Abstract
Free full text

Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1
Abstract
Mutation at the R132 residue of IDH1, frequently found in gliomas and acute myelogenous leukemia, creates a neo-enzyme that produces 2-hydroxyglutarate (2-HG) from α-ketoglutarate (α-KG). We sought to therapeutically exploit this neo-reaction in mutant IDH1 cells which requires α-KG derived from glutamine. Glutamine is converted to glutamate by glutaminase (GLS) and further metabolized to α-KG. Therefore, we inhibited GLS with siRNA or the small molecule inhibitor BPTES (bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide) and found slowed growth of glioblastoma cells expressing mutant IDH1 compared to those expressing wild-type IDH1. Growth suppression of mutant IDH1 cells by BPTES was rescued by adding exogenous α-KG. BPTES inhibited GLS activity, lowered glutamate and α-KG levels, and increased glycolytic intermediates while leaving total 2-HG levels unaffected. The ability to selectively slow growth in cells with IDH1 mutations by inhibiting glutaminase suggests a unique re-programming of intermediary metabolism and a potential therapeutic strategy.
Introduction
Despite the availability of oxygen, cancer cells exhibit high glycolytic rates and increased lactate production, known as the Warburg effect or aerobic glycolysis, rather than high oxidative phosphorylation. Over the past decade, oncogenes (MYC, PI3K, RAS and AKT) and tumor suppressors (VHL and p53) have been documented to reprogram cancer cell metabolism to aerobic glycolysis (1, 2). Cancer cells also consume glutamine for energy or as a carbon skeleton or nitrogen donor (3, 4). Recently, the oncogene MYC was found to induce mitochondrial biogenesis and increase glutamine metabolism, indicating that MYC stimulates both aerobic glycolysis and glutamine oxidation (5).
Before the discovery of isocitrate dehydrogenase 1 (IDH1) and 2 (IDH2) mutations, succinate dehydrogenase (SDH) and fumarate hydratase (FH) mutations, which cause HIF-1α stabilization and hereditary cancer syndromes, were the only known oncogenic mutations of metabolic enzymes. IDH1 and IDH2 mutations occur frequently in malignant low grade gliomas, secondary glioblastomas, and acute myelogenous leukemias (AML) (2, 6–8). These discoveries underscore the importance of metabolic alterations in oncogenesis and suggest the possibility of targeting genetically altered cancer metabolism.
While wild-type (WT) IDH1 converts isocitrate and NADP+ to α-ketoglutarate (α-KG) and NADPH, mutated amino acids in IDH1 and IDH2 reside in the catalytic pocket and result in a neo-enzymatic activity: α-KG + NADPH → D-2-hydroxyglutarate (2-HG) + NADP+ (9). IDH1 mutations in gliomas and AML have, thus far, only been found at residue R132 which is most commonly mutated to a histidine (7). Mutations of IDH2 have been found at both R140 and R172 (10). All documented IDH1 or IDH2 mutations result in the ability to produce 2-HG from α-KG (9, 10).
Although the role of IDH1 mutation in tumorigenesis has not been determined, changes in enzymatic function which result from IDH1 mutationlikely contribute to tumor formation. Decreased NADPH production from loss of IDH1 WT function coupled with increased 2-HG levels could lead to oxidative stress (11, 12). Secondly, 2-HG interferes with the electron transport chain and could alter mitochondrial physiology and drive cells toward aerobic glycolysis (13). Due to structural similarity between 2-HG and α-KG, 2-HG could also interfere with the function of enzymes that utilize α-KG (e.g. histone demethylases). Lastly, 2-HG is produced in inborn errors of metabolism (L-2-or D-2-hydroxyglutaric aciduria) where the enzyme that metabolizes 2-HG (L- or D-2-hydroxyglutarate dehydrogenase) is non-functional (14, 15). Individuals with L-2-hydroxyglutaric aciduria have been documented to develop gliomas (16), but not in those with D-2-hydroxyglutaratic aciduria (the enantiomer produced by mutant IDH1/2).
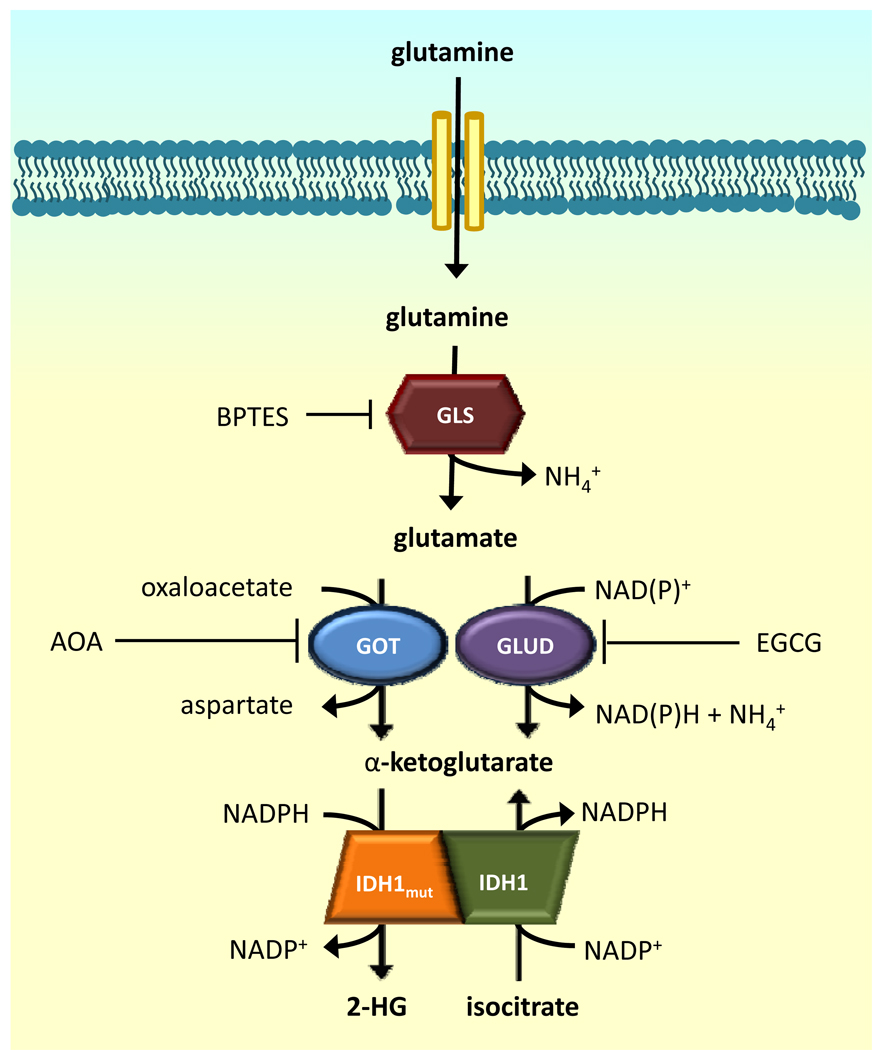
It is not clear if blocking IDH1 mutant activity would be an effective therapy, particularly if the mutant protein is only involved in tumor initiation. However, since mutant IDH1 tumors require α-KG to produce 2-HG, they could consequently be susceptible to alteration of α-KG homeostasis. It has been shown that 2-HG is primarily derived from glutamine (Figure 1) (9). Glutamine is hydrolyzed by glutaminase to produce glutamate which is subsequently converted to α-KG. α-KG is then converted to 2-HG by mutant IDH1. Thus, we sought to determine whether inhibiting glutaminase might perturb α-KG homeostasis and yield a selective response in cancer cells bearing IDH1 mutant enzymes.
Materials and Methods
Standard techniques were used to introduce the R132H mutation into human IDH1 engineered with a 6X-His-tag and produce lentivirus containing either WT or mutant IDH1. D54 cells or transformed normal human astrocytes (TNA) were transduced with virus, and lines were grown from individual cells using limiting dilution. IDH1 expression in response to 0.04 µg/mL doxycycline was confirmed using a 6X-His tag antibody (Millipore). LC/MS was conducted as previously described (9). siRNA was used to knockdown glutaminase, and cells were assessed for knockdown using Western blotting (5). Cell growth assays using alamarBlue were carried out as described following DMSO or BPTES treatment (17). Glutaminase activity was measured after treatment with DMSO or BPTES by incubating cell extracts with [3H]-glutamine. [3H]-glutamate was isolated from the reaction mixture using anion exchange and measured using a scintillation counter. All effects of BPTES were assayed 48 hrs after treatment. Data was evaluated using a two-tailed student’s t-test. A p-value of ≤0.05 was considered significant. Detailed materials and methods are available as supplemental information.
Results
Currently, no established glioblastoma cell lines are reported to possess IDH1 or IDH2 mutations, and our attempts to derive cell lines from patients with an IDH1 mutation have been unsuccessful.Therefore, we created tet-inducible, stable D54 glioblastoma cell lines that overexpress WT or R132H IDH1 (Figure 2A). Expression of R132H IDH1 decreased total IDH activity by 50% and 25% compared to cells overexpressing WT IDH1 and parental D54 cells, respectively (Supplemental Figure 1). This observation corroborates decreased IDH activity in cell culture models and glioblastoma tumor sections (7, 11, 18). 2-HG levels were elevated in mutant IDH1 cells compared to WT IDH1 expressing cells while α-KG levels were not significantly different (Figures 2B and 2C).
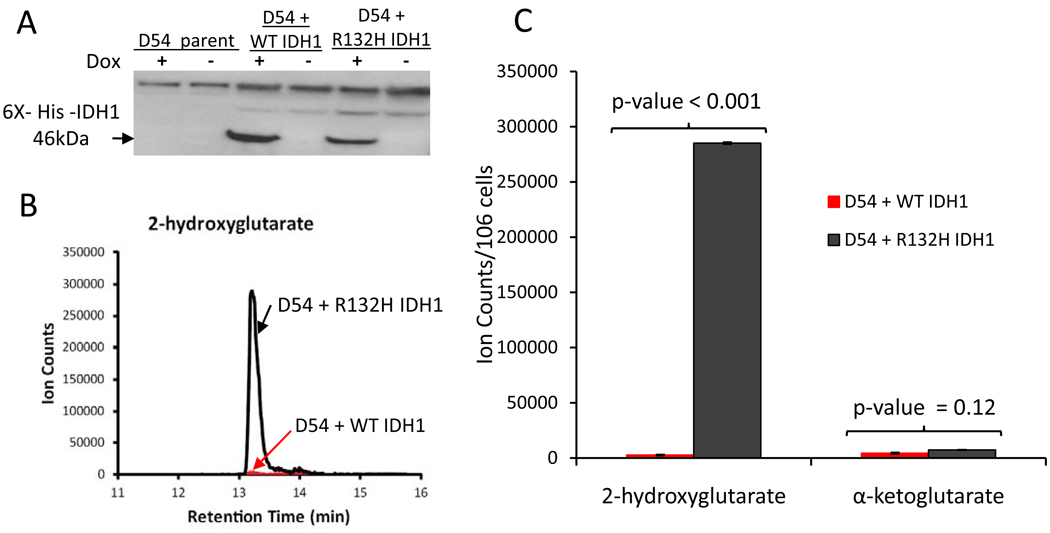
Validation of Tet-Inducible, Stable D54 Glioblastoma Lines. A. Western blot showing doxycyline-induced expression of 6X-His-Tag-IDH1. B. 2-hydroxyglutarate LC/MS retention peaks. C. 2-HG and α-KG levels measured by LC/MS.
Since previous studies demonstrated that α-KG consumed by mutant IDH1 was derived from glutamine (Figure 1) (9), we targeted glutaminase with siRNA to determine if mutant IDH1 cells exhibited decreased growth compared to WT IDH1 cells. siRNA against glutaminase specifically slowed the growth of mutant IDH1 cells but not parental cells or cells overexpressing wildtype IDH1 (Figure 3A). Consistent with the effects of anti-glutaminase siRNA, BPTES (bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide), a glutaminase inhibitor (19), preferentially slowed growth of mutant IDH1 cells without inducing apoptosis (Figure 3B, Supplemental Figures 2 and 3). We also treated TNA which overexpressed R132H or WT IDH1 with BPTES and found that mutant IDH1 cells were also more sensitive to BPTES (Supplemental Figure 4).
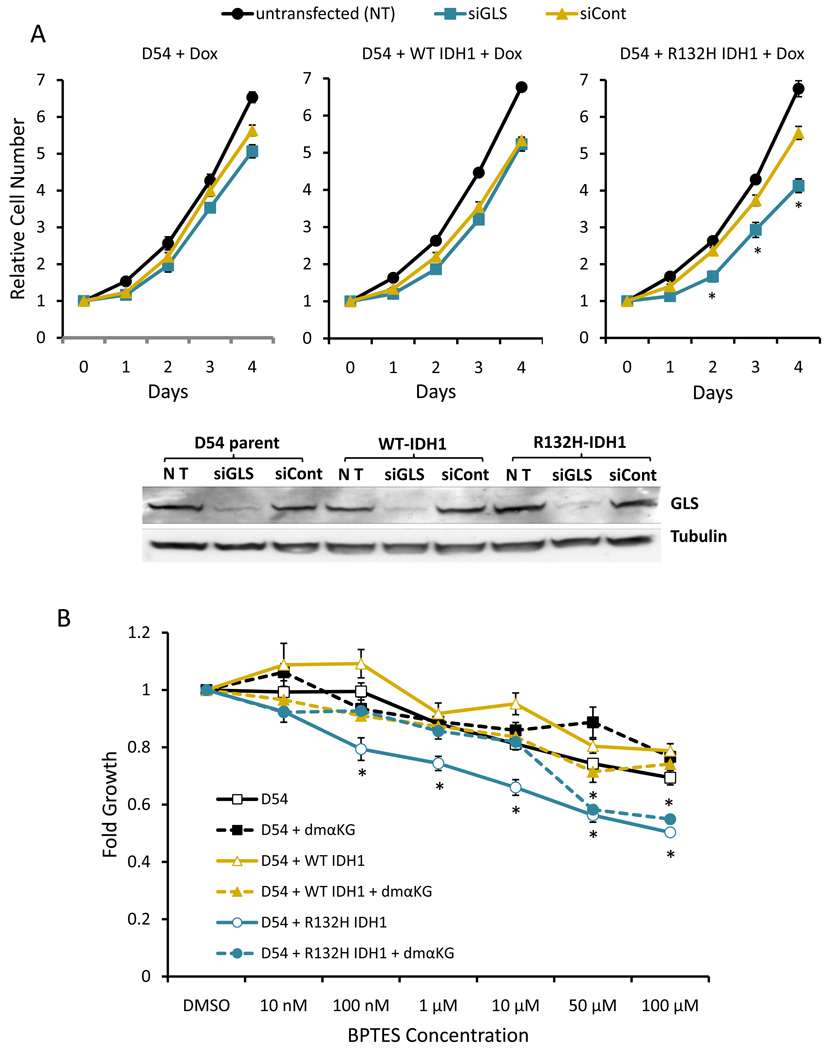
Mutant IDH1 cells depend on glutaminase for cell growth and glutaminase inhibition is negated by dimethyl-α-ketoglutarate. A. Anti-glutaminase siRNA (siGLS) slows growth of mutant IDH1 cells. Western blot shows decreased levels of glutaminase in response to siGLS. B. Effects of BPTES in the absence or presence of 1mM dimethyl-α-ketoglutarate were measured. B shows one representative experiment of three with similar trends and the average and SEM of four replicates at each concentration. *corresponds to p-value ≤ 0.05. For A, p-value is for siGLS compared to siCont. Cell number was normalized to day 0. For B, the p-value was for D54 + R132H compared to D54 and D54 + WT IDH1. Fold growth represents the ratio of alamarBlue fluorescence units of treated cells to vehicle treated cells.
We then sought to restore α-KG levels in BPTES treated cells to determine whether this would ameloriate growth inhibition. Exposing cells to dimethyl-α-ketoglutarate, a cell permeable α-KG precursor, significantly reduced growth inhibition of mutant IDH1 cells by BPTES (Figure 3B, Supplemental Figure 2). These observations suggest that glutaminase inhibition in mutant IDH1 cells decreased α-KG levels, potentially altering intermediary metabolism and consequently inhibiting cell proliferation. The effects of BPTES at higher concentrations, however, were not blocked by 1 mM dimethyl-α-ketoglutarate. Although the reasons for this are unclear, , it is possible 1 mM dimethyl-α-ketoglutarate is not sufficient to rescue the effects of 50 or 100 µM, or BPTES may have additional growth inhibitory effects at elevated concentrations.
Glutaminase activity was significantly reduced in both WT and mutant IDH1 expressing cells (59% and 68% inhibition, respectively) by 10 µM BPTES (Figure 4A). Consistent with decreased glutaminase activity, BPTES treatment diminished glutamate and α-KG levels. Lowered α-KG led to decreases in subsequent TCA cycle intermediates (succinate and malate) as well as aspartate, which is derived from transamination of oxaloacetate with glutamate serving as the nitrogen donor. Surprisingly, 2-HG levels remained unchanged in treated mutant IDH1 cells (Figure 4B, Supplemental Figure 5). However, levels of glycolytic intermediates (fructose-1,6-bisphosphate, dihydroxy-acetone-phosphate, and 3-phosphoglycerate) increased with BPTES treatment indicating that glycolytic flux is altered. Intriguingly, citrate levels changed in diametrically opposite directions between WT and IDH1 mutant cells before and after BPTES treatment. Basal citrate levels were lower but increased in treated mutant IDH1 cells as compared with higher basal levels in WT IDH1 cells that decreased with BPTES treatment (Figure 4C).
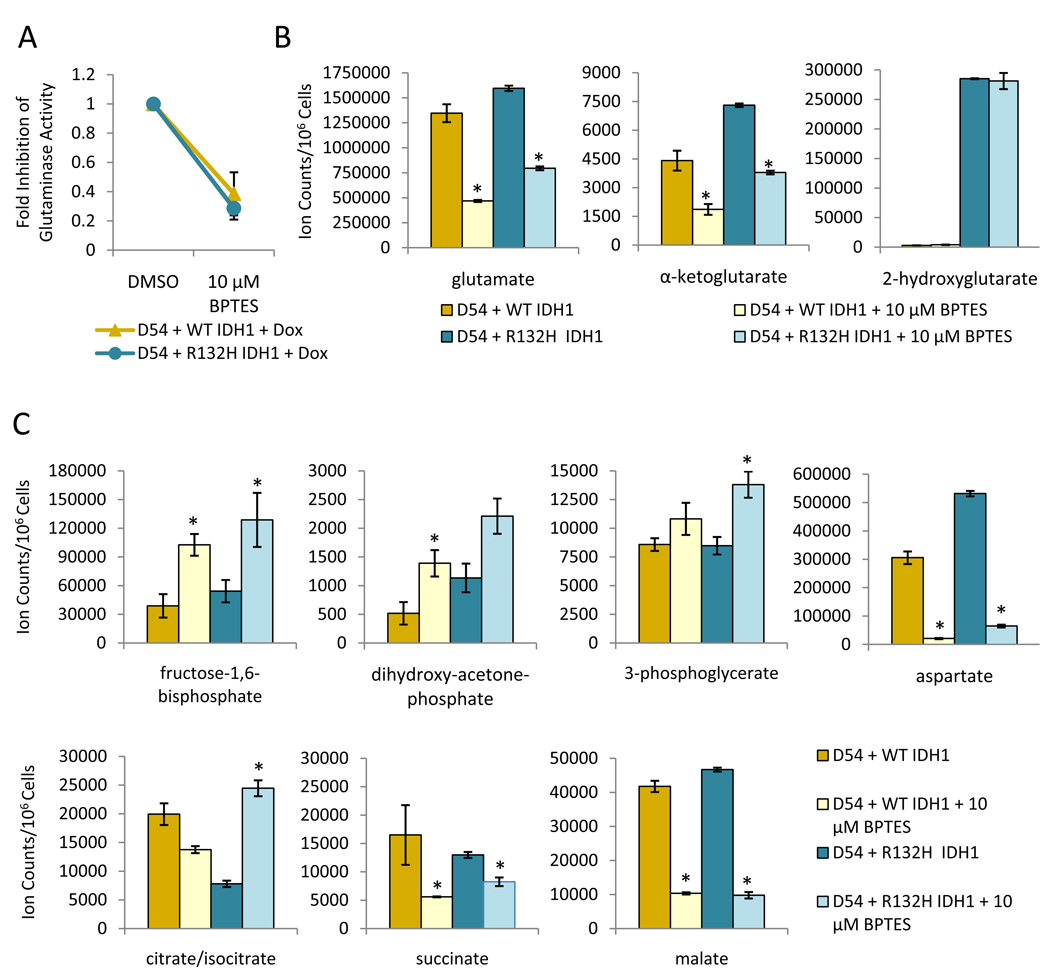
Metabolic changes result from overexpression of mutant IDH1 and 48 hrs of treatment with 10 µM BPTES. Glutaminase activity (A), and glutamate, α-KG, and 2-HG levels (B) were measured in WT and mutant IDH1 cells. C. Levels of other metabolites measured using LC/MS in response to BPTES treatment. For B and C, * represents a p-value ≤0.05. The p-value is for either D54 WT IDH1 DMSO versus 10 µM BPTES or D54 R132H IDH1 DMSO versus 10 µM BPTES.
To further demonstrate the sensitivity of mutant IDH1 cells to inhibition of α-KG synthesis, we inhibited enzymes which convert glutamate to α-KG. Mutant IDH1 cells were more sensitive to both epigallocatechin gallate (EGCG), a glutamate dehydrogenase inhibitor, and amino-oxyacetic acid (AOA), a pan-transaminase inhibitor. However, a reduced glucose concentration in the media was required to see effects of EGCG and AOA, suggesting that reduced glycolytic compensation is necessary to unmask the sensitivity of mutant IDH1 cells to these inhibitors (Supplemental Figure 6). Mutant IDH1 cells were not more sensitive to glutamine deprivation than WT cells (Supplemental Figure 7). Nonetheless, acquisition of mutant IDH1 activity appears sufficient to sensitize cells to inhibition of α-KG synthesis.
Discussion
Therapies that target various aspects of cancer cell metabolism are currently being developed and primarily focused on glucose metabolism (2). The dependence of cancer cells on glutamine for various processes is well documented (2–4) and has also been a target of interest for therapy; however, clinical trials have yielded little success due to a lack of efficacy or undesirable side effects of glutamine analogs (2, 4). Here, we explore an inhibitor of glutamine metabolism, BPTES, which allosterically inhibits glutaminase (GLS, but not GLS2) and is not a glutamine analog (19). Specifically, we studied the dependency of cancer cells with IDH1 mutation on glutaminase activity for maintenance of α-KG homeostasis.
The discovery of IDH1 mutations (6) identified a metabolic genetic alteration present in a large fraction of gliomas and cytogenetically normal AML (6, 8). Genetically, a clustering of heterozygous mutations in IDH1 at a single residue indicates a gain-of-function mutation which is supported by the gain of a new enzymatic activity by mutant IDH1. Rather than converting isocitrate to α-KG, mutant IDH1 consumes α-KG and produces 2-HG. Studies have demonstrated that glutamine serves as a cellular source of α-KG consumed by mutant IDH1 (9). It is not currently understood whether inhibiting mutant IDH1 and reducing 2-HG production would be therapeutically useful, because a role for mutant IDH1 or 2-HG in tumor maintenance has not been established. Thus, we sought to slow mutant IDH1 cell growth by inhibiting glutaminase.
Here we demonstrate that BPTES inhibits glutaminase activity and consequently lowers glutamate and α-KG levels in mutant and WT IDH1 cells, but only growth of mutant IDH1 cells is preferentially slowed in response to BPTES treatment. Intriguingly, BPTES treatment was associated with elevated glycolytic intermediates, which may reflect a compensatory increase in glycolysis to produce α-KG and maintain homeostasis. The notion that glutaminase inhibition perturbs α-KG homeostasis and causes growth inhibition is supported by rescue experiments using a membrane permeable α-KG precursor. Further, inhibition of enzymes which convert glutamate to α-KG showed selectivity for mutant IDH1 cells but only under glucose deprived conditions. Mutant IDH1 cells, however, were not more susceptible to glutamine deprivation than WT IDH1 cells, and withdrawing glutamine altogether will eventually slow the growth of any cell which requires glutamine as an essential nutrient. This result raises the possibility that inhibition of glutaminase may have a different therapeutic result on IDH1 mutant cells compared to inhibition of glutamine uptake.
Our metabolomic studies revealed a number of interesting findings. Even though BPTES treatment lowered glutamate and α-KG levels, 2-HG levels were not significantly decreased. Accordingly, if 2-HG competes with α-KG for binding sites on α-KG-dependent enzymes occupancy of these sites with α-KG would fall with BPTES treatment. While the effect would likely not be significant in wild-type cells, where α-KG presumably fills most sites, it could contribute to impaired cell growth upon BPTES treatment of cells expressing mutant IDH1. Second, BPTES treatment decreased subsequent TCA cycle intermediates, succinate and malate. Additionally, levels of glycolytic intermediates were increased demonstrating that metabolism is perturbed even far from the TCA cycle. We hypothesize that glycolytic intermediates are increased because of increased glycolytic flux to compensate for lowered α-KG levels; however, we have not ruled out the possibility that intermediates build up due to decreased glycolytic flux. One intriguing observation is the diametrically opposite changes in citrate levels between treated WT and R132H IDH1 cells. Currently, the reasons for these differences are not fully understood, and the dissection of these mechanisms is beyond the scope of this paper. Nonetheless, differences in intermediary metabolism between WT and mutant IDH1 cells are sufficient to provoke a gain of sensitivity to glutaminase inhibition in cells with mutant IDH1.
While reduction of glutaminase activity is significant in mutant IDH1 cells, the effects of BPTES on growth are modest (about 20% growth reduction). The modest growth reduction is not particularly surprising, since D54 cells have a genetic background that can tolerate significant siRNA-mediated reduction of glutaminase level. However, the simple overexpression of mutant IDH1 alters intermediary metabolism sufficiently to sensitize mutant IDH1 cells to glutaminase inhibition. We speculate that glioma cells with a naturally occurring IDH1 mutation would demonstrate increased susceptibility to glutaminase inhibition; however, no such cell lines exist at present.
Additionally, cellular metabolism is incredibly dynamic and appears to compensate for changes in intermediary metabolism, such as increased glycolysis, upon BPTES treatment. As a result, we propose that glutaminase inhibition will not be effective as a single arm therapy but will be a part of a more complex strategy that may involve simultaneous inhibition of glycolysis.
Like all treatments, there could be potential disadvantages to this therapeutic strategy since glioblastoma cells engineered with mutant IDH1 or IDH2 could have a pseudohypoxic phenotype (18). Previous studies documented that exogenous α-KG could reactivate prolyl hydroxylase, decrease HIF-1α levels, and inhibit cell growth resulting from pseudohypoxia elicited by mutations in SDH and FH (20). As such, reducing α-KG by glutaminase inhibition could hypothetically enhance pseudohypoxia and favor tumor growth.
Our study demonstrates that glutaminase could be a potential therapeutic target in mutant IDH1 cancer cells. However, further work is needed to investigate the metabolic consequences and biochemical specificity for mutant IDH1 cells in response to glutaminase inhibition. An understanding of these effects will be useful in developing combination therapies to augment the effects of glutaminase inhibition and provide insights into how IDH1 mutation and 2-HG production affect cellular physiology.
Supplementary Material
1
2
3
4
5
6
7
8
9
Acknowledgments
Grant Support
Support provided by the Ludwig Fund; NIH Grants R01NS052507, R01CA57341, R01CA051497; the AACR Stand-Up-to-Cancer grant; Bayer Schering Grants for Targets; the JHU Brain Science Institute; the Irving Sherman Neurosurgery Professorship (to GJR); and the Johns Hopkins Family Professorship in Oncology Research (to CVD).
Footnotes
Disclosure of Potential Conflicts of Interest
CVD and JDR are consultants for Agios Pharmaceuticals, Inc. GJR is a co-inventor on IDH1 related intellectual property managed by JHU.
References
Full text links
Read article at publisher's site: https://doi.org/10.1158/0008-5472.can-10-1666
Read article for free, from open access legal sources, via Unpaywall:
https://aacrjournals.org/cancerres/article-pdf/70/22/8981/2645018/8981.pdf
Free to read at cancerres.aacrjournals.org
http://cancerres.aacrjournals.org/cgi/content/abstract/70/22/8981
Free after 12 months at cancerres.aacrjournals.org
http://cancerres.aacrjournals.org/cgi/reprint/70/22/8981.pdf
Free after 12 months at cancerres.aacrjournals.org
http://cancerres.aacrjournals.org/cgi/content/full/70/22/8981
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1158/0008-5472.can-10-1666
Article citations
A patient-derived cell model for malignant transformation in IDH-mutant glioma.
Acta Neuropathol Commun, 12(1):148, 10 Sep 2024
Cited by: 0 articles | PMID: 39256867 | PMCID: PMC11385154
Emerging roles of the chromatin remodeler MORC2 in cancer metabolism.
Med Oncol, 41(9):221, 08 Aug 2024
Cited by: 0 articles | PMID: 39117768
Review
Discovery of Aloperine as a Potential Antineoplastic Agent for Cholangiocarcinoma Harboring Mutant IDH1.
Int J Mol Sci, 25(17):9226, 25 Aug 2024
Cited by: 0 articles | PMID: 39273177 | PMCID: PMC11395030
Treatment of IDH-mutant glioma in the INDIGO era.
NPJ Precis Oncol, 8(1):149, 19 Jul 2024
Cited by: 0 articles | PMID: 39025958 | PMCID: PMC11258219
Review Free full text in Europe PMC
Glutaminolysis is a Potential Therapeutic Target for Kidney Diseases.
Diabetes Metab Syndr Obes, 17:2789-2807, 23 Jul 2024
Cited by: 0 articles | PMID: 39072347 | PMCID: PMC11283263
Review Free full text in Europe PMC
Go to all (334) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Inhibiting glutaminase in acute myeloid leukemia: metabolic dependency of selected AML subtypes.
Oncotarget, 7(48):79722-79735, 01 Nov 2016
Cited by: 101 articles | PMID: 27806325 | PMCID: PMC5340236
Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations.
Exp Hematol, 42(4):247-251, 11 Dec 2013
Cited by: 105 articles | PMID: 24333121
Quantitative metabolome analysis profiles activation of glutaminolysis in glioma with IDH1 mutation.
Tumour Biol, 35(6):5911-5920, 05 Mar 2014
Cited by: 72 articles | PMID: 24590270
D-2-Hydroxyglutarate in Glioma Biology.
Cells, 10(9):2345, 07 Sep 2021
Cited by: 18 articles | PMID: 34571995 | PMCID: PMC8464856
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (7)
Grant ID: R01 CA051497
Grant ID: R01CA57341
Grant ID: R01 CA057341
Grant ID: R01 CA057341-20
Grant ID: R37 CA051497
Grant ID: R01CA051497
Grant ID: R37 CA051497-17
NINDS NIH HHS (3)
Grant ID: R01 NS052507
Grant ID: R01NS052507
Grant ID: R01 NS052507-04