSCs entering the cell cycle in 1-3 months (24, 25). Although the rate at which human HSCs enter the cell cycle is currently unknown, assuming a comparable rate as in the mouse would result in a very large number of cell divisions over a lifetime and agree with the largest estimates of the number of cell divisions that an HSC can undergo (26).
A requirement for continuously dividing and self-renewing cells, such as HSCs and tumor cells, is the ability to avoid fatal telomere shortening through the action of the telomerase complex (27, 28). In mice, LT-HSCs contain as much telomerase activity as cancer cells do, whereas ST-HSCs and MPPs have significantly less (29). Enriched human stem/progenitor cell populations show telomere shortening with age (27), as do mouse LT-HSCs that undergo many divisions during serial transplantation (30). These results are consistent with the hypothesis that telomere shortening may limit the replicative capacity of HSCs. Serial transplantation has often been taken as a measure of the replicative life span of hematopoietic cells. However, resting bone marrow HSCs that are in the S/G2/M phases of the cell cycle transplant less well than HSCs with 2 M DNA content (31), although they are fully active as LT-HSCs. Furthermore, after transplantation, a much higher frequency of HSCs remain in the cell cycle for several months (23, 31), and whether HSCs have long or short telomeres, they are lost by the fifth serial transplantation generation (32). Therefore, serial transplantation of HSCs may create artefacts that renders it as an inappropriate measure of HSC intrinsic life span.
The number of HSCs, as with many other cell types, is also regulated by programmed cell death or apoptosis (33). Preventing apoptosis by transgenic expression of the antiapoptotic protein Bcl-2 in LT-HSCs has been shown to cause a gradual increase in LT-HSC frequency, which takes place without malignant transformation and despite Bcl-2-mediated decrease of LT-HSC entry into the cell cycle. Different mouse strains also show differences in the number and cell cycle status of HSCs, perhaps indicating differences in susceptibility to apoptosis. Several chromosomal regions have been shown to contribute to these polymorphisms (34, 35), but to date few of these regulator genes have been isolated (36).
HSCs reside predominantly in the bone marrow but low numbers of HSCs are also found in peripheral blood (37). Recent work has shown that HSCs rapidly and constitutively migrate through the blood and play a physiological role in functional reengraftment of unconditioned bone marrow (38). In vivo, LT-HSCs respond to a variety of conditions by entering the cell cycle, expanding their numbers by self-renewing proliferation, and mobilizing into the bloodstream (39). These conditions include myelosuppresion with chemotherapeutic agents such as cyclophosphamide and treatment with the cytokines granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (40, 41). In vitro, several growth factors have been identified to induce a potent proliferation of HSCs but have been unsuccessful in preventing their diffentiation or apoptosis in long-term cultures. Although progress has been made in identifying culture conditions that maintain some of the HSC functions in vitro (42), it has not been possible to identify a combination of growth factors able to expand the number of HSCs in culture with retention of their transplantable activity. Mouse embryonic stem cells can continuously proliferate, without undergoing terminal differentiation, in the presence of leukemia inhibitory factor, which acts, at least in part, via activation of the transcription factor STAT3, an essential mechanism for self-renewal activity in embryonic stem cells (43). It therefore seems likely that cytokines and/or cell-surface molecules expressed by bone marrow stromal cells (44) are necessary for maintaining the self-renewal activity of HSCs.
At the molecular level, the mechanisms controlling HSC self-renewal activity are still poorly understood. Interestingly, the signaling pathways that have been shown to date to be involved in the regulation of HSC self-renewal, i.e., Hox genes and Notch, Sonic hedgehog (Shh), and Wnt signaling pathways, are also hypothesized to be associated with oncogenesis (45-48). Expression of some of the Hox genes, such as HoxB4 and HoxA9, has been intimately tied to HSC self-renewal, because their overexpression in hematopoietic cells causes the selective expansion of the HSC population both in vitro and in vivo (49, 50). Activation of Notch by its ligand Jagged-1 in cultured HSCs also results in an increased amount of primitive progenitor activity, both in vitro and after transplantation in vivo, indicating that Notch activation promotes HSC self-renewal (51, 52). Similarly, HSC-enriched [Lin-, CD34+, CD38-] human cells exhibit increased self-renewal capability in response to Shh stimulation in vitro, indicating that the Shh pathway also plays a role in regulating self-renewal of HSCs as of other types of tissue stem cells (53). Recently, the different components of the Wnt signaling pathway have been elucidated (54). The Wnt proteins usually act by binding to cell-surface Frizzled receptors and activating the dishevelled (Dsh) gene product. Active Dsh inhibits the association of glycogene synthase kinase 3β and axin with β-catenin, leading to reduced phosphorylation and nuclear translocation of β-catenin. Nuclear β-catenin associates with the LEF/TCF family of transcription factors to stimulate transcription of downstream target genes. The expression of Wnt proteins in the bone marrow (55) suggests that they may play a role in hematopoiesis. Furthermore, retroviral transduction of constitutively activated β-catenin into HSCs leads to their expansion in vitro, and retroviral transduction of the Wnt pathway inhibitor axin leads to inhibition of HSC proliferation, increased HSC death in vitro, and reduced reconstitution in vivo (56). Soluble partially purified Wnt proteins obtained from conditioned supernatants have also been shown to influence the proliferation of CD34+ hematopoietic progenitors isolated from mouse fetal livers and human bone marrow (57, 58). Recently, purified Wnt3A has been shown to act on highly purified LT-HSCs to cause up to a 300-fold expansion of LT-HSCs identified both phenotypically and functionally (56, 59). The molecular mechanism by which Wnt signaling influences HSCs remains to be elucidated, as does the question of whether the Wnt, Notch, and Shh pathways interact to regulate stem and progenitor cell self-renewal.
LSCs
Emerging evidence from stem cell biology has recently provided new insights into cancer biology by emphasizing the relationship between stem cells and tumor cells and by formalizing the notion that tumors might contain some cancer stem cells, which are rare cells with indefinite proliferation potential that drive the formation and growth of tumors (47). Leukemias are blood cancers, and the hematopoietic system is one of the best tissues to study the notion of the cancer or LSCs, because normal HSCs are a well-characterized cell population and LSCs have recently been revealed (see below). In the present review we focus on myeloid leukemias (60-62). The current paradigm of myeloid leukemias states that the process giving rise to the aggressive and often fatal acute myeloid leukemias (AMLs) takes place through an ordered progression from the indolent myelodysplastic syndromes or myeloproliferative disorders to the more aggressive chronic myeloid leukemias (CMLs) that, if not inherently fatal, ultimately progress to the blast crisis of AML. Many different translocations and genetic aberrations are found within the various forms of myeloid leukemia and specific translocations are often associated with disease subtypes that manifest themselves through the accumulation of immature myeloid cells at varying stages of differentiation (60, 61).
The Concept of LSCs. Since the early 1970s, the notion of tumorigenic LSCs has emerged based on several studies showing that only a small subset of leukemic cells was capable of extensive proliferation in vitro and in vivo. Using ascites-derived mouse myeloma cells, separated first from normal hematopoietic cells, Park and coworkers (63) showed that only 1 in 10,000 to 1 in 100 leukemic cells were able to form colonies in vitro in clonal colony-forming assays. In addition, only 1-4% of the total number of leukemic cells transplanted in vivo could form spleen colonies, even using different types of leukemic cells (64, 65). Because the clonogenic read-out of the leukemic cells perfectly mirrored the difference in clonogenicity among the normal hematopoietic cells, the clonogenic leukemic cells were described as LSCs. However, not until 1997, in studies published by Blair and colleagues (66) and Bonnet and Dick (67), was there a clear demonstration that most of the leukemic cells were unable to proliferate extensively and only a small, defined subset of cells was consistently clonogenic. In these studies, LSCs for human AML were identified prospectively and purified as [Thy1-, CD34+, CD38-] cells from various patient samples. Although these cells represent a small and variable proportion of the totality of the AML cells (0.2-1% depending on the patient), they were the only cells capable of transferring AML from human patient to nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice and were referred as SCID leukemia-initiating cells or SL-IC. The general concept of LSCs is now well established and serves as a reference for the identification of other cancer stem cells in solid tumors (47, 68).
A given leukemia can be viewed as a newly formed aberrant hematopoietic tissue initiated by tumorigenic leukemic cells that have kept or reacquired the capacity for indefinite proliferation through accumulated mutations. This concept suggests that leukemias are produced by a few LSCs that undergo an aberrant and poorly regulated process of organogenesis analogous to that of normal HSCs. Both cell types have extensive proliferative potential and the ability to give rise to new hematopoietic tissues, normal in the first case and abnormal in the second. Both normal and leukemic tissues are composed of a combination of heterogeneous cells, with different phenotypic characteristics and proliferative potentials. Because most leukemias, similar to most solid tumors, have a clonal origin, tumorigenic leukemic cells must give rise to phenotypically diverse progeny including a few LSCs with indefinite proliferation potential, as well as cells within the leukemia that may have limited or no proliferative potential. Although some of the heterogeneity in the tumor arises as a result of continuing mutagenesis or epigenetic changes, it is likely that it also arises from the aberrant differentiation of the cancer cells. The variable expression of myeloid markers by blast cells in CML further supports the notion that some of the heterogeneity within the leukemic cells results from the abnormal differentiation of malignant myeloid cells.
The Controversy About the Origin of LSCs. For most leukemia, as for most cancers, the target cell of transforming mutations is still unknown. Because normal stem cells and LSCs share the ability to self-renew, as well as various developmental pathways, it has been postulated that LSCs are HSCs that have become leukemic as the result of accumulated mutations. Because normal stem cells and LSCs share the ability to self-renew, as well as various developmental pathways, it is possible that LSCs are HSCs that have become leukemic as the result of accumulated mutations. HSCs have the machinery for self-renewal already activated and therefore may require fewer mutations to maintain it than more differentiated cells would require to activate it ectopically. HSCs also persist throughout life and therefore have much greater opportunities to accumulate mutations than more mature cells, which persist only for a short period. Conversely, LSCs could also be a more restricted progenitor or even a differentiated mature cell, which would have first to reacquire the stem cell capability for self-renewal before becoming tumorigenic to accumulate additional mutations. Restricted progenitors could therefore potentially be transformed either by acquiring mutations that cause them to self-renew like stem cells, or HSCs themselves could be accumulating genetic and epigenetic changes, such as loss of programmed cell death, ultimately leading to the gain of self-renewal activity in a restricted progenitor population (see Fig. 2).
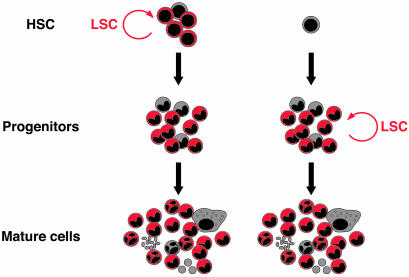
Origin of the LSC. A given leukemia can be viewed as a newly formed abnormal hematopoietic tissue initiated by a few LSCs that undergo an aberrant and poorly regulated process of organogenesis analogous to that of normal HSCs. LSCs can either be HSCs, which have become leukemic as the result of accumulated mutations, or more restricted progenitors, which have reacquired the stem cell capability of self-renewal. Regardless of their origin, both types of LSCs give rise to similar end-stage leukemias.
HSCs as LSCs. There is now evidence that certain subtypes of human AML and CML arise from mutations that accumulate in HSCs. For most AML subtypes, except for the M3 acute promyelocytic leukemia (APML) subtype, the only cells capable of transplanting AML in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice have a [CD34+, CD38-] phenotype, similar to that of normal HSC, whereas the [CD34+, CD38+] leukemic blast cells cannot transfer the disease to mice (66, 67). These observations suggest that for most AML subtypes HSCs rather than committed progenitors are the target for leukemic transformation, regardless of the extent and the lineage of differentiation of the leukemic cells present in the peripheral circulation. The t(8,21) translocation is one of the most frequent chromosomal abnormalities associated with AML (≈15%) (69). This translocation involves the AML-1 gene (AML1 also known as CBFA2, PEBP2αB, and RUNX1) on chromosome 21 and the ETO (MTG8) gene on chromosome 8 and generates an AML1-ETO fusion transcription factor (70). Expression of the AML1-ETO fusion transcript can be detected in leukemic blast cells but also in normal bone marrow cells, including HSCs, obtained from AML patients in remission (71, 72). However, these prospectively isolated AML1-ETO-expressing stem cells and their progeny are not leukemic and could differentiate into normal myelo-erythroid cells in vitro (71). This observation suggests that the translocation occurred originally in normal HSCs and that additional mutation in a subset of these HSCs or their progeny subsequently led to leukemia. Because the original AML1-ETO-expressing normal HSC were [Lin-, CD34+, CD38-, Thy-1+] and the LSC were [Lin-, CD34+, CD38-, Thy-1-], the subsequent transforming mutation might have occurred either in downstream Thy-1 negative progenitors or in HSCs that have lost Thy-1 expression as one of the first consequences of the neoplastic transformation (71, 72). This observation is further supported by the finding of Blair and colleagues (66) that the severe combined immunodeficient leukemia-initiating cells from human AML samples were also Thy-1 (CD90) negative.
Approximately 95% of CML patients possess the Philadelphia (Ph) chromosome, a shortened chromosome 22 arising from a reciprocal translocation t(9q34;22q11), which serves as a cytogenetic hallmark of the disease, and contains the BCR-ABL fusion gene, which produces the 210-kDa BCR-ABL chimeric protein (73). This fusion protein can be found in myeloid, erythroid, B lymphoid, and occasionally T lymphoid cells in the majority of CML patients, suggesting that the original translocation takes place in LT-HSCs. This finding was consistent with the early demonstration, by analysis of allelic X chromosome enzyme isoforms, that CML cells were part of a larger clone that included red blood cells and B cells (74). More recently, the BCR-ABL gene could be detected in endothelial cells produced from cells of a CML patient (75). If confirmed as not the result of cell fusion, these data might place the level of the BCR-ABL mutation in a cell type even earlier than the LT-HSCs, such as the putative hemangioblast cells, a very primitive cell population with both hematopoietic and endothelial differentiation potential that remains to be identified. In chronic phase, the leukemic clone appears to be maintained by a small number of BCRABL-positive [CD34+, CD38-] cells, a population enriched for HSCs, which differentiate normally and amplify slowly. In contrast, as these cells enter the intermediate stages of lineage restriction, their progeny are selectively expanded and generate an enlarged pool of overproduced [CD34-] progeny. Recent analyses of purified subsets of primitive CML cells have provided a coherent explanation for this dichotomous behavior of BCRABL-positive stem and progenitor cells based on the discovery of an unusual autocrine IL-3/granulocyte colony-stimulating factor mechanism (62). This mechanism only partially counteracts in vivo signals that maintain normal HSCs in a quiescent state but, when active in BCR-ABL-positive HSCs, promotes their differentiation in favor of their self-renewal. In more differentiated CML progenitors, the same mechanism has a more potent mitogenic effect that is then extinguished when the cells enter the terminal stages of differentiation. Thus, further expansion of the leukemic clone is limited until additional mutations are acquired that will further alter the regulatory mechanisms still operative in the chronic phase cells. These observations strongly argue for the necessity of additional somatic mutations or epigenetic events in HSCs or myeloid progenitors for the development of fully malignant diseases.
Committed Progenitors as LSCs. Although HSCs are often the target of genetic events leading to malignant transformations, committed progenitors or even differentiated cells may also become transformed. In APML patient samples, the M3 subtype of AML, it has been shown that the APML-associated fusion gene PML/retinoic acid receptor α (RARα), which results from the t(15,17) balanced reciprocal translocation, was present in [CD34-CD38+] cell populations but not in [CD34+CD38-] HSC-enriched cell populations (76). This observation suggests that in APML the transformation process may involve a more differentiated cell type than HSCs and/or pluripotent progenitors that have been implicated in the other AML subtypes (66, 67).
Further evidence indicating that cells devoid of self-renewing activity, such as committed progenitors and mature cells, can also be the target cells for leukemic events came from analyses of leukemia-associated genes in the mouse. The development of transgenic and knockout technologies has led to the creation of a wide variety of physiological disease models of human leukemias, and the promoter elements of several myeloid-specific human genes have been used to target transgene expression specifically to committed myeloid cells. The MRP8 gene encodes a small calcium-binding protein of the S100 family, which is only expressed in neutrophils, monocytes, and their immediate progenitors, CMPs and GMPs, but not in HSCs (77). The use of the human MRP8 (hMRP8) promoter has allowed the generation of several accurate mouse models of human leukemia. Transgenic mice expressing BCR-ABL from this promoter develop a CML-like disease (78), and mice similarly expressing the fusion gene PML/RARα exhibit a preleukemic state, which eventually progresses to APML, similar to human patients with the PML/RARα translocation (79). Transgenic mice expressing the fusion protein AML1-ETO from the MRP8 promoter also develop AML with high frequency, but only after mutagenesis treatment, indicating the strong requirement of AML1-ETO for additional mutations (80). The promoter regions of other myeloid-specific genes have also been used to drive transgene expression in the myeloid lineage, including the CD11b promoter (81), which is exclusively expressed in differentiating and mature monocytes and granulocytes, and the human cathepsin G (hCG) promoter (82), which is expressed in myeloid progenitors but not in mature myeloid cells. The failure of PML/RARα to cause leukemia when expressed from the CD11b promoter during late myeloid differentiation, and the differences in the phenotypes resulting from hCG- and hMRP8-directed PML/RARα expression most likely reflect the difference in the expression profile given by these two promoters and reveal the importance of the developmental stage of the cells targeted by the transgene promoter for the leukemogenic effect of the leukemic-associated fusion gene (83). A recent study using retroviral transduction of highly purified HSCs, CMPs, and GMPs also has shown that the potent leukemic-fusion gene MLL-ENL, which results from the t(11,19) translocation and has been involved in a large subset of AML in childhood (84), can induce the exact same AML not only from totipotent HSCs, but also from restricted CMPs and GMPs (unpublished results). Altogether, these different models demonstrate that myeloid leukemias can also arise from committed progenitors, because leukemic fusion proteins such as BCRABL, PML/RARα, and MLL-ENL, which were previously thought to act only in totipotent HSCs, can be transforming at the level of myeloid progenitors and are able to give rise to leukemia without HSC involvement. However, in the case of spontaneously arising human leukemia, it seems likely that HSCs accumulate the mutations that are necessary for neoplastic transformation, but that these genetic mutations exert their effects in committed progenitors, leading to the generation of LSCs downstream of the HSCs.
Deconstructing the Molecular Pathways Leading to Leukemia Transgenic mouse models have been used to deconstruct in vivo the molecular pathways leading to leukemic transformation, similar to what has been done in normal human epithelial and fibroblast cells (85). The development of cancer is a stepwise process in which increasing numbers of somatic mutations give rise to an increasingly transformed clonal population of cells (86). The multistep model of carcinogenesis was originally postulated to require a clonal event causing increased proliferation, which, together with mutations blocking cellular differentiation, synergizes to cause transformation. More recently, protooncogenes that either suppress or promote programmed cell death, or apoptosis, have been shown to play critical roles in oncogenesis, as well as in regulating hematopoiesis (87, 88). Although genetic rearrangements and leukemic-associated fusion genes have a critical function by interfering with the hematopoietic differentiation programs and thereby dictating the nature of the leukemia, they require additional cooperative mutations to induce fully malignant diseases (see Fig. 3).
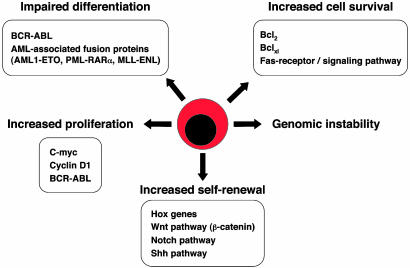
Deregulated pathways leading to leukemia. Although leukemias are heterogeneous in terms of phenotypes, there are general mechanisms underlying leukemic transformation such as increased cell survival, increased proliferation capacity, increased self-renewal capacity, genomic instability, and prevention of differentiation. Examples of such deregulated mechanisms and/or signaling pathways that have been found in various types of leukemias are indicated.
Differentiation and Leukemia. Leukemia-associated fusion proteins generally function as aberrantly activated signaling mechanisms or transcriptional regulators that directly interfere with the hematopoietic differentiation program (18). The 210-kDa BCR-ABL fusion protein differs from the normal 145-kDa c-ABL in its preferential location in the cytoplasm and its constitutively elevated tyrosine kinase activity. Structure-function analyses have shown that both of these properties are critical for the transforming activity of BCR-ABL (73). Concerning the vast collection of AML-associated fusion proteins, one of the two components of each fusion protein is generally a transcription factor (AML1, CBFβ, or RARα) whereas the other partner is more variable in function, but is often involved in the control of cell survival and apoptosis such as the nuclear structure protein PML (89). Moreover, AML-associated fusion proteins have been shown to affect hematopoietic differentiation in a variety of experimental models, and the specific stage of myeloid maturation arrest appears to direct dependent on the nature of the fusion protein expressed. The abnormal network of transcriptional regulation induced by these leukemia-associated fusion genes seems to occur through common mechanisms, including recruitment of aberrant corepressor complexes, alteration in chromatin remodeling, and disruption of specific sub-nuclear compartments (89, 90).
Programmed Cell Death and Leukemia. The Bcl-2 family members, such as Bcl-2, Bcl-xl, Mcl-1, and A1, function as cell death antagonists against a wide array of apoptotic stimuli, whereas their binding partners, like Bax, Bad, and Bak, promote apoptosis (87). Thus, gain-of-function mutations in Bcl-2 family members or loss-of-function mutations in Bax family members would be expected to predispose toward cancer. Bcl-2 was in fact discovered upon characterization of a t(14,18) chromosomal translocation found in follicular lymphoma. Although the role of Bcl-2 in leukemogenesis has been extensively confirmed in lymphoid leukemia, deregulation of Bcl-2 family members also appears to play an important role for the transformation of myeloid cells. The fusion protein AML1-ETO was shown to directly up-regulate expression of Bcl-2 by binding to its promoter elements (91). In fact, cells from most human AML have been found to express Bcl-2 at much higher levels than their normal counterparts (92). Furthermore, activating mutations by retroviral integration in the murine Bcl-xl gene have been reported for both myeloid and T cell leukemias (93). Together, these results suggest that activating mutations in Bcl-2 gene family members may be critical events in the multistep myeloid transformation process. Hence, the increased survival provided by enforced Bcl-2 family member expression may allow sufficient time for the acquisition of additional oncogenic mutations, a mechanism thought to underlie the transition from chronic to acute leukemia (94). Whereas the deregulation of Bcl-2 expression is found in many human cancers, overexpression of Bcl-2 in a transgenic mouse model has been found to be relatively benign in terms of cellular transformation (95). Hence, enforced expression of Bcl-2 in the myeloid lineage with the hMRP8 promoter leads to a disease that is similar to human chronic myelomonocytic leukemia, including monocytosis, splenomegaly and neutropenia as the mice age (96). However, despite decreased survival compared with littermates, these mice rarely progress to acute leukemia and they require additional mutations to promote AML, as has been shown for lymphoid leukemias (97).
Although loss-of-function mutations in the Fas receptor (CD95) or Fas signaling pathway have historically been associated with lymphoid hyperplasia and autoimmunity (98, 99), several clinical reports have recently implicated similar mutations in various human leukemias (100, 101). Granulocytes, and their myeloblastic progenitors, are known to express high levels of Fas (102), and several patients with granulocytic leukemias have been shown to have blasts with functional deficiencies in the Fas signaling pathway (103). Interestingly, intercrosses between hMRP8Bcl-2 and Fas-deficient Faslpr/lpr mice lead to the development of AML in 15% of the Faslpr/lprhMRP8Bcl-2 mice, with an expansion of myeloblasts in all hematopoietic tissues and substantially lower numbers of granulocytes in the bone marrow and blood (96). These results indicate that Bcl-2 and the Fas receptor regulate two distinct apoptosis pathways in the myeloid lineage and that alteration of both of them seems to be required for transformation of myeloblasts in both mouse and human. Furthermore, increased survival provided by enforced Bcl-2 expression greatly increases the incidence of CML-like disease in hMRP8BCR-ABLhMRP8Bcl-2 double transgenic mice (78), as well as the incidence of APML development in hMRP8PML/RARα hMRP8Bcl-2 double transgenic mice (104). Altogether, these observations demonstrate that prevention of cell death is one of the crucial events in myeloid leukemogenesis and may even be the first step that sets the stage for additional mutations.
Self-Renewal and Leukemia. Leukemic cells absolutely require self-renewal capability to propagate the disease. Self-renewal is the default pathway in virtually all single-celled organisms, and regulation of self-renewal versus differentiation or death is a strict requirement for organisms that have tissue specialization. Perhaps the reason not all of the Faslpr/lprhMRP8Bcl-2 mice progress to AML is that the leukemic cells acquire an additional mutation that causes deregulated self-renewal. Current research focuses on gain-of-function mutations that promote constitutive self-renewal, such as stabilization of β-catenin. Stabilized β-catenin has been shown to promote the self-renewal of stem cells and other types of progenitor cells (47, 105), and activation of β-catenin and deregulation of the Wnt signaling pathway is a common phenomenon in cancer (106). Mutations in other signaling pathways that promote progenitor self-renewal, such as Notch and Shh, are also likely to contribute to unregulated self-renewal of leukemic cells and should be further studied.
Although much work is being done to identify the transcription factors and the signal transduction pathways involved in HSC cell-fate decisions and self-renewal regulation, an alternative option is to envisage that self-renewal is a default pathway that can occur only to the extent that death and differentiation are prevented. In that view, it is interesting that the main effect of leukemia-inducing genes is to prevent apoptosis and/or to block cell differentiation. One simplistic view is therefore to assume that whether the target cell of the transformation event is an HSC or a restricted progenitor, the impairment of these cellular pathways will produce self-renewing LSCs. This question has recently been addressed in the study performed by Cozzio and colleagues (unpublished results), which showed that retroviral transduction of HSCs, CMPs, or GMPs by MLL-ENL induced the exact same AML. This result raises several interesting questions: either MLL-ENL interferes at a crucial point in the regulation of the normal self-renewal program, which is currently unknown, leading to leukemic expansion of any of the transduced cells, or MLL-ENL, through oncogenic mechanisms such as a block in apoptosis and/or differentiation, induces leukemic self-renewal in any of the transduced cells. Future use of well-defined highly purified cells as starting populations for leukemogenic transformation will allow more precise transcriptional analysis of these early molecular events by RT-PCR or microarray analysis and provide a deeper understanding of the molecular basis of deregulated self-renewal in leukemic cells.
Conclusions
Emerging evidence indicates that leukemias are initiated by a few LSCs that are heterogeneous in terms of their origin. They can either be HSCs that have become leukemic as the result of accumulated mutations or more restricted progenitors that have reacquired the stem cell capability for self-renewal. Identifying the LSCs for each given leukemia is therefore needed to fully understand their specific biology. Leukemias are also heterogeneous in terms of phenotype, disease progression, prognosis, and response to therapy, but there are general mechanisms underlying leukemic transformation that are starting to be well understood. Future investigation of such deregulated mechanisms in the newly identified LSCs will lead to a considerable increase in our understanding of the molecular mechanisms and signaling pathways that are affected in a given type of leukemia and are likely to provide key insights into more efficient drug design and therapy.
Perspectives
LSC identification and prospective isolation would facilitate the study of gene expression profiles of malignant progenitors compared with their normal counterparts by using microarray technology. The recent development of lentiviral vectors for rapid and efficient transduction of highly purified populations of cells, including the nonproliferating HSCs, will allow efficient testing of candidate genes obtained from the microarray investigations for their roles in leukemogenesis. The identification of genes expressed in LSCs should therefore lead to novel therapies directed toward gene products expressed in LSCs but not in HSCs. Purified LSCs will also provide a powerful substrate for preclinical testing of the efficacy of newly developed chemotherapeutic agents, immune-based therapies, and biologic response modifiers.
The diagnostic evaluation of leukemia currently depends on morphologic and flow cytometric determination of the blast count, which in the light of LSCs being responsible for the propagation of the leukemia, may represent an inaccurate assessment of the disease status. If phenotypic markers were available that facilitated the rapid identification of LSCs within bone marrow or peripheral blood via flow cytometry, then clinicians could more adequately assess the disease status and respond with the most appropriate treatment, including chemotherapy, blood or bone marrow transplant, biologic response modifier therapy, or molecularly targeted therapies. LSC phenotypic identification may also facilitate early diagnosis of disease relapse postchemotherapy or hematopoietic cell transplants and could allow the elimination of LSC contamination from hematopoietic cell transplants with cell sorting technologies.
Acknowledgments
We are grateful to Thomas Serwold for helpful comments and critical reading of this manuscript. E.P. is a fellow of the Jose Carreras Leukemia Foundation, L.E.A. is a fellow of the Canadian Institutes of Health Research, and C.H.M.J. is supported by a Yu-Bechmann fellowship for Genomics and Oncology at the Center for Clinical Immunology at Stanford. This work was supported by the National Institutes of Health Grants CA55209 and CA86017 and a De Villier Award from the Leukemia and Lymphoma Society (to I.L.W.).
Notes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Regenerative Medicine,” held October 18-22, 2002, at the Arnold and Mabel Beckman Center of the National Academies of Science and Engineering in Irvine, CA.
Abbreviations: HSC, hematopoietic stem cell; LT-HSC, long-term HSC; ST-HSC, short-term HSC; MPP, multipotent progenitor; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMP, myelomonocytic progenitor; LSC, leukemic stem cell; CML, chronic myeloid leukemia; AML, acute myeloid leukemia; PML, promyelocytic leukemia; APML, acute PML; Shh, Sonic hedgehog; RARα, retinoic acid receptor α.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.2034201100
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc304096?pdf=render
Citations & impact
Impact metrics
Article citations
Integrins in cancer stem cells.
Front Cell Dev Biol, 12:1434378, 21 Aug 2024
Cited by: 0 articles | PMID: 39239559 | PMCID: PMC11375753
Review Free full text in Europe PMC
Metabolic dependencies of acute myeloid leukemia stem cells.
Int J Hematol, 120(4):427-438, 15 May 2024
Cited by: 0 articles | PMID: 38750343
Review
Bahcc1 is critical for the aberrant epigenetic program in a mouse model of MLL-ENL-mediated leukemia.
Blood Adv, 8(9):2193-2206, 01 May 2024
Cited by: 0 articles | PMID: 38452334 | PMCID: PMC11061229
Single nucleotide polymorphisms conferring susceptibility to leukemia and oral mucositis: a multi-center pilot study of patients prior to conditioning therapy for hematopoietic cell transplant.
Support Care Cancer, 32(4):220, 11 Mar 2024
Cited by: 0 articles | PMID: 38467943
Subverting the Canon: Novel Cancer-Promoting Functions and Mechanisms for snoRNAs.
Int J Mol Sci, 25(5):2923, 02 Mar 2024
Cited by: 0 articles | PMID: 38474168 | PMCID: PMC10932220
Review Free full text in Europe PMC
Go to all (351) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Leukemic stem cells: where do they come from?
Stem Cell Rev, 1(3):181-188, 01 Jan 2005
Cited by: 30 articles | PMID: 17142854
Review
Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9.
Nature, 442(7104):818-822, 16 Jul 2006
Cited by: 968 articles | PMID: 16862118
Metabolism and the leukemic stem cell.
J Exp Med, 207(4):677-680, 05 Apr 2010
Cited by: 58 articles | PMID: 20368582 | PMCID: PMC2856035
A STAT5B-CD9 axis determines self-renewal in hematopoietic and leukemic stem cells.
Blood, 138(23):2347-2359, 01 Dec 2021
Cited by: 18 articles | PMID: 34320169 | PMCID: PMC8777465
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: R01 CA086017
Grant ID: CA55209
Grant ID: CA86017





