Abstract
Free full text

National estimates of the prevalence of Alzheimer's disease in the United States*
Abstract
Several methods of estimating prevalence of dementia are presented. For both Brookmeyer and the Chicago Health and Aging project (CHAP), the estimates of prevalence are derived statistically, forward calculating from incidence and survival figures. Here, the choice of incidence rates upon which to build the estimates may be critical. Brookmeyer used incidence rates from a number of published studies, whereas the CHAP investigators applied the incidence rates observed in their own cohort. The Aging, Demographics, and Memory Study (ADAMS) and the East Boston Senior Health Project (EBSHP) were sample surveys designed to ascertain the prevalence of AD and dementia. ADAMS obtained direct estimates by relying on probability sampling nationwide. EBSHP relied on projection of localized prevalence estimates to the national population. The sampling techniques of ADAMS and EBSHP were rather similar while their disease definitions were not. By contrast, EBSPH and CHAP have similar disease definitions internally, but use different calculation techniques, and yet arrive at similar prevalence estimates, which are considerably greater than those obtained by either Brookmeyer or ADAMS. Choice of disease definition may play the larger role in explaining differences in observed prevalence between these studies.
1. Introduction
Prevalence is a straightforward concept: it is simply the count or proportion of persons who have disease at a single point in time, in a defined population. As a proportion, prevalence does not imply a person's risk or probability of becoming affected by the disease in question. Instead, prevalence portrays the potential burden—for care, services, and other things—that the disease places on the population. The specific quality of the healthcare burden is also dictated by the duration, disability, and family resources (public and private) required to treat the disease or to cope with it. Because dementias are relatively common, have relatively long duration, and lead to marked impairment in social and occupational functioning, their burden level is high. Epidemiologists have routinely observed that persons who live longer with the disease are also more likely to be the ones captured in a cross-sectional survey and counted as “prevalent” cases.
Since 1980, most but not all of the prevalence figures for Alzheimer's disease (AD) that have been generated in the United States have come from community-based, cohort studies where the primary purpose was to investigate risk factors and protective factors based on incident cases. The prevalence figures were a by-product of the baseline effort to identify a disease-free cohort. Although these figures could be published as prevalence estimates for a specific community, many were not intended to represent the full-range of disease from very mild to severe cases [1]. More recently, the research field has taken a keen interest in mild cases of AD and in other forms of age-related, mild cognitive impairment.
US national prevalence estimates of AD have been produced using projection methodology [2–4], and they have also been obtained directly from a national cross-sectional survey known as the Aging, Demographics, and Memory Study (ADAMS) [5]. ADAMS produced prevalence figures, not only for AD and other dementia, but also for cognitive impairment not dementia (CIND) [6,7]. The current article focuses on some major prevalence estimates and how they were obtained. Brookmeyer provides an overview of forward calculation projection methodology and uses that approach to generate various national prevalence estimates for comparison purposes based on data from different populations—to note similarities and differences. Evans and Hebert, who always had an interest in including mild AD in addition to moderate and severe AD, describe their use of projection methodology, including the forward calculation method, and present national prevalence projections based on data from the East Boston Senior Health Project (EBSHP) and the Chicago Health and Aging Project (CHAP). Langa, Heeringa, and Plassman describe the ADAMS sampling methodology and provide prevalence figures for AD, other dementia, and CIND that were produced in ADAMS. In the Discussion, Kukull provides a synthesis of this material and gives some perspective.
2. Approaches for estimating AD prevalence in the United States population
National estimates of US AD prevalence have differed by a factor of more than two. It is important to understand the sources of uncertainty underlying the various methodologies for prevalence estimation. There are two main approaches for estimating the national prevalence of AD. The first approach is essentially a forward calculation method that relies on AD incidence rates [3]. The second approach relies on representative cross-sectional prevalence surveys of AD [2,6]. Both approaches have important sources of uncertainty. One of the most significant sources of uncertainty with the forward calculation approach concerns the critical assumptions about the age-specific incidence of AD. One of the most significant sources of uncertainty with the prevalence survey approach concerns the representativeness of the survey sample.
In this section the forward calculation approach is discussed and various comparisons are made of national prevalence estimates AD. Specifically there is an attempt to reconcile differences in the prevalence estimates produced using the forward calculation approach. In addition, forward calculation prevalence estimates are compared to corresponding ones based on a national prevalence survey.
The forward calculation method requires several critical inputs. One input is the age-specific incidence rates of AD. Another input is the survival rates among persons with AD. These two inputs are then used in a competing risks model. The competing risks model is a multi-state model which includes a healthy state, AD, and death. The AD state may be further subdivided into an early stage and advanced stage of disease. Fig. 1 illustrates the multi-state model and the transition rates from one state to the next. The prevalence estimates can be expressed in terms of the transition rates (e.g., see equation 5 in [8]). Once the prevalence estimates have been calculated (i.e., the percent of people with AD), the numbers of persons in a population living with AD are obtained by multiplying those prevalence estimates by population sizes.
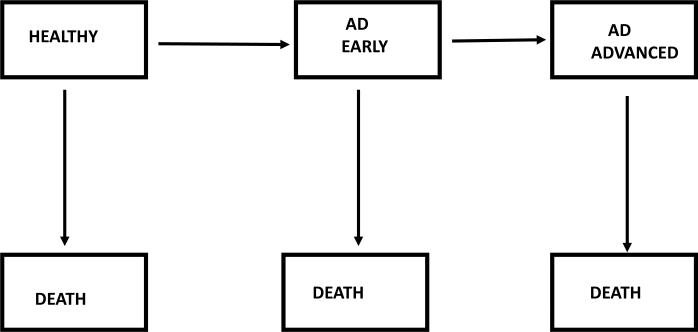
Multi-state model of progression of Alzheimer's disease. Transition rates from one state to the next may depend on age, gender, and calendar year
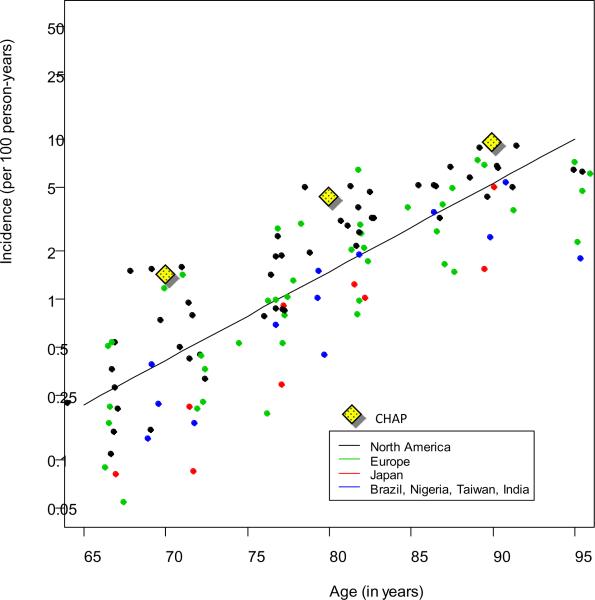
In order to implement forward calculation, the transition rates in Fig.1 must be specified. Brookmeyer and colleagues performed a forward calculation to estimate the global prevalence of AD [9]. Their calculation was based on systematic reviews of 27 published studies to determine the transition rates in Fig. 1 [10]. Here the transition rates used in those calculations are briefly reviewed. The age-specific incidence rate per 100 person-years versus age was found to increase linearly on a log scale, doubling about every 5 years (Fig. 2). Advanced-stage disease was defined as a state which takes on average 6 years to reach following diagnosis, which corresponds to an annual disease progression rate of about 16% per year. National vital statistics were used to determine background mortality rates (the transition rates from healthy state to death). The mortality among persons with advanced-stage AD was determined by adding 11% per year to the background mortality rates [9]. That determination was based on an additive model for death rates in which the parameters were calibrated from a published study on AD survival [11]. The results were consistent with several other published studies on AD survival. With these inputs placed into the competing risks model, the Brookmeyer calculations estimated that there were approximately 2.8 million Americans living with AD in 2008.
An alternative to forward calculation for determining national prevalence is based on nationally representative cross-sectional prevalence surveys. ADAMS is a nationally probability-based representative sample [5,6]. ADAMS estimated that in 2002 the US prevalence of dementia among persons over the age of 70 years was 3.4 million. With the ADAMS results, there is the opportunity to compare forward calculation estimates to survey estimates. However, the ADAMS figures are not directly comparable to the Brookmeyer figures because the ADAMS figures cited above refer to all dementia in 2002 among persons over the age of 70 years, while the Brookmeyer figure refers to AD cases in 2008. An update of the ADAM's study suggested that the US prevalence of AD in 2008 among persons over the age of 70 years was 2.6 million (see Appendix 5, p 131, in [12]). When the Brookmeyer analysis is restricted to persons over the age of 70 years, a prevalence of 2.65 million is obtained. Thus, the two approaches (the Brookmeyer forward calculation and ADAMS survey approach) give fairly consistent prevalence estimates (2.65 versus 2.60 million).
Another forward calculation by Hebert and colleagues yielded somewhat different results [4]. That calculation was based on AD incidence from CHAP, which was conducted in biracial neighborhoods in Chicago. Hebert calculated that in 2000 there were 4.5 million Americans living with AD who are over the age of 65 years. When those numbers were updated to 2008 and also included cases under the age of 65 years, it produced AD prevalence in 2008 of 5.2 million (see page 130 in [12]). That number is about two times larger than suggested either by the Brookmeyer forward calculation or the ADAMS prevalence survey.
If the Brookmeyer and Hebert estimates both use forward calculation methods, then why do they give such different estimates? The main reason is that different assumptions about AD incidence were used. Fig. 2 illustrates the incidence estimates from the systematic review [10] and the CHAP estimates [13]. The CHAP incidence rates were provided in 10-year age intervals [13], and here they have been plotted at the midpoint of the intervals. The CHAP incidence rates used in the Hebert calculation are considerably higher than the incidence rates used in the Brookmeyer calculation. For example, at age 80 years, the rates were 4.73% per year for CHAP versus 1.48% per year from the systematic review. The differences are even more extreme at the younger ages (at age 70 years the rates were 1.45% per year from CHAP versus 0.13% per year from the review).
There were other differences with how the Hebert and Brookmeyer forward calculation estimates were produced, including different assumptions about survival of AD patients, and differences in the computing software used to perform the calculations. To determine the main reasons for the discrepancy between the two forward calculation estimates, the CHAP AD incidence was inserted into the Brookmeyer forward calculation software (keeping everything else the same), and it was found that the AD prevalence estimate increased by about a factor of 2.5. The conclusion from this sensitivity analysis is that the difference between the two forward calculation (Brookmeyer and Hebert) estimates is driven mainly by differences in the AD incidence rates that are input into the calculations.
One advantage of forward calculation is that it can be used to forecast future prevalence by disease severity, and evaluate the potential effect of preventive and therapeutics advances. Different scenarios can be investigated by modifying the transition rates. For example, forward calculation indicates that the number of people currently living with AD is 26.6 million and that number would grow to 106 million by 2050 [9]. Worldwide about 62% of persons with AD are women. About 43% have “advanced disease” where “advanced disease is defined as a state that takes on average six years to reach following the initial diagnosis of AD. If disease onset could be delayed an average of two years, then the worldwide prevalence in 2050 of AD would decrease by 22 million, with the decrease roughly evenly divided between early and advanced stage disease. If the onset of disease is not delayed but the progression of disease to advance stage is delayed, then prevalence actually increases. If disease progression and disease onset are both delayed by one year, then there is over a 9 million decrease in worldwide prevalence, with almost all of that decrease being among persons with advanced disease. The take-home message from these calculations is that even small delays in disease onset and progression can significantly reduce the global burden of disease. A web-based software application that implements the forward calculation methodology is available, which enables researchers to project the burden of AD, to investigate the sensitivity to input assumptions, and to evaluate the effect of potential interventions [14].
In closing, there are two main approaches for estimating prevalence, cross-sectional prevalence surveys and forward calculation. Both approaches have uncertainties. An important issue with cross-sectional prevalence surveys concerns their representativeness. Important sources of uncertainty with forward calculation concern the transition rates including the incidence rates of AD and also mortality rates. Both methodologies rely critically on the diagnostic criteria for AD case definition. Different thresholds for case definition will lead to different prevalence estimates. Having said that, it is also worth noting that while the absolute prevalence number is sensitive to the threshold used for case definition, relative changes are not. Worldwide AD prevalence will quadruple in the next 50 years due to the aging of the world population and the quadrupling conclusion is relatively insensitive to the diagnostic threshold of case definition.
3. AD projections from East Boston Senior Health Project (EBSHP) and Chicago Health and Aging Project (CHAP) data
Projections of AD prevalence in the US population were made from two similar source studies. The first [2] used estimates of AD prevalence from the EBSHP and was published in 1990. The second [4] used estimates of AD incidence from CHAP and was published in 2003. The most basic design elements were similar to those of previous approaches: estimates of current AD prevalence or incidence were taken from one or more studies. Estimates of the future growth of the US population were taken from the US Census. As in previous estimates, multiple other variables were considered: age, gender, education, race, and survival.
The source studies [13,15] for the two estimates used similar methods. Both were large population-based studies of people over age 65 years. Both achieved strong participation, 85% of all age eligible residents of a geographically defined community for the EBSHP and 78% of all age eligible residents for CHAP. Both were of clinically diagnosed rather than pathologically diagnosed AD. Ascertainment of AD in both studies was by direct evaluation and independent of receipt of health care. Both studies used the NINCDS-ADRDA diagnostic criteria except that individuals meeting criteria for probable AD and also having another condition potentially impairing cognition were retained [1,16]. As will be discussed subsequently, both used two-stage designs—that is, a first stage of population interviewing followed by a second stage of detailed clinical evaluation. In neither study was the second-stage sample restricted to the subjects who scored poorly on the first-stage cognitive testing. Rather, those evaluated at the second stage were a stratified random sample of all first-stage participants. This sample was from all levels of performance on the first stage tests, i.e., first-stage cognitive performance was used as a tool for stratifying the random sample for clinical evaluation rather than a screening test. In both studies, second-stage examiners were masked to first-stage cognitive performance. Uniform, fully specified criteria and procedures were used by examiners in both studies. In both studies, the criteria and procedures used detected mild as well as moderate and severe AD. Both studies considered age (by single year), education, and gender in forming estimates.
There were also differences both in the methods used in these two source studies and in the methods used to apply these source-study estimates to the US population. The source-study differences included the obvious differences in location and dates of the studies. The relevant portion of the EBSHP was conducted in East Boston, an almost exclusively White community of Boston, Massachusetts in 1982, and 1980 US Census figures were used for the estimates of the US population. CHAP was conducted in a biracial (African-American and non-Hispanic White) community of the southwest side of the City of Chicago. The relevant portion of the study was conducted from 1996 to 2000, and the 2000 US Census was used for US population estimates. In the 1990 estimates from the EBSHP, the prevalence of AD in the US population was estimated directly from the prevalence of AD observed in the source study.
In the 2003 estimates from the CHAP study, the prevalence of AD in the US population was estimated from the incidence of AD observed in the source study using the methods of Brookmeyer et al. [3]. Use of incidence estimates from the source study with conversion to prevalence in population allows better consideration of changes in survival over the projection period. Such changes in survival among people both with and without AD, and the aging of the population during the projection period affect projected prevalence. These estimates assume that the general increase in survival will affect both people with and without AD, but that the excess mortality associated with the disease will continue to be elevated to the same degree as it is now. The estimates were not sensitive to excluding a term for African-American race/ethnicity (not significant) from the 2003 estimates or to including a term for gender in either estimate. Adjustment for education and age was significant, but modeling educational level in different groups made little difference, and modeling age as a single linear term produced results fairly close to those presented.
The 1990 projections from the EBSHP are shown in Fig. 3 and Fig. 4, and the 2003 projections from CHAP are shown in Fig. 5 and Fig. 6. The results are reasonably similar: The 1990 estimates using EBSHP data and 1980 US Census population estimates suggest that AD prevalence in the US population will increase from 2.88 million in 1980 to 10.2 million in 2050 and will equal 5.12 million in the current year, 2010. The 2003 estimates using CHAP data and 2000 US Census population estimates suggest that AD prevalence in the US population will increase from 4.5 million in 2000 to 13.2 million in 2050 and will equal 5.1 million in the current year, 2010.
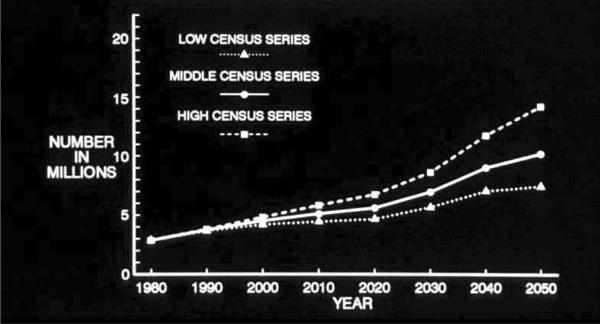
Projected number of people age 65 years or older with clinically diagnosed probable Alzheimer's disease (AD) in the US population from 1980 through 2050 using low, middle, and high US Census projections of population growth and AD prevalence data from the East Boston Senior Health Project in East Boston, Massachusetts [15].
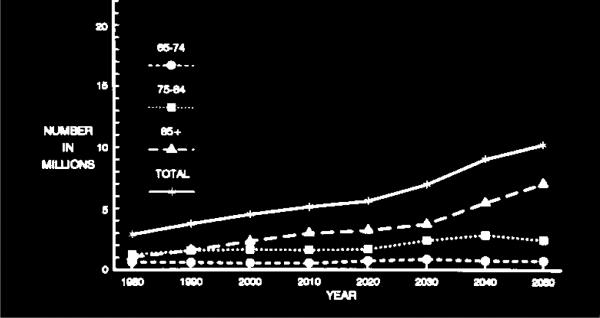
Projected number of people age 65 years or older with clinically diagnosed probable Alzheimer's disease (AD) in the US population from 1980 through 2050 by three age subgroups using the US Census middle series projection of population growth and AD prevalence data from the East Boston Senior Health Project (EBSHP) [15].
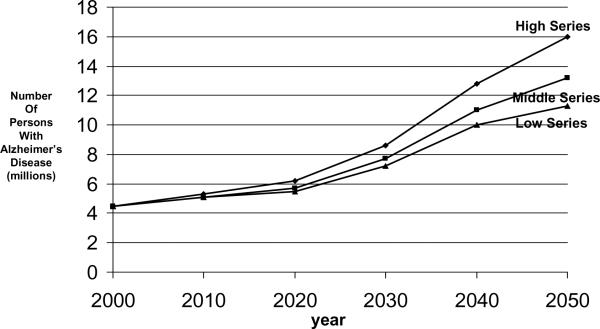
Projected number of people age 65 years or older with clinically diagnosed probable Alzheimer's disease (AD) in the US population from 2000 through 2050 using low, middle, and high US Census projections of population growth and AD incidence data from the Chicago Health and Aging Project (CHAP) [13].
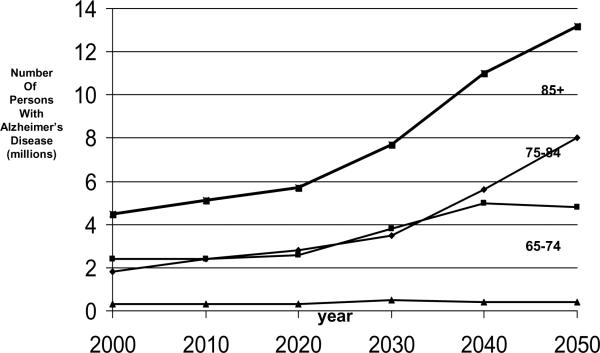
Projected number of people age 65 years or older with clinically diagnosed probable Alzheimer's disease (AD) in the US population from 2000 through 2050 by three age subgroups using the US Census middle series projection of population growth and AD incidence data from the Chicago Health and Aging Project (CHAP) [13].
In the 2003 projections, the number of affected persons projected in 2050 is higher than in the 1990 estimates as the methodology used in 2003 assumes that projected increases in survival will affect both persons with and without AD, with the ratio of survival among unaffected persons to survival among affected persons remaining constant at its present level whereas the 1990 methods did not. Some of the closeness of the estimates is likely due to chance. Some of the closeness, despite the diagnosis of AD being made by different skilled clinicians in the EBSHP and CHAP, is likely due to both studies using NINCDS-ADRDA criteria for the clinical diagnosis of AD and implementing these criteria in a very similar way in the field with strong emphasis on fully structured and specified methods.
Three series of US Census population growth estimates—the high, middle, and low series—are used to form the projections both in the 1990 effort (Fig. 3) and in the 2003 effort (Fig. 5). Use of these three series of estimates may be thought of as very roughly analogous in purpose to the use of a confidence interval about an estimate: the middle series represents the best estimate of population growth with current knowledge and reasonable expectations. The high and low series are projections that “do not represent likely scenarios in themselves, but purport to represent the extremes between which most likely outcomes should fall” [17]. Thus, the high and low series estimates represent brackets about the more likely middle series estimate of population growth.
Similar points deserve emphasis about both sets of estimates. The projected increase in the number of people affected by AD in the US population over age 65 years by 2050 is large. In the 1990 projections, the projected increase is from 2.88 million in 1980 to 10.2 million (middle series) by 2050. In the 2003 projections the projected increase is from 4.5 million in 2000 to 13.2 million (middle series) by 2050. Perhaps most importantly, in the two sets of projections presented here, this increase in number of people affected by AD is solely attributable to the increase in size of the oldest age groups of the population. These projections assume that the factors underlying the occurrence of AD will remain constant over this period of time. That is, they represent what will happen if society does not take any effective steps to prevent this disease over this period. Projected declines in death rates among people older than 65 years will both increase the number and proportion of people who will survive to the ages at which this disease become most prevalent and result in increased survival of people affected by AD.
As illustrated by Fig. 4 and Fig. 6, the projected overall increase in the number of people with AD in the US population is heavily driven by the growth in size of the over-85 subgroup of the population over age 65 years, and by the high occurrence of AD in this subgroup. The over-85 subgroup is the oldest subgroup shown in the figures and the oldest population subgroups are the ones increasing most rapidly in the populations of the United States and of all other developed countries. While the number of cases of AD in this subgroup increases sharply, the number of cases in the 75–84 year-old subgroup increases moderately, and the number of cases in the 65–74 year-old subgroup remains nearly constant. A substantial limitation of both the 1990 and the 2003 estimates is that both are based on AD estimates from a single population study, and no single community, including either East Boston, Massachusetts or Chicago, Illinois, is likely to be truly representative of the entire US population in many ways.
Projections of AD prevalence in the US population have varied substantially. In general, the differences between various estimates reflect primarily differences in the source studies, not differences in projection methodology. These differences include whether a single source study or multiple studies were used, whether the source study or studies are population-based, study size, and study design features. In studies using a two-stage design, two differences are especially important: first, whether second-stage evaluation for AD is wholly or mostly restricted to subjects who fail a brief first-stage cognitive screening procedure, or whether selection for second-stage evaluation is random from all strata of first-stage cognitive performance, and, second, whether second-stage clinical evaluators are efficiently masked to subjects' first-stage cognitive test performance. The assumption that efficiently masked skilled clinical examiners will detect AD solely among study subjects who fail a brief cognitive tests and never or very rarely among those who score above a cut-off is unlikely, especially as, for efficiency, the proportion failing the test is generally set to be substantially smaller than the proportion passing [18]. Thus source studies using screening test failure to identify subjects for further evaluation are very likely to result in lower estimates of disease occurrence. Another important design feature of the source study is whether receipt of health care is a necessary feature of AD ascertainment, as AD and other dementing illnesses are typically substantially under-recognized in health care settings [19]. The criteria used in the diagnosis of AD and their implementation can also affect the estimates reached. This source of variation is increased because reasonable investigators can implement the same criterion system for AD in very different fashions. An especially difficult matter is that the clinical and pathological manifestations of AD typically develop and progress by minute degrees, often over a long period. AD prevalence or incidence estimates require placing a diagnostic cut point along the continuum between normality and disease. Because it is not clear exactly where this cut point should be placed, different investigators, each using the same diagnostic criteria, will place the cut point differently. In contrast, the methods of referring the source study estimates to the US population vary somewhat, but not greatly, and estimates of future US population growth vary little; usually US Census estimates are used.
Comparing the results of a previous projection [3] with the ones presented here is instructive about how the source studies used influence projection results. Brookmeyer et al. [3] used four source studies [20–23], strong methods of referring estimates from these studies to the US population, and US Census estimates of population growth. One of the source studies used [23] was the incidence phase of the EBSHP, which used stratified random sampling to ascertain disease. (The prevalence phase of the EBSHP was also the source study used for the 1990 projections described here.) The other three studies, however, used methodologies that gave lower estimates of AD, especially in the oldest age groups. One [21] used only medical records to identify cases; another [22] eliminated the low-scoring 10% of persons from the dementia-free cohort and used a restrictive protocol to detect incident disease. Another [20] examined a highly educated volunteer cohort which was likely more healthy than the total population. These differences in the source studies resulted in fairly large differences in current estimates of AD prevalence in the US population. The 1997 estimate by Brookmeyer et al. [3] was 2.32 million, substantially lower than the 2000 estimate by Hebert et al. [4] of 4.5 million. Both studies used similar US Census estimates of US population growth and similar projection methods, however, and Brookmeyer et al. estimated that US AD prevalence would increase to 8.64 million by 2047, an increase of 372% while Hebert et al. estimated US AD prevalence would increase to 13.2 million by 2050, an increase of 293%.
In conclusion, there is substantial variation in current estimates of AD prevalence in the US population. Much of this variation is likely due to methodologic variations in the source studies used to form these estimates or is intrinsic to the nature of the disease. AD, like many other common chronic diseases, usually arises over time by minute degrees so that it is difficult for even highly skilled examiners to place a cut point between normality and disease precisely and uniformly even when using the same disease criteria. There is much less variation in estimating future changes in AD prevalence. With rapid increase in the oldest population age groups reliably forecast for both the US population and for the populations of all other developed countries, the future increase in AD prevalence will be large and strongly emphasizes the need to find preventive measures for this disease in the near future.
4. Informative probability sampling and major prevalence findings from the Aging, Demographics, and Memory Study (ADAMS)
ADAMS was designed to provide nationally representative data on the antecedents, prevalence, outcomes, and costs of dementia and CIND, using a unique study design based on the Health and Retirement Study (HRS). ADAMS is the first population-based study of dementia and CIND in the United States to include subjects from all regions of the country, while at the same time using a single standardized diagnostic protocol in a community-based sample. A sample of 856 individuals age 71 years or older who were participants in the ongoing HRS received an extensive in-home clinical and neuropsychological assessment to determine a diagnosis of normal, CIND or dementia [1]. Linkage of data from the ADAMS with detailed HRS longitudinal data on health, health care utilization, informal care, and economic resources and behavior, allows for in-depth investigations into the risk factors and outcomes of CIND and dementia, as well as the lifetime costs of dementia in the population.
4.1. ADAMS sampling methodology
The nationally representative HRS sample provided the sample frame for ADAMS [24,25]. From the larger nationally representative sample of approximately 7,000 HRS respondents age 70 years or older, a stratified, random subsample of 1,770 individuals was selected for participation in ADAMS. The ADAMS goal was to obtain clinical assessments on 850 individuals. ADAMS sample selection, initial consent, and final data management for the project were conducted by staff of the University of Michigan Survey Research Center, Ann Arbor. In-home evaluations for cognitive impairment and consensus conferences to establish final diagnoses were directed by experienced teams at the Duke University Program in Epidemiology of Dementia [5].
Early in the design stage of ADAMS, the investigators recognized that a field period of two or more years would be required to complete 850 in-home assessments with the nationally distributed subsample of HRS panel members. To maximize efficiencies in the field and to minimize the elapsed time between an HRS cognitive assessment and the ADAMS evaluation, the baseline sample was drawn in two phases. Each phase was based on a random ½ sample of the full HRS multi-stage sample design. All HRS respondents age 70 years or older at the time of the HRS 2000 interview were eligible for the initial ADAMS selection in Phase 1 sample areas. Likewise, all HRS respondents in Phase 2 areas who were age 70 years or older at the time of the 2002 HRS interview were eligible for Phase 2 sample selection.
To achieve a sufficient number of ADAMS respondents across the full range of cognitive ability, the Phase 1 and 2 samples were stratified based on cognitive test scores, gender, and age. Respondents were classified into major cognitive strata based on their performance on the cognitive measures in the designated HRS interview (either 2000 or 2002, depending on the ADAMS Phase assignment). Self-respondents were classified into cognition strata based on the full set of HRS cognitive tests (aggregate scores ranging from 0– 35). Proxy respondents were classified based on scores ranging from 1.0 to 5.0 on the IQCODE scale. (More details on the self- and proxy-respondent scales are available in documentation at the HRS website [26].) The combination of stratification criteria—HRS self or proxy interview status, cognition stratum, age and gender—yielded 18 explicit strata for the ADAMS baseline sample selection. The stratified sample for ADAMS Phase 1 areas was selected based on HRS 2000 cognition scores. A final stratified sample for the Phase 2 areas was selected based on updated cognition measures obtained in the HRS 2002 interview. A detailed description of the ADAMS sample design is available in a documentation report at the HRS website [27].
Table 1 summarizes the final disposition of the full sample of n=1,770 ADAMS selectees. In the total sample, ADAMS clinical evaluations and diagnostic assessments were completed with a total of n=856 sample individuals (48.4% of the sample, 55.6% of persons known to be alive at the time the ADAMS contact was attempted). In the time window between the 2000 or 2002 HRS interview and the subsequent ADAMS assessment attempt, 228 (12.9%) of the designated sample members died. An additional 59 (3.3%) sample members were believed to be alive but could not be located at the time of the scheduled assessment. A total of 499 (28.2%) sample individuals refused to participate in the ADAMS baseline assessment and an additional 128 (7.2%) could not participate for other reasons (including health and lack of a suitable proxy).
Table 1
ADAMS baseline sample dispositions by respondent type
| Sample disposition | Total | Self Respondent | Proxy Respondent |
|---|---|---|---|
| n=1770 | n=1238 | n=532 | |
| Assessed | 48.4% | 53.1% | 37.3% |
| No contact | 3.3% | 2.8% | 4.7% |
| Non-interview, other | 7.2% | 7.8% | 6.0% |
| Refused | 28.2% | 28.6% | 27.2% |
| Deceased | 12.9% | 7.8% | 24.8% |
| Cooperation among survivors | 56% | 57% | 50% |
Table 1 also illustrates the very different pattern of ADAMS sample dispositions for persons who were self-respondents or proxy respondents in the 2000 and 2002 HRS interviews that determined their sample stratum and sample selection status. The percentages of original sample cases that proved to be no contact, other non-interview, or refusals are very similar for the two respondent type groups but as expected the short-term mortality rates were much higher for persons who required a proxy respondent in the preceding HRS interview.
Since the in-depth ADAMS assessments occurred at varying lengths of time after the 2000 or 2002 HRS interview used to determine an individual panel member's eligibility and sample stratum assignment and the sample was subject to nonresponse, an in-depth analysis was performed of the potential selection bias due to differential mortality or other sources of selective attrition in the ADAMS baseline sample [27]. These analyses suggested that the natural process of mortality among the members of the original ADAMS sample did not introduce significant selection/attrition bias into the final sample of 856 ADAMS assessments for surviving members of the 70+ age cohort. Among the surviving members of the ADAMS sample, an investigation into factors associated with nonresponse identified being a man and pre-existing mental health conditions as significant factors in increased response propensity for sample persons who were self-reporters in the preceding HRS wave. For ADAMS sample members who required a proxy reporter at the previous HRS interview, lower cognitive functioning status is associated with slightly increased likelihood of an ADAMS assessment. To attenuate potential selection bias due to differential participation, the final ADAMS analysis weights include an attrition adjustment that controls for original cognitive stratum, gender, and age.
4.2. Prevalence findings
Table 2 provides sample characteristics for the 856 ADAMS participants based on dementia status. The sample is well-distributed across the range of age and education levels with a significant number of individuals age 90+ years and also a large percentage with 8 or fewer years of education.
Table 2
Characteristics of the ADAMS sample that completed a baseline assessment*
| All | All demented N (%) | AD N (%) | VaD N (%) | Dementia, undetermined etiology N (%) | Non-demented N (%) | |
|---|---|---|---|---|---|---|
| Overall | 856 (100%) | 308 (100%) | 229 (100%) | 48 (100%) | 23 (100%) | 548 (100%) |
| Age, yrs | ||||||
 71–79 71–79 | 355 (58.6%) | 62 (20.9%) | 37 (14.0%) | 14 (23.7%) | 8 (64.2%) | 293 (64.7%) |
 80–89 80–89 | 366 (33.7%) | 158 (58.6%) | 119 (62.7%) | 25 (56.8%) | 10 (29.1%) | 208 (29.7%) |
 90+ 90+ | 135 (7.7%) | 88 (20.5%) | 73 (23.3%) | 9 (19.5%) | 5 (6.7%) | 47 (5.6%) |
| Gender | ||||||
 Men Men | 355 (39.3%) | 95 (31.5%) | 59 (28.5%) | 20 (37.9%) | 13 (43.0%) | 260 (40.6%) |
 Women Women | 501 (60.7%) | 213 (68.5%) | 170 (71.5%) | 28 (62.1%) | 10 (57.0%) | 288 (59.4%) |
| Education, yrs | ||||||
 0–8 0–8 | 291 (17.4%) | 125 (33.5%) | 93 (32.2%) | 18 (33.8%) | 12 (48.0%) | 166 (14.7%) |
 9–11 9–11 | 144 (16.1%) | 53 (15.3%) | 39 (16.1%) | 7 (9.9%) | 4 (17.4%) | 91 (16.3%) |
 12 12 | 203 (29.4%) | 71 (27.2%) | 55 (29.2%) | 10 (32.2%) | 4 (6.4%) | 132 (29.8%) |
 >12 >12 | 218 (37.1%) | 59 (24.0%) | 42 (22.5%) | 13 (24.1%) | 3 (28.2%) | 159 (39.2%) |
| Race/ethnicity | ||||||
 Non-Hispanic White Non-Hispanic White | 613 (87.1%) | 218 (83.4%) | 162 (82.1%) | 36 (87.3%) | 15 (86.4%) | 395 (87.7%) |
 Non-Hispanic African-American Non-Hispanic African-American | 159 (7.6%) | 67 (12.4%) | 49 (12.9%) | 9 (10.5%) | 7 (12.3%) | 92 (6.9%) |
 Hispanic Hispanic | 84 (5.2%) | 23 (4.2%) | 18 (5.0%) | 3 (2.2%) | 1 (1.3%) | 61 (5.4%) |
AD- Alzheimer's disease, VaD – vascular dementia,
Standard design-based methods were used to estimate the population prevalence of AD, vascular dementia (VaD), all dementia, and CIND based on the initial in-home assessments of the ADAMS sample. Descriptive estimates of prevalence and model-based analyses of risk factors incorporated survey weights to reflect the differential probability of selection for the subjects in the ADAMS cognition strata and for non-response in the ADAMS assessment. The final weighted ADAMS prevalence estimates were initially post-stratified to 2000 Census population controls for household and nursing facility/extended care populations and then to July 2002 total population controls by age and gender. Standard errors for all descriptive estimates and model parameters account for the ADAMS complex sample design.
4.2.1. AD, VaD, and Dementia
Diagnoses were anchored by DSM-III-R and DSM-IV criteria for dementia and other currently accepted diagnostic criteria for AD and other subtypes of dementia were used. Table 3 shows the overall national prevalence estimates for AD and all dementia, stratified by gender and 9 or 10-year age ranges. As expected, the national prevalence of AD and all dementia increased with age, reaching 37.2% dementia prevalence among individuals age 90 years or older.
Table 3
National prevalence of dementia and Alzheimer's disease, by age categories
| Age,yrs | All dementia | Alzheimer's disease | ||||
|---|---|---|---|---|---|---|
| Combined | Men | Women | Combined | Men | Women | |
| 71–79 | 4.97 (2.61–7.32) | 5.25 (1.25–9.25) | 4.76 (1.82–7.70) | 2.32 (1.26–3.37) | 2.30 (0.80–3.81) | 2.33 (0.95–3.70) |
| 80–89 | 24.19 (19.28–29.11) | 17.68 (11.66–23.70) | 27.84 (20.41–35.28) | 18.10 (13.47–22.74) | 12.33 (5.82–18.84) | 21.34 (14.44–28.24) |
| 90+ | 37.20 (25.36–49.03) | 44.59 (21.70–67.47) | 34.69 (23.36–46.02) | 29.60 (18.59–40.61) | 33.89 (10.00–57.77) | 28.15 (17.61–38.69) |
| Total | 13.67 (11.21–16.12) | 10.80 (7.55–14.50) | 15.53 (12.23–18.83) | 9.51 (7.41–11.61) | 6.77 (4.25– 9.85) | 11.29 (8.35–14.23) |
Weighted percentages and (95% confidence interval).
Overall, AD accounted for approximately 69.9% of all dementia, while VaD accounted for 17.4%. Other types of dementia such as `dementia, undetermined etiology,' Parkinson's dementia, normal pressure hydrocephalus, frontal lobe dementia, alcoholic dementia, traumatic brain injury, and Lewy body dementia accounted for the remaining 12.7% of cases. With increasing age, AD accounted for progressively more of the dementia cases so that in the age group 90 years or older, AD accounted for 79.5% of the dementia cases compared to 46.7% among those age 71–79.
The estimated number of individuals nationwide age 71 years or older with AD was 2.3 million (95% CI: 2.8 to 4.0 million) and an estimated 577,000 (319,000 – 834,000) had VaD. The estimated number of individuals age 71 years or older in the United States in 2002 with any type of dementia was 3.3 million (2.8 to 4.0 million).
4.2.2. CIND
CIND was defined as: (1) mild cognitive or functional impairment reported by the participant or informant that did not meet criteria for dementia; or (2) performance on neuropsychological measures that was both below expectation and ≥ 1.5 standard deviations below published norms on any test. Diagnostic subcategories for CIND were used in an effort to reflect the variation in clinical presentation and potential differences in the etiology of the impairment. Further details are available in this volume on how the definition of CIND was operationalized in ADAMS [1].
Table 4 shows the national prevalence estimates for CIND and some of its more frequent subtypes, stratified by 9 or 10-year age ranges. The overall prevalence of CIND in the United States for individuals aged 71 years or older was 22%. CIND prevalence increased with age, affecting nearly 39% of individuals age 90 years or older. The prevalence of prodromal AD increased substantially with age, accounting for 8% of those over age 70 years.
Table 4
National prevalence of cognitive impairment not dementia (CIND), by age categories
| Age,yrs | All CIND | Prodromal AD* | Vascular CIND and stroke | Medical conditions |
|---|---|---|---|---|
| N=241 | N = 98 | N = 54 | N = 55 | |
| 71–79 | 16.0% (11.5–20.5) | 5.5% (2.6–8.4) | 3.57% (1.4–5.6) | 4.7% (1.2–8.3) |
| 80–89 | 29.2% (24.3–34.1) | 9.7% (6.4–13.1) | 10.1% (6.4–13.9) | 5.4% (2.1–8.7) |
| 90+ | 38.8% (25.6–52.0) | 22.1% (11.8–32.3) | 2.7% (0.00–6.0) | 9.4% (0.2–18.6) |
| Total | 22.0% (18.5–25.5) | 8.1% (6.3–9.8) | 5.7% (3.8 – 7.6) | 5.3% (2.5–8.0) |
Unweighted N's. Weighted percentages and (95% confidence interval).
4.3 Discussion
ADAMS has produced the first prevalence estimates of dementia, AD, VaD, and CIND in a nationally representative sample in the United States that included individuals from all regions of the country. To allow comparison with findings from previous studies using a lower minimum age (i.e., either age 60+ years or 65+ years), the estimates from ADAMS for ages 71 years or older were combined with those from other studies for ages 60 to 70 years. This resulted in an estimated total of 3.7 million individuals with dementia and just over 2.5 million with AD in the United States. The sole previous national estimate of dementia prevalence was 2.9 million, based on a Delphi consensus review of previously published studies in the United States [28]. The four previous national estimates of AD prevalence differed by greater than two-fold and ranged from 2.1 million [29] to 4.5 million [2,4]. The lowest estimate came from a meta-analysis of 18 US and European studies; the highest from the East Boston and Chicago community studies [2,4]. Variability in prevalence estimates of AD due to geographic factors has been discussed. In addition to the issue of extrapolation from regional samples, one likely source for variation among AD prevalence estimates is the use of different criteria for dementia. Some studies used criteria that do not require evidence of impaired functional performance [16], while most used criteria requiring significant impairment in social or occupational functioning. Another likely source of study variation is the use of different methods to identify the “border” between CIND and dementia. To explore this point, additional analyses of ADAMS data were performed that included longitudinal 18-month follow-up assessments of those diagnosed with CIND. For these analyses, all of those individuals who progressed to AD at follow-up were considered to have had `AD at baseline.' Because the ADAMS sample consisted of individuals aged 71 years or older, prevalence estimates of dementia for age 60–71 years were obtained from other studies and combined with estimates from the present study. This resulted in an estimate of 3.1 million individuals age 60 years or older with AD in the United States, up from the previous estimate of 2.3 million. These figures are still substantially lower than the highest AD prevalence estimates of 4.5 million [2,4].
In ADAMS the prevalence of CIND is 22% or about 5.3 million individuals in the United States age 71 years or older. These results suggest that the number of individuals with CIND in the United States is about 70% higher than the number with dementia. In the 71–79 year old age group, 16% had CIND, while an additional 5% had dementia, suggesting that more than one in five individuals in this age group has cognitive impairment as well as considerable life expectancy. To date, there are no other national estimates of the number of individuals with CIND in the United States to compare to these ADAMS estimates. Studies of the frequency of other medical conditions, such as stroke, hypertension, and cancer, suggest substantial regional variation throughout the United States. Thus, similar regional differences for cognitive impairment are possible. Reviews often report CIND prevalence ranging widely from 5% to 29% [30,31]. Even so, it is striking that estimates from the few available US regional and Canadian samples report CIND prevalence figures of 17% to 23% [32–34], closely bracketing the ADAMS estimate of 22%. Selected European population studies using different CIND criteria report prevalence rates ranging from 21% to 27% [35,36].
ADAMS has several strengths: a representative, directly assessed sample of the US population age 71 years or older; the inclusion of large numbers of individuals with few years of education; a sizeable sample over age 90 years; and the inclusion of long-term care residents. All of these groups have a high prevalence of CIND and dementia. In addition, employing a single, experienced assessment team, successfully used in other population studies, and one common expert case review panel likely minimized diagnostic variability.
Some limitations also exist. The participation rate was lower than hoped for but comparable to other population studies of this age group, such as the Cardiovascular Health Study (participation rate of 57.3%) and the Canadian Study of Health and Aging (68.5%). Both studies have made major scientific contributions toward improved understanding of health and memory in late life. Nonparticipation in all such studies could result in selection bias. ADAMS has addressed potential non-response bias using detailed archived information from prior interviews, although models based on measures collected 6–18 months prior to the ADAMS assessment may not fully capture selection bias. However, given the range of available measures, it is likely that the response propensity models and the associated weighting adjustments do capture the major factors that could contribute to any significant selection bias in population estimates based on the ADAMS data.
As the elderly US population grows, the number of individuals with CIND and dementia will also increase, making planning for the long-term care needs of these individuals increasingly important. The value of ADAMS, the first study of CIND and dementia in a nationally representative sample in the United States, extends beyond just estimating prevalence to being able to address many key questions in preparing for the care of older adults with CIND and dementia. These prevalence estimates provide the framework necessary to assess the impact of treatment advances as they become available. In the years to come, the ADAMS methodology can provide a marker of how well the country is doing with respect to the control and treatment of AD and other dementias. Regional studies in the United States will now have a national estimate with which to compare when exploring regional differences in disease patterns. The ADAMS data also can be enriched with other data collected from the ongoing HRS and with linked Medicare administrative records, allowing researchers to explore questions that might increase our understanding of, and ability to successfully address, the needs of an aging US population.
The ADAMS prevalence findings and accounts of how they were produced have been described previously [5–7].
5. Discussion
In principle, to determine prevalence one enumerates the underlying population and counts all its members who meet criteria for disease on a given day. Simple as it sounds that task is not easily accomplished. Sampling design, response factors, and diagnostic procedures all play key roles in determining the level of prevalence and its validity when proportions are applied to the US population structure over a span of calendar years. Stratified sampling schemes designed to estimate a proportion, like prevalence, have their precision and variability tied to the number of strata, the expected prevalence in each stratum, and the allocation or sampling fraction within each stratum [37]. Researchers may opt for proportional sampling from strata, for so-called “optimal” sampling, or for other designs to achieve the overall estimate.
Before undertaking to estimate prevalence, it is important to ask why one would want to know prevalence. In other words, what would be the purpose of estimating prevalence of AD or dementia? Focusing on the prevalence of severe cognitive impairment and disability might allow the estimation of the number of nursing home beds, or demand for institutional care or home healthcare. Focusing on newly diagnosed cases might reveal the need for family and caregiver counseling and training to develop coping skills. Expanding beyond currently available data to estimate prevalence of asymptomatic cases might indicate “treatment demand” (should disease modifying treatments become available). Current prevalence data are tied to estimates of clinically diagnosable dementia and, therefore, reflect a more general disease-disability burden or cost-and-suffering index visited on society. Average prevalence proportions can be used to project numbers of beds or other resources needed to adequately care for affected persons. Age-specific prevalence estimates have tended to vary depending on the method used to ascertain cases and statistical methods used to calculate them. However, regardless of variation in prevalence proportions, the slopes of the age-prevalence curves appear to be rather similar. Specifically, estimated prevalence is reported to approximately double for every 5 years of age regardless of study [38].
Several elegant methods of estimating the prevalence of dementia and its subtypes were presented in this article. The first, Brookmeyer's forward calculation method (Section 2 and [8]), uses a carefully selected set of disease incidence estimates from existing published studies, coupled with survival estimates and competing risk data, to produce overall and age-specific prevalence proportions. The resulting prevalence estimates are then a function of both the rate at which new cases occur (incidence), and how long individuals live with the disease (duration or survival). Survival with the disease is also associated with their risk of dying from another disease (competing risks). Competing risks of death also vary by age, in kind and magnitude, and influence the observed dementia disease duration through their effects.
Both Brookmeyer (Section 2 and [3]) and Evans and Hebert (Section 3 and [4]) applied the forward calculation method to produce US national prevalence estimates of AD. Brookmeyer uses source data from multiple studies, whereas Evans and Hebert use source data only from CHAP. Conceivably the use of source data from multiple studies leads to an “average” definition of dementia and its onset may have some stability—so the composite prevalence is roughly equal to the incidence times the disease duration. The Evans and Hebert prevalence estimates were substantially higher than those of Brookmeyer.
Evans and Hebert, using a different projection approach, produced a second set of national prevalence estimates of AD, based on source data from EBSHP. Essentially they projected prevalence proportions from EBSHP to the larger national setting, taking into account various demographic variables. The resulting national estimates were very similar to those produced using CHAP incidence data and the forward calculation method (Section 3 and [2]). It is worth noting that both EBSHP and CHAP were completed by virtually the same team of investigators in different cities (Boston and Chicago) using very similar, if not identical, disease criteria and definitions. Both studies employed accepted sampling designs and techniques to conduct a two-stage case-finding approach.
ADAMS resulted from a national probability sample of persons over age 70 years (Section 4 and [5,6]. Prevalence estimates were produced that were more similar to those of Brookmeyer (Section 2 and [3]) than they were to those generated from EBSHP and CHAP (Section 3 and [2,4]). Despite careful design, only about half (n=856) of the 1,700 persons sampled agreed to participate, 308 of those were found to be demented, and an additional 241 were diagnosed as cognitively impaired. Accounting for sampling design, however, the estimated prevalence proportions were somewhat lower than given by these raw numbers alone. Diagnosis was assigned primarily by an expert panel review of data collected through in-home neuropsychological testing and interview of the subjects selected. Despite its strong points and detailed arguments to the contrary, some skepticism is still raised by the relative low response rates, potentially causing bias in the estimates and the relatively small sample overall used to project nationwide numbers of affected persons. Brief in-home cognitive testing as a basis for case ascertainment could also have affected the determination of prevalence in unpredictable ways. In addition, the disease definition applied by the expert panel was somewhat different from those used in EBSHP and CHAP [13,15,39]. The ADAMS investigators realized many of these potential limitations and attempted to address and explain their likely impact.
For ADAMS, EBSHP, and CHAP, all participants were screened with a cognitive battery, and on that basis were stratified into those more likely to have dementia and those less likely to have the condition. Other demographic variables were also used to refine further the stratification. Then a stratified sample of persons (across all strata) was selected and given detailed evaluations. Strata with lowest scores (i.e., more likely dementia) may have had higher sampling fractions that those with higher scores (i.e., less likely dementia). While the actual sampling fractions and stratum weights are somewhat unclear, it raises the possibility that, for EBSHP and CHAP, a few “cases” identified through clinical evaluation among those included in a stratum defined by higher scores, younger ages etc., could cause the estimated overall prevalence estimate to be greater because of the weight they add to the stratum. This is presented only as a technical caveat for interpretation or explanation of why prevalence estimates could differ due to sampling methodology. Certainly the sampling methods and techniques used in part to ascertain prevalent or incident cases in ADAMS, EBSHP, and CHAP were well established, and justified; thus, it may be more likely that differences in other factors could have lead to differences in estimates.
So, which estimate is “right” and which are left? It may come back to the purpose in formulating such estimates. Each study described earlier in this article has strong points as well as limitations. Agreement of figures from ADAMS and the forward calculation results of Brookmeyer, while interesting, does not diminish the greater estimated prevalence from EBSHP and CHAP. CHAP uses the same forward calculation method as the Brookmeyer study, yet CHAP and EBSHP use the same disease definitions and arrive at similar prevalence estimates. Thus, differences in disease definition/threshold appear to drive differences in prevalence.
Adding earlier stage or milder cases from along the clinical disease continuum, or broadening the definition of disease could certainly affect projections of disease burden. More recently, it has also been hypothesized, through the results of imaging and biomarker studies (e.g., [40]) as well as neuropathological studies, that certain pathologic features of AD may form many years before any symptoms are observable clinically. This has led the research field to conceive of asymptomatic prevalence, but prevalence studies of that construct appear far off in the future. Or maybe not.
While delaying symptom onset may decrease clinical prevalence (primarily due to competing risks of death), delaying symptom progression could potentially increase prevalence if the force of competing mortality risks were not as strong in those affected with dementia. Thus, how the neurodegeneration of AD affects body systems, other than simply cognition, may contribute to the estimated prevalence. For example, neurodegeneration and vascular pathology may interact synergistically [41], resulting in an increased symptom progression and a decreased survival. These hypothetical scenarios await substantiation but at the same time raise new potential prevalence targets for estimation. Estimating prevalence by disease severity strata for example, consistent with the Clinical Dementia Rating [42] or other common metric, may be additionally informative. Competing risks are also likely to vary by ethnicity and other factors. As more is learned and researchers are able to apply diagnostic biomarkers together with the probability of clinical disease expression, more useful and consistent prevalence estimates should become evident and would be tailored to specific purposes.
Acknowledgments
This study was supported by National Institute on Aging grants R01AG011101, U01AG009740, R01AG027010, U01AG016976, and by National Institute on Aging contracts N01AG12106 and N01AG02107.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement for authors The authors have no conflicts to disclose. The sponsors had neither a role in the analysis or interpretation of these data, nor in the content of the paper. Appropriate approval procedures were used concerning human subjects.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jalz.2010.11.007
Read article for free, from open access legal sources, via Unpaywall:
http://deepblue.lib.umich.edu/bitstream/2027.42/153120/1/alzjjalz201011007.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.jalz.2010.11.007
Article citations
Senescence-related genes and proteins in the development of Alzheimer's disease: evidence from transcriptomic and Mendelian randomization analysis.
Front Aging Neurosci, 16:1423725, 02 Aug 2024
Cited by: 0 articles | PMID: 39156738 | PMCID: PMC11327092
Protocol for a telephonic mixed methods study to understand needs and find solutions for bereaved dementia caregivers.
BMJ Open, 14(7):e086559, 22 Jul 2024
Cited by: 0 articles | PMID: 39038859 | PMCID: PMC11268041
Development of SERS Active Nanoprobe for Selective Adsorption and Detection of Alzheimer's Disease Biomarkers Based on Molecular Docking.
Int J Nanomedicine, 19:8271-8284, 14 Aug 2024
Cited by: 0 articles | PMID: 39161360 | PMCID: PMC11330857
Revised criteria for diagnosis and staging of Alzheimer's disease: Alzheimer's Association Workgroup.
Alzheimers Dement, 20(8):5143-5169, 27 Jun 2024
Cited by: 49 articles | PMID: 38934362 | PMCID: PMC11350039
Amyloid β accelerates age-related proteome-wide protein insolubility.
Geroscience, 46(5):4585-4602, 16 May 2024
Cited by: 0 articles | PMID: 38753231 | PMCID: PMC11335993
Go to all (190) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Sources of variability in estimates of the prevalence of Alzheimer's disease in the United States.
Alzheimers Dement, 7(1):74-79, 01 Jan 2011
Cited by: 43 articles | PMID: 21255745 | PMCID: PMC3145367
Operationalizing diagnostic criteria for Alzheimer's disease and other age-related cognitive impairment-Part 2.
Alzheimers Dement, 7(1):35-52, 01 Jan 2011
Cited by: 45 articles | PMID: 21255742 | PMCID: PMC3039838
Review Free full text in Europe PMC
Design of the Chicago Health and Aging Project (CHAP).
J Alzheimers Dis, 5(5):349-355, 01 Oct 2003
Cited by: 134 articles | PMID: 14646025
Prevalence of Alzheimer's disease dementia in the 50 US states and 3142 counties: A population estimate using the 2020 bridged-race postcensal from the National Center for Health Statistics.
Alzheimers Dement, 19(10):4388-4395, 17 Jul 2023
Cited by: 13 articles | PMID: 37458371 | PMCID: PMC10593099
Funding
Funders who supported this work.
NIA NIH HHS (14)
Grant ID: R01 AG011101-09
Grant ID: U01 AG009740
Grant ID: N01AG12106
Grant ID: R01AG011101
Grant ID: U01AG009740
Grant ID: N01AG02107
Grant ID: U01 AG009740-10
Grant ID: U01 AG016976
Grant ID: U01 AG016976-09
Grant ID: R01 AG027010
Grant ID: R01 AG027010-01
Grant ID: R01 AG011101
Grant ID: U01AG016976
Grant ID: R01AG027010
National Institute on Aging (6)
Grant ID: N01AG12106
Grant ID: R01AG027010
Grant ID: R01AG011101
Grant ID: U01AG009740
Grant ID: U01AG016976
Grant ID: N01AG02107