Abstract
Free full text

Effect of premature termination of translation on mRNA stability depends on the site of ribosome release.
Abstract
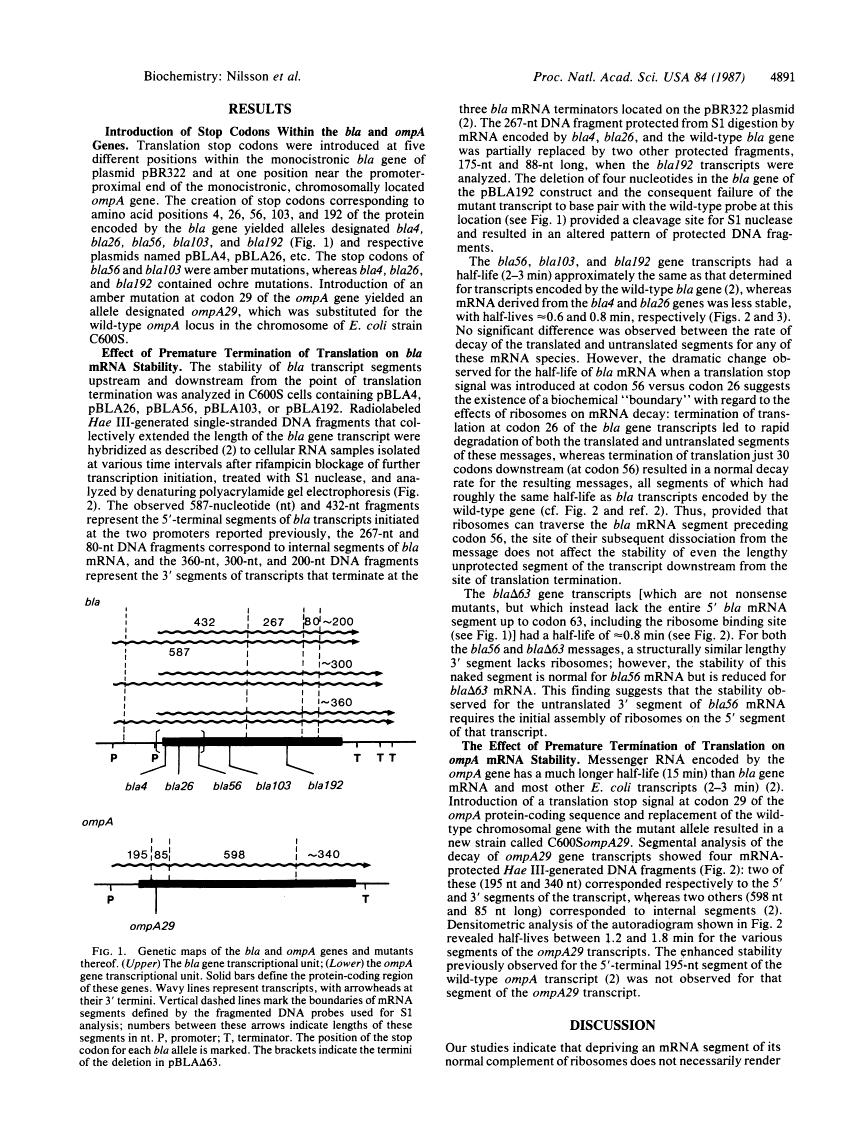
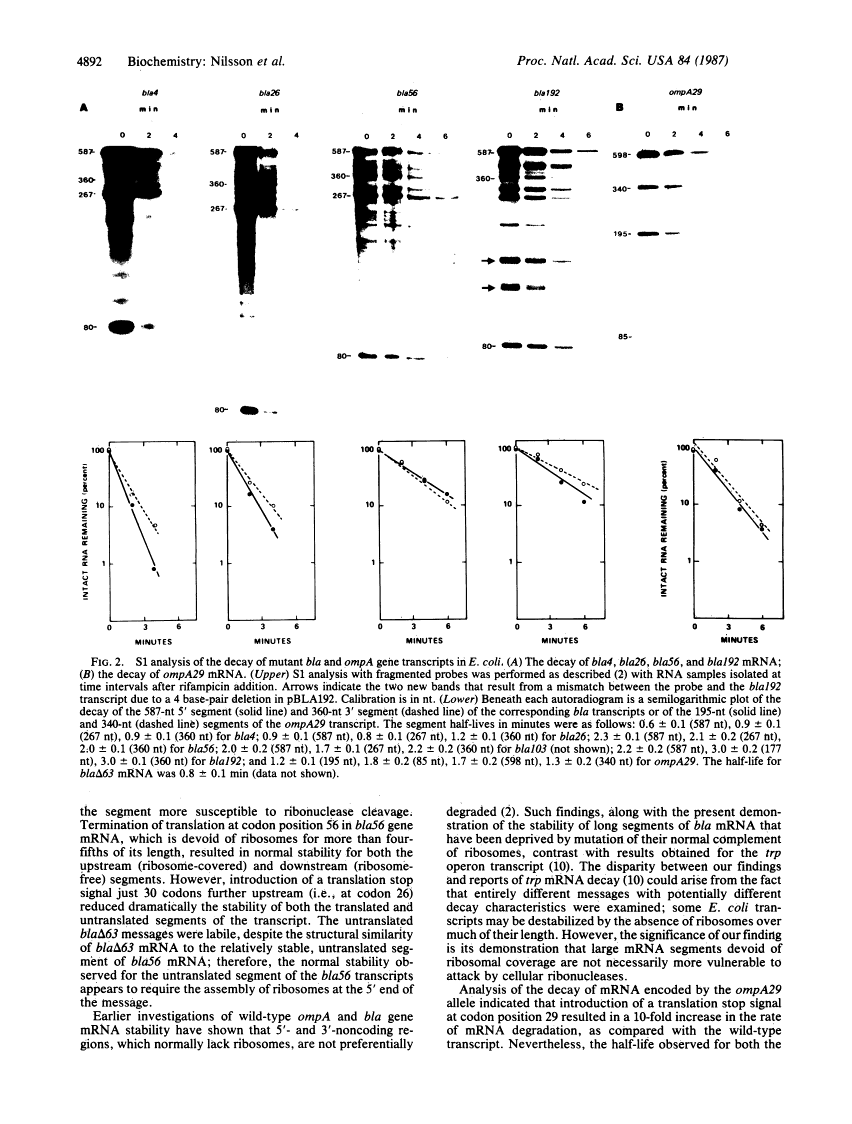
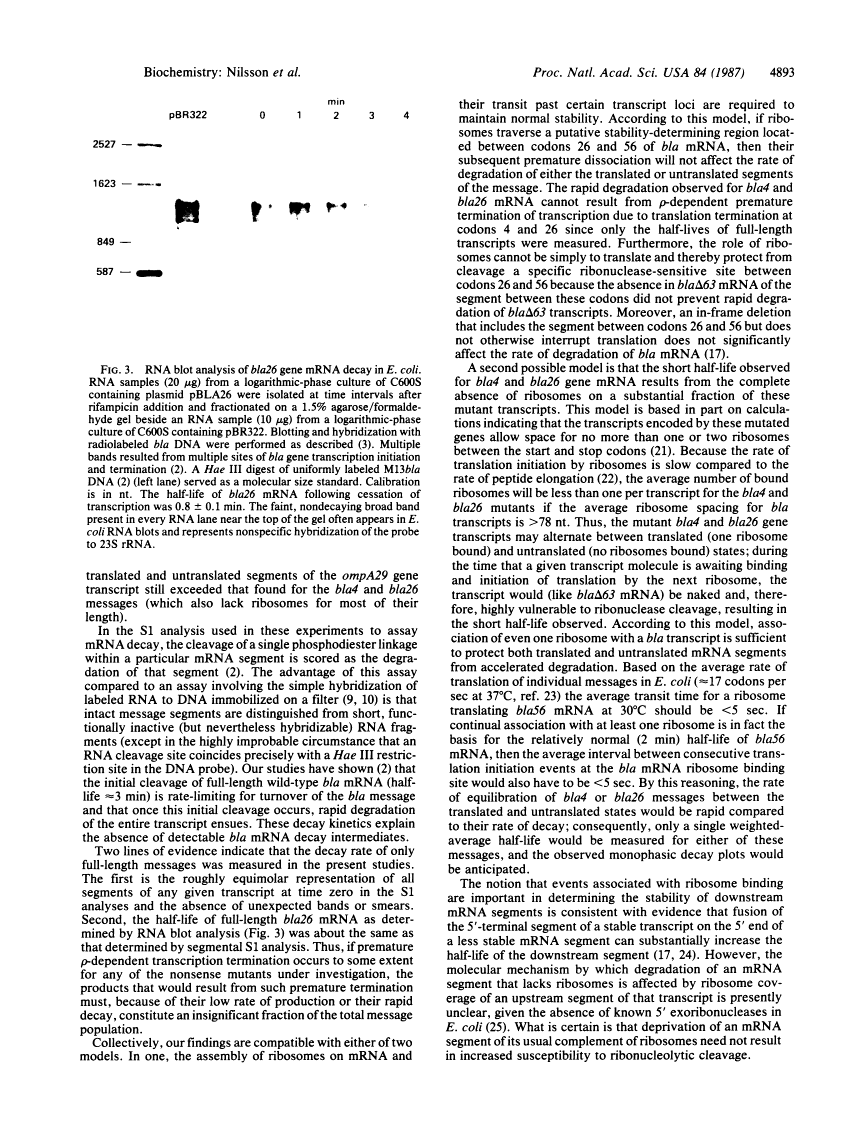
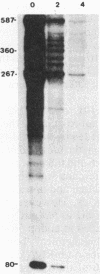
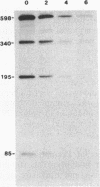
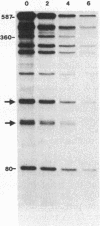
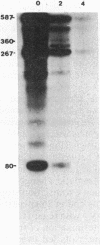
Translational stop codons were introduced at various locations in the protein-coding regions of the monocistronic bla and ompA gene transcripts of Escherichia coli, and the decay characteristics of the upstream and downstream mRNA segments were analyzed. Premature termination of translation at codon position 26 reduced the stability of both the translated and ribosome-free segments of bla mRNA, whereas release of ribosomes just 30 codons further downstream resulted in normal stability for both segments. Normal stability of an untranslated bla gene mRNA segment required its linkage to a ribosome-bound segment of bla gene mRNA. These findings indicate that depriving an mRNA segment of ribosomes does not necessarily render it more susceptible to degradation. However, premature termination of translation at a location that allows ribosomes to traverse only a short segment of bla mRNA can lead to destabilization of the entire transcript.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Pedersen S, Reeh S. Functional mRNA half lives in E. coli. Mol Gen Genet. 1978 Nov 9;166(3):329–336. [Abstract] [Google Scholar]
- von Gabain A, Belasco JG, Schottel JL, Chang AC, Cohen SN. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. [Europe PMC free article] [Abstract] [Google Scholar]
- Nilsson G, Belasco JG, Cohen SN, von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature. 1984 Nov 1;312(5989):75–77. [Abstract] [Google Scholar]
- Bartlett BO, Scott Russell R, Jenkins W. Improved relationship between the deposition of strontium-90 and the contamination of milk in the United Kingdom. Nature. 1972 Jul 7;238(5358):46–48. [Abstract] [Google Scholar]
- Newbury SF, Smith NH, Robinson EC, Hiles ID, Higgins CF. Stabilization of translationally active mRNA by prokaryotic REP sequences. Cell. 1987 Jan 30;48(2):297–310. [Abstract] [Google Scholar]
- Belasco JG, Beatty JT, Adams CW, von Gabain A, Cohen SN. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. [Abstract] [Google Scholar]
- Brock ML, Shapiro DJ. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. [Abstract] [Google Scholar]
- Darnell JE., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. [Abstract] [Google Scholar]
- Schneider E, Blundell M, Kennell D. Translation and mRNA decay. Mol Gen Genet. 1978 Apr 6;160(2):121–129. [Abstract] [Google Scholar]
- Morse DE, Yanofsky C. Polarity and the degradation of mRNA. Nature. 1969 Oct 25;224(5217):329–331. [Abstract] [Google Scholar]
- Beck E, Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. [Europe PMC free article] [Abstract] [Google Scholar]
- Shortle D, Koshland D, Weinstock GM, Botstein D. Segment-directed mutagenesis: construction in vitro of point mutations limited to a small predetermined region of a circular DNA molecule. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5375–5379. [Europe PMC free article] [Abstract] [Google Scholar]
- Kadonaga JT, Gautier AE, Straus DR, Charles AD, Edge MD, Knowles JR. The role of the beta-lactamase signal sequence in the secretion of proteins by Escherichia coli. J Biol Chem. 1984 Feb 25;259(4):2149–2154. [Abstract] [Google Scholar]
- Miller JH, Ganem D, Lu P, Schmitz A. Genetic studies of the lac repressor. I. Correlation of mutational sites with specific amino acid residues: construction of a colinear gene-protein map. J Mol Biol. 1977 Jan 15;109(2):275–298. [Abstract] [Google Scholar]
- Sutcliffe JG. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. [Abstract] [Google Scholar]
- Belasco JG, Nilsson G, von Gabain A, Cohen SN. The stability of E. coli gene transcripts is dependent on determinants localized to specific mRNA segments. Cell. 1986 Jul 18;46(2):245–251. [Abstract] [Google Scholar]
- Meacock PA, Cohen SN. Genetic analysis of the inter-relationship between plasmid replication and incompatibility. Mol Gen Genet. 1979 Jul 13;174(2):135–147. [Abstract] [Google Scholar]
- Manning PA, Puspurs A, Reeves P. Outer membrane of Escherichia coli K-12: isolation of mutants with altered protein 3A by using host range mutants of bacteriophage K3. J Bacteriol. 1976 Sep;127(3):1080–1084. [Europe PMC free article] [Abstract] [Google Scholar]
- D'Ari R, Jaffé-Brachet A, Touati-Schwartz D, Yarmolinsky MB. A dnaB analog specified by bacteriophage P1. J Mol Biol. 1975 May 25;94(3):341–366. [Abstract] [Google Scholar]
- Kang CW, Cantor CR. Structure of ribosome-bound messenger RNA as revealed by enzymatic accessibility studies. J Mol Biol. 1985 Jan 20;181(2):241–251. [Abstract] [Google Scholar]
- Gorski K, Roch JM, Prentki P, Krisch HM. The stability of bacteriophage T4 gene 32 mRNA: a 5' leader sequence that can stabilize mRNA transcripts. Cell. 1985 Dec;43(2 Pt 1):461–469. [Abstract] [Google Scholar]
- Deutscher MP. E. coli RNases: making sense of alphabet soup. Cell. 1985 Apr;40(4):731–732. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.84.14.4890
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc305211?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Environment modulates protein heterogeneity through transcriptional and translational stop codon readthrough.
Nat Commun, 15(1):4446, 24 May 2024
Cited by: 1 article | PMID: 38789441 | PMCID: PMC11126739
First description of antimicrobial resistance in carbapenem-susceptible Klebsiella pneumoniae after imipenem treatment, driven by outer membrane remodeling.
BMC Microbiol, 20(1):218, 20 Jul 2020
Cited by: 12 articles | PMID: 32689945 | PMCID: PMC7372807
Universally high transcript error rates in bacteria.
Elife, 9:e54898, 29 May 2020
Cited by: 14 articles | PMID: 32469307 | PMCID: PMC7259958
Resistance to imatinib in patients with chronic myelogenous leukemia and the splice variant BCR-ABL1(35INS).
Leuk Res, 49:108-112, 12 Aug 2016
Cited by: 21 articles | PMID: 27658269 | PMCID: PMC5625826
Messenger RNA degradation in bacterial cells.
Annu Rev Genet, 48:537-559, 01 Oct 2014
Cited by: 133 articles | PMID: 25292357 | PMCID: PMC4431577
Review Free full text in Europe PMC
Go to all (73) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The differential stability of the Escherichia coli ompA and bla mRNA at various growth rates is not correlated to the efficiency of translation.
Gene, 72(1-2):141-149, 01 Dec 1988
Cited by: 24 articles | PMID: 3072245
The ompA 5' untranslated RNA segment functions in Escherichia coli as a growth-rate-regulated mRNA stabilizer whose activity is unrelated to translational efficiency.
J Bacteriol, 172(8):4472-4481, 01 Aug 1990
Cited by: 111 articles | PMID: 1695894 | PMCID: PMC213277
Synonymous codon selection controls in vivo turnover and amount of mRNA in Escherichia coli bla and ompA genes.
J Bacteriol, 178(9):2718-2720, 01 May 1996
Cited by: 20 articles | PMID: 8626345 | PMCID: PMC178002
Translation initiation and the fate of bacterial mRNAs.
FEMS Microbiol Rev, 30(6):967-979, 21 Sep 2006
Cited by: 95 articles | PMID: 16989654
Review
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: GM27241
Grant ID: GM35769