Abstract
Unlabelled
Acetaminophen (APAP) hepatotoxicity is the most frequent cause of acute liver failure in many countries. The mechanism of cell death is initiated by formation of a reactive metabolite that binds to mitochondrial proteins and promotes mitochondrial dysfunction and oxidant stress. Manganese superoxide dismutase (SOD2) is a critical defense enzyme located in the mitochondrial matrix. The objective of this investigation was to evaluate the functional consequences of partial SOD2-deficiency (SOD2+/-) on intracellular signaling mechanisms of necrotic cell death after APAP overdose. Treatment of C57Bl/6J wild type animals with 200mg/kg APAP resulted in liver injury as indicated by elevated plasma alanine aminotransferase activities (2870±180U/L) and centrilobular necrosis at 6h. In addition, increased tissue glutathione disulfide (GSSG) levels and GSSG-to-GSH ratios, delayed mitochondrial GSH recovery, and increased mitochondrial protein carbonyls and nitrotyrosine protein adducts indicated mitochondrial oxidant stress. In addition, nuclear DNA fragmentation (TUNEL assay) correlated with translocation of Bax to the mitochondria and release of apoptosis-inducing factor (AIF). Furthermore, activation of c-jun-N-terminal kinase (JNK) was documented by the mitochondrial translocation of phospho-JNK. SOD2+/- mice showed 4-fold higher ALT activities and necrosis, an enhancement of all parameters of the mitochondrial oxidant stress, more AIF release and more extensive DNA fragmentation and more prolonged JNK activation.Conclusions
the impaired defense against mitochondrial superoxide formation in SOD2+/- mice prolongs JNK activation after APAP overdose and consequently further enhances the mitochondrial oxidant stress leading to exaggerated mitochondrial dysfunction, release of intermembrane proteins with nuclear DNA fragmentation and more necrosis.Free full text

THE IMPACT OF PARTIAL MANGANESE SUPEROXIDE DISMUTASE (SOD2)-DEFICIENCY ON MITOCHONDRIAL OXIDANT STRESS, DNA FRAGMENTATION AND LIVER INJURY DURING ACETAMINOPHEN HEPATOTOXICITY
Abstract
Acetaminophen (APAP) hepatotoxicity is the most frequent cause of acute liver failure in many countries. The mechanism of cell death is initiated by formation of a reactive metabolite that binds to mitochondrial proteins and promotes mitochondrial dysfunction and oxidant stress. Manganese superoxide dismutase (SOD2) is a critical defense enzyme located in the mitochondrial matrix. The objective of this investigation was to evaluate the functional consequences of partial SOD2-deficiency (SOD2+/−) on intracellular signaling mechanisms of necrotic cell death after APAP overdose. Treatment of C57Bl/6J wild type animals with 200 mg/kg APAP resulted in liver injury as indicated by elevated plasma alanine aminotransferase activities (2870±180 U/L) and centrilobular necrosis at 6h. In addition, increased tissue glutathione disulfide (GSSG) levels and GSSG-to-GSH ratios, delayed mitochondrial GSH recovery, and increased mitochondrial protein carbonyls and nitrotyrosine protein adducts indicated mitochondrial oxidant stress. In addition, nuclear DNA fragmentation (TUNEL assay) correlated with translocation of Bax to the mitochondria and release of apoptosis-inducing factor (AIF). Furthermore, activation of c-jun-N-terminal kinase (JNK) was documented by the mitochondrial translocation of phospho-JNK. SOD2+/− mice showed 4-fold higher ALT activities and necrosis, enhancement of all parameters of the mitochondrial oxidant stress, more AIF release and more extensive DNA fragmentation and more prolonged JNK activation. Conclusions: The impaired defense against mitochondrial superoxide formation in SOD2+/− mice prolongs JNK activation after APAP overdose and consequently further enhances the mitochondrial oxidant stress leading to exaggerated mitochondrial dysfunction, release of intermembrane proteins with nuclear DNA fragmentation and more necrosis.
INTRODUCTION
Acetaminophen (APAP) is a widely used analgesic and antipyretic drug, which is safe at therapeutic doses. An overdose, however, can trigger liver injury and even liver failure in animals and humans (Larson et al., 2005). Liver injury depends on the formation of a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which initially can be detoxified by conjugation with glutathione (Mitchell et al., 1973; Nelson, 1990) but eventually binds to cellular proteins (Jollow et al., 1973; Cohen et al., 1997). However, it appears that not random binding to cellular proteins but binding to mitochondrial proteins is a critical early event in the pathophysiology (Tirmenstein and Nelson, 1989; Qiu et al., 2001). This triggers mitochondrial dysfunction as indicated by inhibition of respiration and declining ATP levels (Meyers et al., 1988; Jaeschke, 1990; Tirmenstein and Nelson, 1990). Most importantly, there is increased formation of reactive oxygen (Jaeschke 1990; Tirmenstein and Nelson, 1990; Knight et al., 2001) and peroxynitrite (Cover et al., 2005) inside the mitochondria. Because of the extensive depletion of cytosolic and mitochondrial glutathione levels during the early phase of APAP metabolism (Knight et al., 2001), the detoxification of these reactive oxygen and reactive nitrogen species is severely impaired leading to activation of c-jun-N-terminal kinase (JNK) (Hanawa et al., 2008; Saito et al., 2010a), mitochondrial DNA damage (Cover et al., 2005), and the mitochondrial membrane permeability transition (MPT) pore opening with collapse of the mitochondrial membrane potential (Kon et al., 2004; Ramachandran et al., 2011). The characteristic nuclear DNA fragmentation (Ray et al., 1990) is caused by the nuclear translocation of mitochondrial intermembrane proteins such as apoptosis-inducing factor (AIF) and endonuclease G (Bajt et al., 2006). The release of the intermembrane proteins is facilitated initially by JNK-induced translocation of Bax to the mitochondria and formation of Bax pores (Gunawan et al., 2006; Jaeschke and Bajt, 2006; Bajt et al., 2008) but later is dominated by mitochondrial swelling and rupture of the outer membrane (Bajt et al., 2008). In addition, both JNK activation and the MPT further amplify the mitochondrial oxidant stress (Saito et al., 2010a, Ramachandran et al., 2011). The central role of this mitochondrial oxidant stress and peroxynitrite formation for hepatocyte cell death was shown by the profound protective effect of interventions that accelerate the recovery of mitochondrial GSH levels and restore the capacity to scavenge these reactive metabolites (Knight et al., 2002, James et al., 2003, Bajt et al., 2003, Jaeschke et al., 2003; Saito et al., 2010b).
Manganese superoxide dismutase (MnSOD, SOD2) is located predominantly in the mitochondrial matrix and plays an important role in the detoxification of mitochondrial superoxide (Macmillan-Crow and Cruthirds, 2001; Zelko et al., 2002). As all SODs, SOD2 converts 2 molecules of superoxide into 1 molecule of hydrogen peroxide and 1 molecule of molecular oxygen in a reaction that is only limited by the diffusion of superoxide to the enzyme (Fridovich, 1995). The vital importance of this enzyme for cell survival was documented by the fact that homozygous SOD2-deficient mice die shortly after birth due to cardiomyopathy and neuropathy caused by mitochondrial damage (Li et al., 1995, Lebovitz et al., 1996). In contrast, heterozygous SOD2-deficient (SOD2+/−) mice are viable (Williams et al., 1998; Van Remmen et al., 1999) but show increased susceptibility to chemicals that target mitochondria in the liver including troglitazone (Ong et al., 2007, Lee et al., 2008), nimesulide (Ong et al., 2006), ethanol (Larosche et al., 2010), flutamide (Kashimshetty et al., 2009), trovafloxacin (Hsiao et al., 2010) and APAP (Fujimoto et al., 2009). In addition, SOD2 gene knock-down experiments in rats demonstrated increased APAP-induced liver injury in these animals (Yoshikawa et al., 2009). In the mouse study it was demonstrated that mitochondrial GSH levels at 6 h after APAP were significantly lower in SOD2+/− mice compared to wildtype animals and hepatocytes isolated from SOD2+/− mice had lower ATP levels after exposure to 10 mM APAP providing indirect evidence for a mitochondrial stress in SOD2+/− mice (Fujimoto et al., 2009). Although both APAP studies convincingly documented that lower SOD2 levels enhanced the susceptibility to APAP hepatotoxicity in rats and in mice, the detailed mechanistic implications for APAP hepatotoxicity remained unclear. Therefore, the goal of this investigation was to evaluate the effect of partial SOD2 deficiency on the modulation of mitochondrial oxidant stress and peroxynitrite formation, JNK activation, mitochondrial Bax translocation and other mitochondrial events involved in the mechanism of APAP-induced liver injury.
MATERIAL and METHODS
Animals
Male heterozygous MnSOD (SOD2)-deficient (SOD2+/−) mice, which are on a C57Bl/6 background, along with control C57Bl/6J mice, were used in this study (Van Remmen et al., 1999; Van Remmen et al., 2003). The SOD+/− mice, which were originally obtained from Dr. Ting-Ting Huang (Stanford University, Stanford, CA), were bred in our facilities at KUMC. Age-matched wild type C57Bl/6J mice were obtained from Jackson Laboratories (Bar Harbor, Maine). All animals were housed in an environmentally controlled room with 12 h light/dark cycle and allowed free access to food and water. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals in research. All chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA) unless stated otherwise.
Experimental design
Mice were injected intra-peritoneally with 200 mg/kg APAP (dissolved in warm saline) after overnight fasting. The animals were killed after 3 or 6 h after APAP treatment and blood was withdrawn from the vena cava into a heparinized syringe for measurement of alanine aminotransferase (ALT) activities (Kinetic Test Kit 68-B, Biotron Diagnostics, Inc., Hernet, CA, USA). The liver was removed and was rinsed in saline; liver sections were fixed in 10% phosphate-buffered formalin for histological analyses. A portion of the liver was used for mitochondrial isolation and the remaining liver was snap-frozen in liquid nitrogen and stored at −80 °C.
Histology and immunohistochemistry
Formalin-fixed tissue samples were embedded in paraffin and 4 µm sections were cut. Replicate sections were stained with hematoxylin and eosin (H&E) for evaluation of necrosis (Gujral et al., 2002). For the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, sections of liver were stained with the In Situ Cell Death Detection Kit, AP (Roche Diagnostics, Indianapolis, IN) as described in the manufacturer's instructions (Gujral et al., 2002).
Measurement of GSH and GSSG
Total soluble GSH and GSSG were measured in the liver homogenate and mitochondria with a modified method of Tietze as described in detail (Jaeschke et al., 1990; Knight et al., 2001). Briefly, the frozen tissue was homogenized at 0 °C in 3% sulfosalicylic acid containing 0.1 mM EDTA. For GSSG measurement, GSH was trapped with 10 mM N-ethylmaleimide. After dilution with 0.01 N HCl, the sample was centrifuged and the supernatant was diluted with 100 mM potassium phosphate buffer (KPP), pH 7.4. The samples were assayed using dithionitrobenzoic acid. All data are expressed in GSH-equivalents.
Isolation of subcellular fractions and western blotting
Mitochondria and cytosolic fractions were isolated as described (Cover et al., 2005). Briefly, the liver was homogenized in ice cold isolation buffer (pH7.4) containing 220 mM mannitol, 70 mM sucrose, 2.5 mM HEPES, 10 mM EDTA, 1 mM EGTA, and 0.1% bovine serum albumin. Mitochondria were isolated by differential centrifugation (20,000 ×g) and washed with 2 ml of isolation buffer. The 20,000 ×g supernatant was used to evaluate the release of mitochondrial factors such as apoptosis-inducing factor (AIF) by western blotting. Western blotting was carried out as described in detail (Bajt et al., 2000) using the following antibodies: a rabbit anti-AIF antibody (Abcam, Cambridge, MA), a rabbit anti-Bax polyclonal antibody (Cell Signaling Technology, Danvers, MA), a rabbit anti-nitrotyrosine antibody (Invitrogen, Carlsbad, CA), a rabbit anti-phospho-JNK polyclonal antibody (Cell signaling Technology, Danvers, MA), a rabbit anti-JNK polyclonal antibody (Cell signaling Technology, Danvers, MA) and a rabbit anti-MnSOD polyclonal antibody (Upstate/Millipore, Billerca, MA). Each lane was loaded with 50µg protein for all experiments except the probing of mitochondrial phospho-JNK, where 75µg/lane was used. A horseradish peroxidase-coupled anti-rabbit IgG (Santa Cruz)) was used as secondary antibody. Proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech. Inc., Piscataway, NJ).
Protein carbonyl assay
Protein carbonyl content in the sample was measured using dinitrophenyl hydrazine (DNPH) as described (Sohal et al., 1993). Briefly, samples were treated with 10 mM DNPH in 2 N HCl and incubated for 1 hour at room temperature. After incubation, the mixture was precipitated with 10% trichloro acetic acid and the precipitate was washed with a mixture of ethanol:ethylacetate (1:1). The precipitate was then dissolved in 6 M guanidine HCl and absorbance read at 366 nm.
Statistics
All results were expressed as mean ± SE. Comparisons between multiple groups were performed with one-way ANOVA followed by a post hoc Bonferroni test. If the data were not normally distributed, we used the Kruskal-Wallis Test (nonparametric ANOVA) followed by Dunn’s Multiple Comparisons Test. P < 0.05 was considered significant.
RESULTS
Partial deficiency of SOD2 exacerbates acetaminophen-induced liver injury
To investigate the role of SOD2 in APAP hepatotoxicity, a moderate overdose of 200 mg/kg was selected. Initial experiments evaluated levels of SOD2 (MnSOD) in livers of mice before and after APAP administration. MnSOD levels in livers of SOD2+/− mice were reduced by approximately 35% when compared to wild type animals (Figure 1A); SOD2 levels did not show significant alterations after APAP administration either in wild type or in SOD2+/− animals. Liver injury due to APAP administration was then examined at 6 h (Figure 1B,C). APAP treatment resulted in significant liver injury as indicated by the increase in plasma ALT activities (Figure 1B) and the development of centrilobular necrosis (Figure 1C) in wild type animals. However, liver injury in SOD2+/− mice treated with APAP was significantly exacerbated (Figure 1B,C). It has been demonstrated earlier that nuclear DNA fragmentation as visualized by the TUNEL assay is a consequence of mitochondrial dysfunction (Cover et al., 2005). Consistent with this, a substantial number of TUNEL-positive cells were observed in centrilobular regions of APAP-treated wild-type mice, which correlated well with the areas of necrosis (Figure 1D). However, nuclear DNA fragmentation was further elevated in SOD2+/− mice when compared to wild type animals after APAP treatment (Figure 1D).
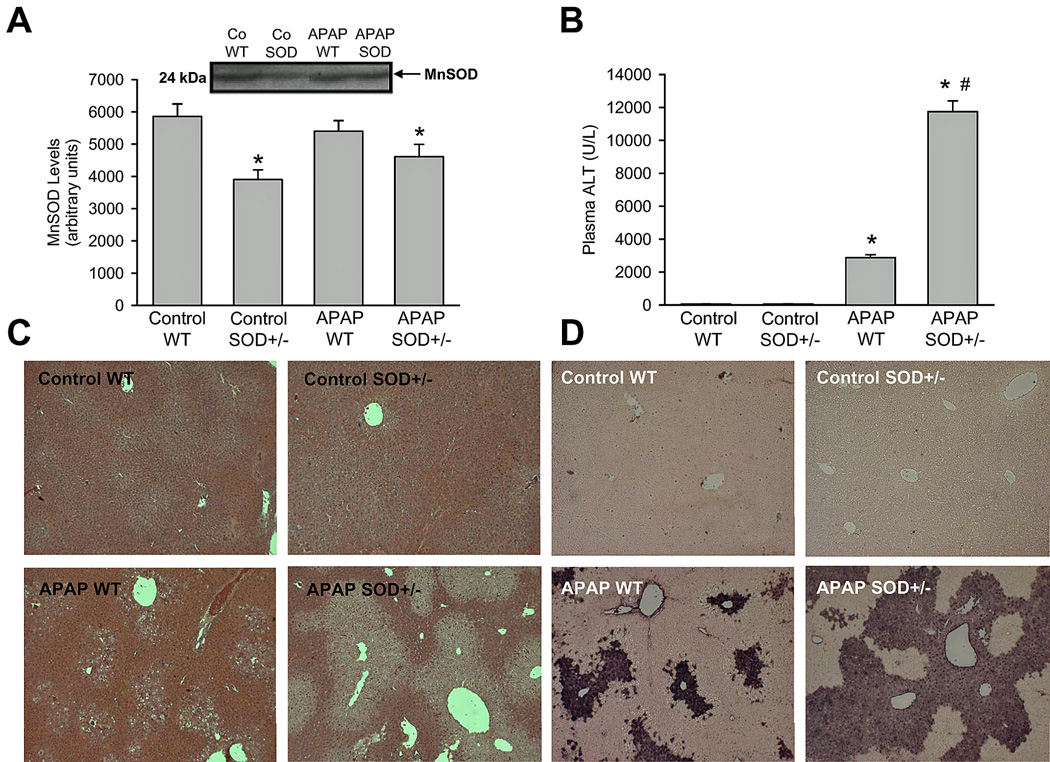
Acetaminophen (APAP)- induced liver injury in heterozygous SOD2-deficient mice. Manganese superoxide dismutase protein levels (A) as well as plasma alanine aminotransferase (ALT) activities (B), liver histology (C) and DNA fragmentation by TUNEL assay (D) as an indicator for APAP-induced hepatotoxicity were measured in C57Bl/6J wild type (WT) and SOD2-deficient (SOD+/−) mice 6 h after 200 mg/kg APAP administration. Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to untreated controls), #P<0.05 (compared to APAP treated wild type mice).
Partial deficiency of SOD2 aggravates acetaminophen-induced oxidative stress
Previous studies have documented that the increase in the hepatic GSSG-to-GSH ratio is caused almost exclusively by enhanced mitochondrial GSSG levels reflective of a mitochondrial oxidant stress (Jaeschke, 1990; Knight et al., 2001; Jaeschke et al., 2003). Consistent with previous experiments (Ramachandran et al., 2011), hepatic glutathione levels were completely recovered in wild type animals 6 h after 200 mg/kg APAP (Figure 2A). However, SOD2+/− mice recovered only to 42% of baseline despite the initially higher GSH levels in control animals (Figure 2A). Although both wild type and SOD2+/− mice showed moderately elevated GSSG levels, the GSSG-to-GSH ratio was substantially higher in SOD2+/− mice compared to wild type animals 6 h after APAP (Figure 2B,C).
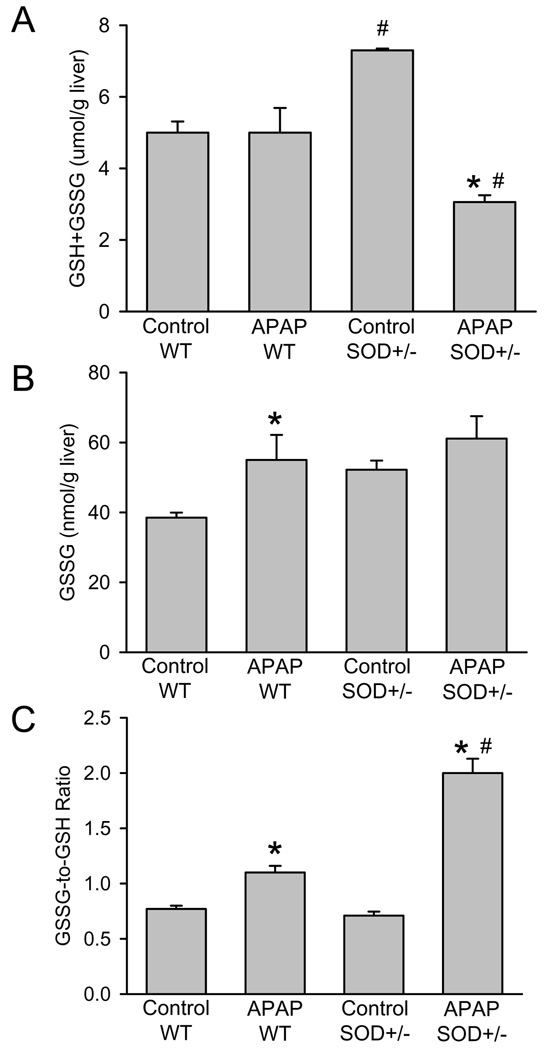
Acetaminophen (APAP)-induced oxidant stress in wild type (WT) and in SOD2-deficient (SOD+/−) mice. Liver content of total glutathione (GSH+GSSG) (A), glutathione disulfide (GSSG) (B) and the GSSG-to-GSH ratio (C) were measured in untreated controls or animals treated with 200mg/kg APAP for 6 hours. Data represent means ± SE of n = 5 animals per group. *P<0.05 (compared to untreated controls), #P<0.05 (compared to APAP treated wild type mice).
In order to assess more specifically events in mitochondria, glutathione levels were measured before and after APAP treatment. Similar to the GSH levels in the overall tissue, mitochondrial GSH levels decline more than 90% within the first hour and gradually recover thereafter (Saito et al., 2010b). In wild type animals, mitochondrial GSH levels recovered to 54% of baseline compared to only 33% in SOD2+/− mice (Figure 3A) suggesting a higher oxidant stress in the SOD2+/− animals. The mitochondrial protein carbonyl content as indicator of oxidant damage to mitochondrial proteins was significantly higher in SOD2+/− mice compared to wild type animals, particularly after APAP treatment (Figure 3B). Consistent with the higher oxidant stress in SOD2-deficient mitochondria, the increase in protein carbonyls due to APAP treatment was twice as high as the increase in wild type animals (Figure 3B). Similarly, a significant increase in mitochondrial nitrotyrosine protein adducts was observed in SOD2+/− mice compared to wild type animals (Figure 3C).
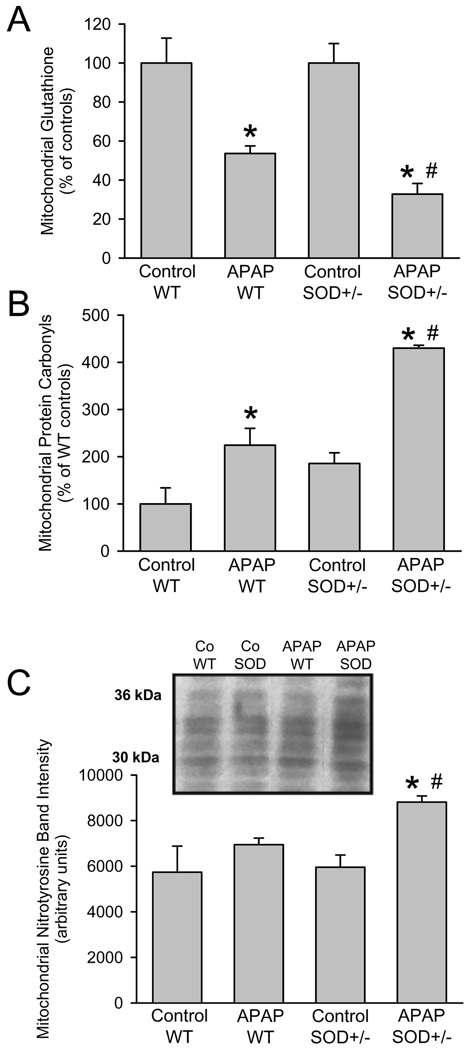
Acetaminophen (APAP)-induced mitochondrial oxidant stress in wild type and SOD2 deficient (SOD+/−) mice. Mitochondrial concentrations of total glutathione (GSH+GSSG) (A), protein carbonyl content (B) and densitometric quantitation (all bands included) of western blotting for nitrotyrosine protein adducts (C) in wild type or SOD2+/− mice treated with 200mg/kg APAP for 6 hours. Data represent means ± SE of n = 3 animals per group. *P<0.05 (compared to untreated controls), #P<0.05 (compared to APAP treated wild type mice).
Mitochondria-related cellular signaling events during acetaminophen hepatotoxicity
A number of cellular signaling events are now known to be initiated by mitochondrial oxidative stress after APAP administration. Previous studies provided evidence that the mitochondrial dysfunction after APAP overdose triggers AIF and endonuclease G release from the mitochondrial intermembrane space and translocation to the nucleus where these proteins are involved in nuclear DNA fragmentation (Cover et al., 2005; Bajt et al., 2006). In wild type animals, AIF was released from the mitochondria during APAP toxicity as indicated by the elevated levels of the protein in the cytosol (Figure 4A). Despite the already higher baseline AIF levels in the cytosol of control SOD2+/− mice, the increase of AIF release induced by APAP in SOD2+/− mice was even higher than in wild type animals (Figure 4A). Since the initial release of intermembrane proteins after APAP overdose is facilitated by mitochondrial translocation of Bax (Bajt et al., 2008), the levels of Bax proteins in the mitochondria were evaluated. As previously reported (Adams et al., 2001; El-Hassan et al., 2003, Jaeschke and Bajt, 2006; Bajt et al., 2008), APAP overdose triggers mitochondrial Bax translocation (Figure 4B). Baseline Bax levels in SOD2+/− mice were similar to wild type animals but the relative increase was only slightly higher in SOD2+/− animals (Figure 4B) suggesting that AIF release requires more than just Bax pore formation.
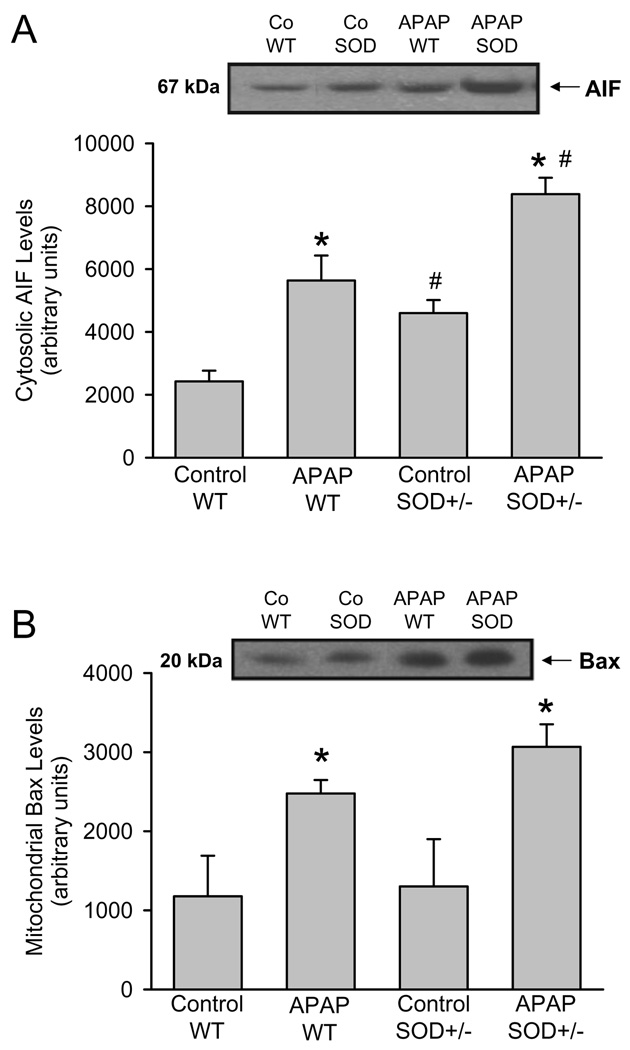
Western blot analysis and densitometric quantitation of apoptosis-inducing factor (AIF) in the cytosolic fraction (A) and of Bax in the mitochondrial fraction (B) from untreated controls and 6 h after 200 mg/kg APAP in wild type (WT) and SOD2-deficient (SOD+/−) mice. Data represent means ± SE of n = 3 animals per group. *P<0.05 (compared to untreated controls), #P<0.05 (compared to APAP treated wild type mice).
Previous studies demonstrated that the mitochondrial oxidant stress after APAP overdose triggers JNK activation and translocation of P-JNK to the mitochondria (Hanawa et al., 2008; Saito et al., 2010a). In both untreated wild type and SOD2+/− mice there was minimal JNK detectable in mitochondria (Figure 5A). APAP overdose caused JNK translocation to the mitochondria in wild type and SOD2+/− animals (Figure 5A), with the increase in SOD2+/− animals being higher when compared to the wild type mice (based on quantitation of both bands). As expected from the JNK data, western blotting for the active phosphorylated form of JNK indicated that in both untreated wild type and SOD2+/− mice there was no P-JNK detectable in mitochondria (Figure 5B). APAP overdose caused P-JNK translocation to the mitochondria in wild type and SOD2+/− animals (Figure 5B). However, the levels of P-JNK was more than 4-times higher in SOD2+/− animals compared to wild type mice at 6 h (Figure 5B).
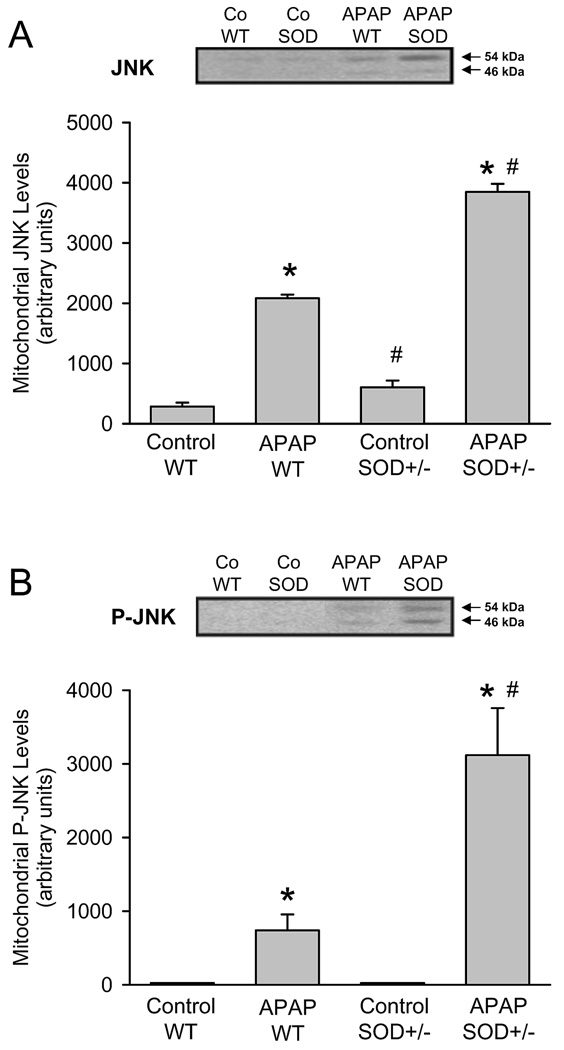
Western blot analysis and densitometric quantitation of c-Jun-N-terminal Kinase (JNK) (A) and the activated form phospho-JNK (P-JNK) (B) in the mitochondrial fraction from untreated controls and 6 h after 200 mg/kg APAP in wild type (WT) and SOD2-deficient (SOD+/−) mice. Data represent means ± SE of n = 3 animals per group. *P<0.05 (compared to untreated controls), #P<0.05 (compared to APAP treated wild type mice).
To evaluate the temporal relationship between the APAP-induced signaling events in the cytosol and mitochondria, experiments were repeated at an earlier time point, namely 3 hours after APAP treatment. At this time, plasma ALT levels were similar in wild type (2190 ± 320 U/L; n=8) and in SOD2+/− animals (2430 ± 575 U/L; n=6). In addition, glutathione levels were similarly low in both wild type (0.24 ± 0.09 µmol/g liver) and SOD2+/− mice (0.32 ± 0.16 µmol/g liver). Elevated levels of AIF in the cytosol were evident in both wild type as well as SOD2+/− animals at 3 hours, though there was no significant difference between the genotypes (Figure 6A). By 6 hours, AIF levels in the wild type animals showed a further minor increase, which was significantly amplified in the SOD2+/− animals (Figure 4A, ,6A).6A). Mitochondrial levels of Bax, on the other hand were highly elevated by 3 hours in both groups of animals and decreased by 6 hours, though at the later time point, the SOD2+/− animals had slightly higher levels of protein than wild type mice (Figure 6B).
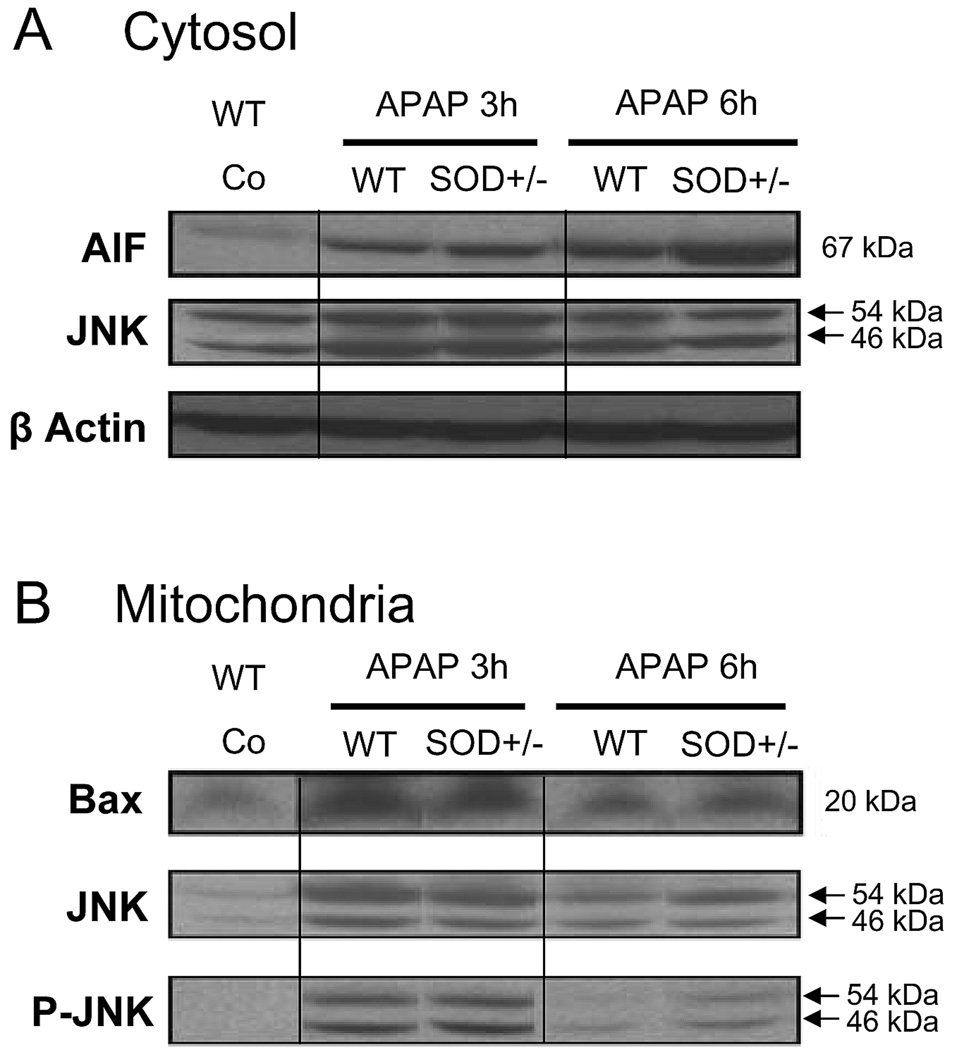
Western blot analysis of apoptosis-inducing factor (AIF) and c-Jun-N-terminal Kinase (JNK) in the cytosolic fraction and of Bax, JNK and the activated form phospho-JNK (PJNK) in the mitochondrial fraction from untreated controls and 3 and 6 h after 200 mg/kg APAP in wild type (WT) and SOD2-deficient (SOD+/−) mice. Beta actin was used as a loading control for cytosolic proteins.
Mitochondrial levels of total JNK as well as the phosphorylated protein were significantly elevated at 3 hours after APAP but no significant differences were evident between wild type and SOD2+/− animals. By 6 hours, the levels of both JNK and P-JNK were lower than that at 3 hours in both groups, with the wild type animals showing a much steeper decrease than the SOD2+/−mice. Thus, at the later time point the SOD2+/− mice had significantly higher levels of protein when compared to wild type animals (Figure 5B, ,6B).6B). To determine if differences in JNK levels between wild type and SOD2+/− mice were restricted to the mitochondria, cytosolic levels of JNK were also evaluated. At 3 hours after APAP, total JNK levels were slightly elevated in the cytosol and there was a more pronounced increase in the mitochondria (Figure 6). JNK levels decreased by 6 hours. However, at both time points, no significant difference in cytosolic JNK was evident between wild type and SOD2+/− animals.
DISCUSSION
SOD2 and mitochondrial oxidant stress in APAP hepatotoxicity
The objective of this investigation was to evaluate the mechanistic implications of partial SOD2-deficiency for APAP hepatotoxicity. Similar to a recent report (Fujimoto et al., 2009), our data clearly showed a substantial aggravation of APAP-induced liver injury in mice with reduced SOD2 activity compared to wild type animals. In addition to the reduced levels of liver and mitochondrial GSH levels in SOD2+/− mice reported previously (Fujimoto et al., 2009), our data show that the higher injury in these animals was correlated with increased oxidation of the hepatic GSH content, which mainly reflects a mitochondrial oxidant stress (Jaeschke, 1990; Knight et al., 2001; Jaeschke et al., 2003). Consistent with this conclusion, a delayed recovery of mitochondrial GSH levels and increased formation of protein carbonyls in mitochondria was observed in SOD2+/− mice. Furthermore, higher levels of nitrated proteins were detected. These findings are in agreement with previous evidence of a predominantly mitochondrial formation of peroxynitrite (Cover et al., 2005). Together these results demonstrate the critical importance of SOD2 in protecting against a mitochondrial oxidant stress after APAP overdose. Even a partial deficiency of this mitochondrial enzyme drastically enhanced the susceptibility to APAP-induced mitochondrial oxidant stress and peroxynitrite formation. Interestingly, SOD2 also appears to be a target of nitration in the mitochondria leading to a further reduction of the enzyme activity after APAP overdose (Hinson et al., 2010).
SOD2 and nuclear DNA fragmentation in APAP hepatotoxicity
DNA fragmentation is a hallmark of APAP hepatotoxicity (Ray et al., 1990; Lawson et al., 1999; Gujral et al., 2002). The frequently reported DNA ladder formation suggests that the DNA fragmentation is an endonuclease-mediated process (Ray et al., 1990; Shen et al., 1992; Cover et al., 2005). The absence of caspase activity during APAP hepatotoxicity (Lawson et al., 1999; El-Hassan et al., 2003; Jaeschke et al., 2006) excludes the involvement of the caspase-activated DNase, which is the classical endonuclease responsible for apoptotic DNA fragmentation (Nagata et al., 2003). In contrast, our recent observations showed translocation of AIF and endonuclease G from the mitochondria to the nucleus as being critical for DNA damage (Bajt et al., 2006). In addition, lysosomal DNase1 may also be involved at later time points (Napirei et al., 2006). The mitochondrial release of AIF and endonuclease G is facilitated by the initial formation of Bax pores and later by mitochondrial matrix swelling and rupture of the outer membrane (Bajt et al., 2008). The fact that the increased DNA fragmentation in SOD2+/− compared to wild type mice as indicated by the TUNEL assay correlates with the appearance of higher levels of AIF in the cytosol suggests that the enhanced mitochondrial release of AIF was at least in part responsible for the increased DNA damage. Interestingly, mitochondrial Bax translocation was only modestly affected indicating the main cause of the increased AIF release was not enhanced Bax pore formation. However, even in the absence of Bax, the mitochondrial oxidant stress and peroxynitrite formation was able to trigger the MPT with matrix swelling, which induced the accelerated release of AIF and endonuclease G and consequently nuclear DNA fragmentation (Bajt et al., 2008). Consistent with this conclusion, preventing the MPT pore formation by scavenging reactive oxygen and peroxynitrite (Knight et al., 2002; Bajt et al., 2003) or by eliminating cyclophilin D, a critical regulator of the MPT pore, drastically reduced DNA damage (Ramachandran et al., 2011). Based on these findings, we conclude that the accelerated DNA damage observed in SOD2+/− mice is most likely caused by the enhanced MPT pore formation due to the enhanced oxidant stress and impaired defense mechanisms against reactive oxygen and nitrogen species.
SOD2 and JNK activation in APAP hepatotoxicity
Activation of JNK is a critical event in APAP-induced liver injury (Gunawan et al., 2006; Hendersen et al., 2007; Latchoumycandane et al., 2007; Saito et al., 2010a). JNK activation can be induced by the initial mitochondrial oxidant stress (Hanawa et al., 2008; Saito et al., 2010a). The most relevant effect of activated JNK is the translocation of P-JNK to mitochondria (Hanawa et al., 2008) where it is involved in amplifying ROS formation (Saito et al., 2010a). Our findings confirm the mitochondrial translocation of P-JNK in wild type animals. However, there was no difference in overall JNK levels in the cytosol between genotypes and the initial mitochondrial translocation of JNK and PJNK was very similar. The substantial difference between wild type and SOD2+/− mice at 6 h was caused by the delayed loss of P-JNK and of JNK in SOD2+/− animals indicating a more prolonged JNK activation in these animals. This may have been caused by the impaired defense against the mitochondrial oxidant stress. The resulting prolonged presence of P-JNK in the mitochondria in turn may be responsible for the higher oxidant stress observed in these mice.
Modulations of signaling pathways in SOD2+/− mice – cause or effect?
A critical question is whether some of the observed effects are caused by SOD2 deficiency, are secondary effects or are even unrelated to SOD2. For example, the injury could have been modulated by differences in the expression of enzymes involved in drug metabolism. Although we did not specifically address this issue, the early depletion of GSH at 1 h after APAP administration was similar in wild type and SOD2+/− mice (Fujimoto et al., 2009) suggesting that NAPQI formation was not significantly different between genotypes. Moreover, the fact that the early injury (3 h) was the same in wild type and SOD2+/− mice indicates that aggravation of liver injury in SOD2+/− mice occurred during the progression phase, which is mainly driven by oxidant stress and peroxynitrite formation in mitochondria (Jaeschke and Bajt, 2006). Consistent with this conclusion is the observation that the major differences in signaling events between the genotypes were predominantly postmitochondrial, i.e. activation of JNK, which is dependent on the mitochondrial oxidant stress (Hanawa et al., 2008; Saito et al., 2010a), and AIF release, which is initially dependent on Bax translocation and later on the MPT (Bajt et al., 2008). Together these findings support the conclusion that the aggravation of APAP-induced liver injury and the effects on intracellular signaling events in SOD2+/− mice is caused by the limited defense mechanisms against mitochondrial oxidant stress.
In summary, our experiments demonstrated that SOD2+/− mice are much more susceptible to APAP-induced liver injury compared to wild type animals, which correlated with enhancement of all parameters of the mitochondrial oxidant stress, more AIF release and more extensive DNA fragmentation and a more prolonged JNK activation. Thus, the impaired defense against mitochondrial superoxide formation in SOD2+/− mice prolonged JNK activation after APAP overdose and consequently further enhanced the mitochondrial oxidant stress leading to exaggerated mitochondrial dysfunction, accelerated release of intermembrane proteins with nuclear DNA fragmentation and more cellular necrosis.
ACKNOWLEDGMENT
We thank Dr. Ting-Ting Huang (Stanford University) for providing the breeder pairs of the SOD+/− mice. This investigation was supported in part by National Institutes of Health Grants R01 DK070195 and R01 AA12916 to H.J., R01 AA012863 to S.W., and by grants P20 RR016475 and P20 RR021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors do not have any conflict of interest to disclose.
REFERENCES
- Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol. Pharmacol. 2001;60:907–915. [Abstract] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006;94:217–225. [Abstract] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2008;324:8–14. [Abstract] [Google Scholar]
- Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminopheninduced liver injury in mice. J. Pharmacol. Exp. Ther. 2003;307:67–73. [Abstract] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol. Sci. 2000;58:109–117. [Abstract] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 1997;143:1–12. [Abstract] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005;315:879–887. [Abstract] [Google Scholar]
- El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol. Appl. Pharmacol. 2003;191:118–129. [Abstract] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. [Abstract] [Google Scholar]
- Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol. Pathol. 2009;37:193–200. [Abstract] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol. Sci. 2002;67:322–328. [Abstract] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. [Abstract] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283:13565–13577. [Europe PMC free article] [Abstract] [Google Scholar]
- Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure. Gut. 2007;56:982–990. [Europe PMC free article] [Abstract] [Google Scholar]
- Hinson J, Agarwal R, Burke A, Saba H, James L, Macmillan-Crow LA. New insights into the mechanisms of acetaminophen toxicity (abstract) Toxicol. Lett. 2010;196 Supplement 1:S26–S27. [Google Scholar]
- Hsiao CJ, Younis H, Boelsterli UA. Trovafloxacin, a fluoroquinolone antibiotic with hepatotoxic potential, causes mitochondrial peroxynitrite stress in a mouse model of underlying mitochondrial dysfunction. Chem. Biol. Interact. 2010;188:204–213. [Abstract] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J. Pharmacol. Exp. Ther. 1990;255:935–941. [Abstract] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 2006;89:31–41. [Abstract] [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol. Lett. 2003;144:279–288. [Abstract] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. [Abstract] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA. Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol. Sci. 2003;75:458–467. [Abstract] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 1973;187:195–202. [Abstract] [Google Scholar]
- Kashimshetty R, Desai VG, Kale VM, Lee T, Moland CL, Branham WS, New LS, Chan EC, Younis H, Boelsterli UA. Underlying mitochondrial dysfunction triggers flutamide-induced oxidative liver injury in a mouse model of idiosyncratic drug toxicity. Toxicol. Appl. Pharmacol. 2009;238:150–159. [Abstract] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol. Sci. 2001;62:212–220. [Abstract] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J. Pharmacol. Exp. Ther. 2002;303:468–475. [Abstract] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. [Abstract] [Google Scholar]
- Larosche I, Lettéron P, Berson A, Fromenty B, Huang TT, Moreau R, Pessayre D, Mansouri A. Hepatic mitochondrial DNA depletion after an alcohol binge in mice: probable role of peroxynitrite and modulation by manganese superoxide dismutase. J. Pharmacol. Exp. Ther. 2010;332:886–897. [Abstract] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. [Abstract] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. [Abstract] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol. Appl. Pharmacol. 1999;156:179–186. [Abstract] [Google Scholar]
- Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93:9782–9787. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee YH, Chung MC, Lin Q, Boelsterli UA. Troglitazone-induced hepatic mitochondrial proteome expression dynamics in heterozygous Sod2(+/−) mice: two-stage oxidative injury. Toxicol. Appl. Pharmacol. 2008;231:43–51. [Abstract] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. [Abstract] [Google Scholar]
- Macmillan-Crow LA, Cruthirds DL. Manganese superoxide dismutase in disease. Free Radic. Res. 2001;34:325–336. [Abstract] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol. Appl. Pharmacol. 1988;93:378–387. [Abstract] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973;187:211–217. [Abstract] [Google Scholar]
- Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ. 2003;10:108–116. [Abstract] [Google Scholar]
- Napirei M, Basnakian AG, Apostolov EO, Mannherz HG. Deoxyribonuclease 1 aggravates acetaminophen-induced liver necrosis in male CD-1 mice. Hepatology. 2006;43:297–305. [Abstract] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. [Abstract] [Google Scholar]
- Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol. Sci. 2007;97:205–213. [Abstract] [Google Scholar]
- Ong MM, Wang AS, Leow KY, Khoo YM, Boelsterli UA. Nimesulide-induced hepatic mitochondrial injury in heterozygous Sod2(+/−) mice. Free Radic. Biol. Med. 2006;40:420–429. [Abstract] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL. Identification of hepatic protein targets of the reactive metabolites of the non-hepatotoxic regioisomer of acetaminophen, 3'-hydroxyacetanilide, in the mouse in vivo using two-dimensional gel electrophoresis and mass spectrometry. Adv. Exp. Med. Biol. 2001;500:663–673. [Abstract] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011 Oct 13; in press; [Epub ahead of print] [Europe PMC free article] [Abstract] [Google Scholar]
- Ray SD, Sorge CL, Raucy JL, Corcoran GB. Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol. Appl. Pharmacol. 1990;106:346–351. [Abstract] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010a;246:8–17. [Europe PMC free article] [Abstract] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010b;51:246–254. [Europe PMC free article] [Abstract] [Google Scholar]
- Shen W, Kamendulis LM, Ray SD, Corcoran GB. Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: effects of Ca(2+)-endonuclease, DNA repair, and glutathione depletion inhibitors on DNA fragmentation and cell death. Toxicol. Appl. Pharmacol. 1992;112:32–40. [Abstract] [Google Scholar]
- Sohal RS, Agarwal S, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7255–7259. [Europe PMC free article] [Abstract] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 264:9814–9819. [Abstract] [Google Scholar]
- Tirmenstein MA, Nelson SD. Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides. J. Biol. Chem. 1990;265:3059–3065. [Abstract] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genomics. 2003;16:29–37. [Abstract] [Google Scholar]
- Van Remmen H, Salvador C, Yang H, Huang TT, Epstein CJ, Richardson A. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knockout mouse. Arch. Biochem. Biophys. 1999;363:91–97. [Abstract] [Google Scholar]
- Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J. Biol. Chem. 1998;273:28510–28515. [Abstract] [Google Scholar]
- Yoshikawa Y, Morita M, Hosomi H, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Knockdown of superoxide dismutase 2 enhances acetaminophen-induced hepatotoxicity in rat. Toxicology. 2009;264:89–95. [Abstract] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33:337–349. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.taap.2011.01.004
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3050115?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Hepsin as a potential therapeutic target for alleviating acetaminophen-induced hepatotoxicity via gap-junction regulation and oxidative stress modulation.
Cell Biol Toxicol, 40(1):80, 18 Sep 2024
Cited by: 0 articles | PMID: 39292286 | PMCID: PMC11410999
Effect of ferroptosis inhibitors in a murine model of acetaminophen-induced liver injury.
J Biochem Mol Toxicol, 38(8):e23791, 01 Aug 2024
Cited by: 0 articles | PMID: 39082238
The Role of Mechanistic Biomarkers in Understanding Acetaminophen Hepatotoxicity in Humans.
Drug Metab Dispos, 52(8):729-739, 16 Jul 2024
Cited by: 2 articles | PMID: 37918967
Review
Central Mechanisms of Acetaminophen Hepatotoxicity: Mitochondrial Dysfunction by Protein Adducts and Oxidant Stress.
Drug Metab Dispos, 52(8):712-721, 16 Jul 2024
Cited by: 7 articles | PMID: 37567742
Review
Deletion of Glyoxalase 1 Exacerbates Acetaminophen-Induced Hepatotoxicity in Mice.
Antioxidants (Basel), 13(6):648, 25 May 2024
Cited by: 0 articles | PMID: 38929087
Go to all (89) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity.
Toxicol Sci, 122(2):598-605, 13 May 2011
Cited by: 76 articles | PMID: 21572097 | PMCID: PMC3155087
c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity.
Toxicol Appl Pharmacol, 246(1-2):8-17, 25 Apr 2010
Cited by: 179 articles | PMID: 20423716 | PMCID: PMC2885557
Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase.
Toxicol Appl Pharmacol, 281(1):58-66, 16 Sep 2014
Cited by: 79 articles | PMID: 25218290 | PMCID: PMC4362889
Central Mechanisms of Acetaminophen Hepatotoxicity: Mitochondrial Dysfunction by Protein Adducts and Oxidant Stress.
Drug Metab Dispos, 52(8):712-721, 16 Jul 2024
Cited by: 7 articles | PMID: 37567742
Review
Funding
Funders who supported this work.
NCRR NIH HHS (2)
Grant ID: P20 RR021940
Grant ID: P20 RR016475
NIAAA NIH HHS (5)
Grant ID: R01 AA012863-11
Grant ID: R01 AA012916
Grant ID: R01 AA012863
Grant ID: R01 AA12916
Grant ID: R01 AA012916-08
NIDDK NIH HHS (3)
Grant ID: R01 DK070195
Grant ID: R01 DK070195-05
Grant ID: R01 DK070195-05S1




