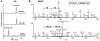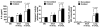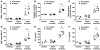| PMC full text: | Published online 2011 Feb 1. doi: 10.1172/JCI43004
|
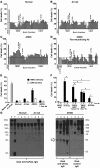
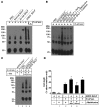
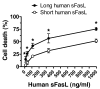
Figure 10
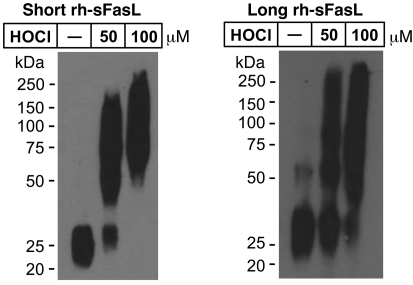
Oxidation-mediated multimerization of short and long sFasL.
Western blot analyses of short and long rh-sFasL incubated in PBS alone or in the presence of 2 different concentrations of HOCl (μM). After 15 minutes, the reaction was terminated with 2.5 nM l-methionine. Both short and long human sFasL have the same aggregation status in basal conditions. Following oxidation with HOCl, short and long rh-sFasL showed similar multimerization (50-kDa and larger molecular weight bands). Western blot analyses were performed using SDS-PAGE with reducing conditions (2-ME and heat).