Abstract
Purpose
Aromatase inhibitors (AIs) are increasingly used as adjuvant treatment of postmenopausal women with hormone receptor-positive breast cancer. AIs are commonly associated with musculoskeletal symptoms. The primary objective of this study was to describe the musculoskeletal symptoms that developed in the first 100 subjects enrolled who had at least 6 months follow-up.Methods
Women with early stage hormone receptor-positive breast cancer were recruited into a multicenter randomized clinical trial to study the pharmacogenomics of two AIs, exemestane, and letrozole. Patients completed the Health Assessment Questionnaire (HAQ) and Visual Analog Scale (VAS) at baseline, 1, 3, 6, and 12 months to assess changes in function and pain, respectively. Patients were referred for evaluation by a rheumatologist if their HAQ and/or VAS scores exceeded a predefined threshold.Results
Forty-four of 97 eligible patients (45.4%) met criteria for rheumatologic referral. Three patients were ineligible because of elevated baseline HAQ (2) and failure to initiate AI therapy (1). No baseline characteristics were significantly associated with referral. Median time to onset of symptoms was 1.6 months (range 0.4-10 months). Clinical and laboratory evaluation of patients evaluated by rheumatology suggested that the majority developed either non-inflammatory musculoskeletal symptoms or inflammation localized to tenosynovial structures. Thirteen patients discontinued AI therapy because of musculoskeletal toxicity after a median 6.1 months (range 2.2-13 months).Conclusions
Musculoskeletal side effects were common in AI-treated patients, resulting in therapy discontinuation in more than 10% of patients. There are no identifiable pre-therapy indicators of risk, and the etiology remains elusive.Free full text

Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors
Abstract
Purpose
Aromatase inhibitors (AIs) are increasingly used as adjuvant treatment of postmenopausal women with hormone receptor-positive breast cancer. AIs are commonly associated with musculoskeletal symptoms. The primary objective of this study was to describe the musculoskeletal symptoms that developed in the first 100 subjects enrolled who had at least 6 months follow-up.
Methods
Women with early stage hormone receptor-positive breast cancer were recruited into a multicenter randomized clinical trial to study the pharmacogenomics of two AIs, exemestane, and letrozole. Patients completed the Health Assessment Questionnaire (HAQ) and Visual Analog Scale (VAS) at baseline, 1, 3, 6, and 12 months to assess changes in function and pain, respectively. Patients were referred for evaluation by a rheumatologist if their HAQ and/or VAS scores exceeded a predefined threshold.
Results
Forty-four of 97 eligible patients (45.4%) met criteria for rheumatologic referral. Three patients were ineligible because of elevated baseline HAQ (2) and failure to initiate AI therapy (1). No baseline characteristics were significantly associated with referral. Median time to onset of symptoms was 1.6 months (range 0.4–10 months). Clinical and laboratory evaluation of patients evaluated by rheumatology suggested that the majority developed either non-inflammatory musculoskeletal symptoms or inflammation localized to tenosynovial structures. Thirteen patients discontinued AI therapy because of musculoskeletal toxicity after a median 6.1 months (range 2.2–13 months).
Conclusions
Musculoskeletal side effects were common in AI-treated patients, resulting in therapy discontinuation in more than 10% of patients. There are no identifiable pre-therapy indicators of risk, and the etiology remains elusive.
Introduction
Aromatase inhibitors (AIs) are increasingly used for the adjuvant treatment of hormone receptor-positive breast cancer instead of or in sequence with tamoxifen [1–6]. These medications are more effective than tamoxifen and have less risk of potentially life-threatening toxicities, including thromboembolic disease and endometrial cancer. However, AIs can cause short- and long-term side effects, including menopausal symptoms, bone loss, and musculoskeletal symptoms.
Arthralgias and myalgias have been noted in up to one-third of patients treated with an AI [1–5, 7–9]. The reported incidence of musculoskeletal side effects varies widely across studies due to differences in definitions of toxicity. Based on cross-study comparisons the three AIs appear similar in both the frequency of these symptoms and their characteristics when used in the adjuvant setting. In a direct comparison of letrozole and anastrozole in the metastatic setting, a similar incidence of bone pain was reported for the two drugs [10]. The reported symptoms can substantially impact quality of life, and occasionally lead to early discontinuation of AI therapy in patients who develop severe symptoms [11, 12].
The mechanisms underlying the development of this toxicity remain unknown, and few studies have carefully evaluated those who developed this type of toxicity using a pre-defined threshold for referral and a standardized rheumatologic evaluation. The COnsortium on BReast cancer phArmacogenomics (COBRA) is currently conducting a multicenter, prospective randomized trial primarily to evaluate the pharmacogenomics of two AIs, letrozole and exemestane, in postmenopausal women with early stage hormone receptor positive breast cancer. A secondary objective of this trial, and the focus of this manuscript, was a prospective evaluation of consecutive individuals who developed new or worsening musculoskeletal symptoms while on therapy. Since few data regarding these side effects were available at the time the clinical trial was developed, the purpose of this preliminary analysis was to characterize the musculoskeletal symptoms that develop in breast cancer patients treated with an AI, and to determine if modifications should be made to the assessment methods being utilized in this clinical trial. This report summarizes the musculoskeletal symptoms and rheumatologic findings in the first 100 women enrolled in this prospective clinical trial.
Subjects and methods
Subjects
Eligible patients were recruited from August 2005 through September 2006 at the following COBRA-member institutions: Indiana University Cancer Center, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, and the University of Michigan Comprehensive Cancer Center. Women 18 years and older with stage I–III hormone receptor positive breast cancer were eligible for enrollment. Patients were required to be postmenopausal, defined as one of the following: (1) age>60 years, (2) prior bilateral oophorectomy, (3) amenorrhea for 1 year with intact uterus and ovaries, or (4) serum estradiol and FSH concentrations consistent with postmenopausal status and either amenorrhea for 6 months or prior hysterectomy at the time of enrollment. Patients were ineligible if they had bilateral mastectomy, radiation to the contralateral breast, prior AI therapy, or prior history of ovarian, endometrial, or fallopian tube carcinoma and/or primary peritoneal carcinomatosis. Prior tamoxifen was allowed. All indicated surgery, radiation therapy, and chemotherapy for breast cancer was completed prior to study enrollment. The protocol was approved by the Institutional Review Boards of all three participating study sites, and all enrolled patients provided written informed consent. The clinical trial was reviewed on a bi-annual basis by an independent Data and Safety Monitoring Committee.
Study design
This prospective rheumatologic evaluation was conducted as a component of an ongoing multicenter, open-label randomized clinical trial of women initiating treatment with an AI for adjuvant therapy of early stage breast cancer. Total accrual to the trial is anticipated to be 500 patients. The rheumatologic characterization of the first 100 patients enrolled who had all completed at least 6 months of follow-up was performed to more thoroughly evaluate the musculoskeletal signs and symptoms and rheumatologic diagnoses associated with AI therapy, and to assess the utility of the measures being used to evaluate musculoskeletal toxicity in this clinical trial.
Prior to initiating therapy, all patients underwent evaluation by history, physical examination, and laboratory assessment (complete blood counts and liver function tests). All patients completed a baseline modified Health Assessment Questionnaire (HAQ), a well-validated tool that has been used to evaluate the functional status of patients with rheumatic disorders for the past two decades [13]. HAQ scores were calculated as previously described, without accounting for the use of functional aides [13]. All patients also completed a pain Visual Analog Scale (VAS) at baseline, in which patients rated their overall pain level over the previous week.
Subjects were then randomly assigned to exemestane 25 mg orally daily or letrozole 2.5 mg orally daily for 2 years. Patients were reevaluated at 1, 3, 6, and 12 months to assess changes in medical history and concomitant medications and for physical examination. Patients completed HAQ and VAS questionnaires at each of these timepoints.
Rheumatology referral and evaluation
Patients were referred for a structured and detailed rheumatologic evaluation based on the presence of at least one of the following criteria: (1) HAQ score increased by more than 0.2 over baseline [14], (2) Pain VAS value ≥50 mm (of 100 mm VAS) for patients with no pain (VAS = 0 mm) at baseline, and (3) Pain VAS value increased and pain rated much worse or very much worse pain on self-rated clinical global impression scale (graded as 6 or 7) for patients reporting pain at baseline (VAS>0 mm) [15]. Patients were referred based on scores on questionnaires completed at routine follow-up visits or at interim times if they contacted study personnel about new or worsening symptoms. Patients were not referred to rheumatology if baseline HAQ score was >1.50 because of presumed clinically significant pre-existing functional incapacity.
Initially, the investigators predicted that 20% of patients would be referred for evaluation. In a predefined analysis planned for 6 months after study initiation, the actual rate of referral using the original criteria was approximately 30%, substantially higher than the anticipated level. Therefore, the criteria for referral based on HAQ score were made more stringent. Patients with baseline HAQ score between 0 and 0.8 were required to have an increase in HAQ score of 0.4, whereas those with baseline HAQ score of >0.8 were required to have an increase of 0.2. No change was made to the criteria used to refer patients based on increased pain.
Those patients who were referred underwent a single structured evaluation, including a comprehensive history and physical examination performed by a board-certified rheumatologist. In addition, the following laboratory studies were obtained at the time of the evaluation: complete blood count, comprehensive metabolic panel, thyroid stimulating hormone (TSH), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anti-nuclear antibodies (ANA), rheumatoid factor (RF), and creatine kinase (CK). If indicated, patients underwent additional evaluation with radiographs or arthrocentesis.
Statistical analyses
Study subjects were categorized according to whether rheumatology referral was made. Medians and ranges for continuous variables were calculated according to referral category and compared using the Wilcoxon rank-sum test. Counts and proportions were calculated for categorical variables according to referral category and compared using the Chi-square goodness-of-fit test or Fisher’s exact test (where appropriate). Statistical calculations were performed with SAS, Version 9.1 (SAS Institute, Inc, Cary, NC, USA). Two-tailed tests are reported for Wilcoxon and Fisher’s exact tests. For all comparisons, an alpha of 0.05 was defined as the level of statistical significance. Differences due to treatment assignment were not examined because of inadequate power to determine differences between the two drugs in this preliminary analysis.
Results
Patient characteristics
The first 100 patients enrolled in the clinical trial were followed for at least 6 months from initiation of AI therapy, with a 12-month median time of follow-up (range 6.5–20.1 months). Baseline patient characteristics are listed in Table 1. Prior breast cancer therapies included surgical resection (100%), adjuvant chemotherapy (54%), adjuvant radiation therapy (76%), and adjuvant tamoxifen therapy (54%). Of the patients treated with tamoxifen, the median duration of therapy was 2 years (range 0.1–13 years).
Table 1
Baseline characteristics of patients according to referral for rheumatologic evaluation
| Characteristic | All n = 100 | Referred n = 44 | Not referred n = 53 | p valued |
|---|---|---|---|---|
| Demographics | ||||
| Age, years; median (range) | 59 (38–83) | 58 (47–70) | 60 (38–83) | 0.5839e |
| Race | ||||
 Caucasian, n (%) Caucasian, n (%) | 89 | 38 (86.4) | 48 (90.6) | 0.8633e |
 African-American, n (%) African-American, n (%) | 9 | 5 (11.4) | 4 (7.6) | |
 Asian, n (%) Asian, n (%) | 2 | 1 (2.3) | 1 (1.9) | |
| Weight, kg; median (range) | 74.0 (40.7–150.6) | 75.3 (55.8–135.4) | 73.3 (40.7–150.6) | 0.3670e |
| BMI, kg/m2; median (range) | 28.8 (17.7–53.4) | 29.6 (22.2–53.4) | 28.1 (17.7–48.5) | 0.6506e |
| Prior breast cancer therapy | ||||
| Axillary surgery, n (%)a | ||||
 Sentinel LN biopsy Sentinel LN biopsy | 64 (76.2) | 28 (75.7) | 35 (79.5) | 0.6765f |
 Axillary LN dissection Axillary LN dissection | 35 (41.7) | 18 (48.6) | 15 (34.1) | 0.1841f |
| Chemotherapy, n (%) | 54 (54.0) | 22 (50.0) | 29 (54.7) | 0.9865f |
| Taxane, n (%)b | 29 (31.8) | 13 (34.2) | 14 (28.0) | 0.5315f |
| Radiation, n (%) | 76 (76.0) | 35 (79.6) | 40 (75.5) | 0.6334f |
| Tamoxifen, n (%) | 54 (54.0) | 25 (56.8) | 27 (50.9) | 0.5635f |
| Medical comorbidities | ||||
| Diabetes, n (%) | 8 (8.0) | 5 (11.4) | 3 (5.7) | 0.4620g |
| Thyroid disorder, n (%) | 12 (12.0) | 5 (11.4) | 6 (11.3) | 1.0000g |
| Any arthritis, n (%) | 36 (36.0) | 18 (40.9) | 16 (30.2) | 0.2706f |
| Prior fracture, n (%)c | 22 (22.2) | 12 (27.9) | 9 (17.0) | 0.1978f |
LN lymph node
Ninety-seven patients were eligible for referral to rheumatology; two were ineligible because of elevated baseline HAQ scores and one discontinued participation in the study prior to initiating protocol-directed therapy because of poor venous access. Forty-four patients (45.4%) qualified for referral to rheumatology, and 38 (39.2%) were evaluated. Four patients initially declined referral but later consented to see the rheumatologist because of persistent or worsening symptoms. Six patients declined referral and never underwent rheumatologic evaluation; one of these subjects subsequently discontinued participation in the study because of musculoskeletal toxicity.
Of the first 100 patients enrolled, 23 discontinued therapy with an AI. Rheumatologic toxicity was the identified cause for 13 of the discontinuations. Patients who stopped therapy or switched to a different AI because of musculoskeletal toxicity did so after a median 6.1 months (range 2.2–13 months). The remaining patients discontinued therapy for other reasons, including recurrent menses (3), non-compliance with therapy (3), dizziness, restlessness, depression, and inability to obtain blood samples.
There were no obvious baseline characteristics that distinguished those patients who met criteria for referral versus those who did not (Table 1). The patients included in the referred subset included all patients who met objective criteria for referral, regardless of whether they consented to rheumatologic evaluation. There was no statistically significant difference in baseline weight, body mass index, or concomitant medical illness. Similarly, there was no statistically significant difference in prior therapy for breast cancer, including type of axillary surgery, radiation therapy, prior tamoxifen, or prior chemotherapy, including taxanes.
Symptom characterization
Median time from initiation of AI to onset of symptoms was 1.6 months (range 0.4–10 months). Symptoms in four patients pre-dated study enrollment. Median time to rheumatology referral was 3.7 months (range 0.9–12.6 months), and 59% of patients were referred to rheumatology after taking an AI for 3–6 months (Fig. 1). Four patients were referred specifically because of symptoms that developed or worsened between scheduled clinic visits. Increased HAQ score led to referral in 17 patients. New onset pain or pain reported as much worse or very much worse led to referral of 20 patients. Six patients were referred because of a combination of pain and decreased function.
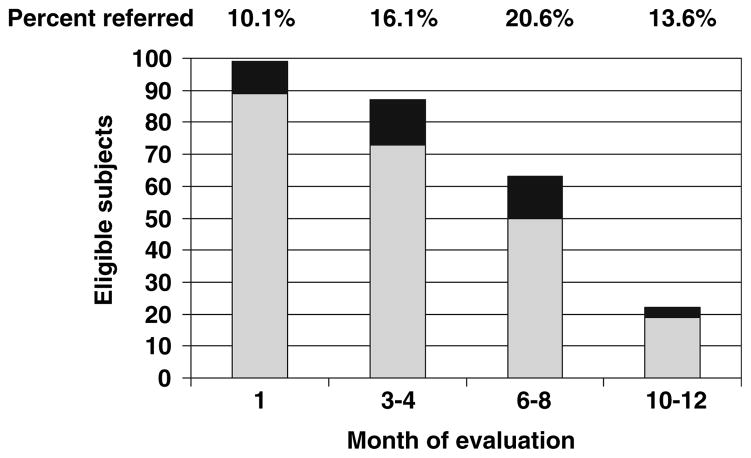
Distribution of rheumatology referrals over time. Light gray bars represent patients eligible for referral who did not qualify for referral at a given timepoint. Black bars represent patients eligible for referral who did qualify for referral at a given timepoint. Percentage at top of each bar represents percent of patients referred to rheumatology at each timepoint
All patients completed HAQ and VAS assessments at baseline (Table 2). Referred patients had statistically significantly higher baseline HAQ scores (p = 0.0440), although the median baseline HAQ score was 0 for both the referred and not referred cohorts. There was a trend toward higher baseline VAS scores for referred patients (p = 0.0664). At the time of referral, the median HAQ score for referred patients was 0.375, with a median absolute increase in HAQ score of 0.3125. Median VAS score at time of referral was 51, with a median absolute increase in VAS score of 32.5.
Table 2
Rheumatologic parameters of patients according to referral for rheumatologic evaluation
| Characteristic | Referred (n = 44) | Not referred (n = 53) | p value |
|---|---|---|---|
| Health Assessment Questionnaire | |||
| Baseline | 0 (0–1.0) | 0 (0–1.0) | 0.0440b |
| At referral | 0.375 (0–1.625) | – | |
| Absolute change | 0.3125 (–0.375 to 1.375) | – | |
| Visual Analog Scale | |||
| Baseline | 10 (0–59) | 2.5 (0–80) | 0.0664b |
| At referral | 51 (0–79) | – | |
| Absolute change | 32.5 (–12 to 78) | – | |
| Months to symptomsa | 1.6 (0.4–10.0) | ||
| Months to referral | 3.7 (0.9–12.6) | ||
| Months to evaluation | 5.4 (1.3–13.0) | ||
| Months to study discontinuation | 6.1 (2.2–13.0) | ||
Median values given, with ranges in parentheses
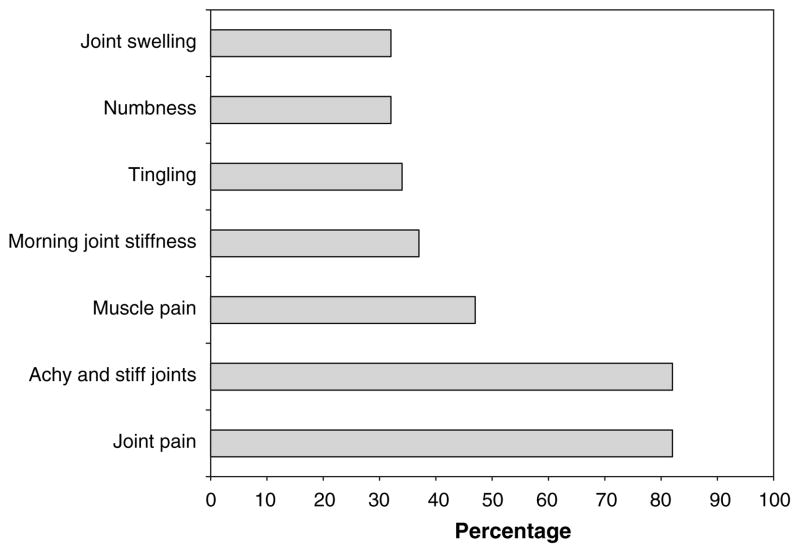
At time of rheumatology evaluation, the primary symptoms were joint pain and achy and stiff joints (Fig. 2). Other reported symptoms included muscle pain, morning stiffness, tingling, numbness, and joint swelling. In descending order, the joints primarily involved per patient report were hands and wrists (14 patients), shoulders (12), knees (11), feet and ankles (9), spine (7), and hips (5). Four patients reported specific muscle pain.
For management of symptoms, the vast majority of patients had used non-steroidal anti-inflammatory medications or acetaminophen. In addition, four patients had been prescribed tramadol or opioids. A majority of patients reported an increase in analgesic usage after initiating therapy with an AI.
Laboratory evaluation
None of the laboratory studies suggests a rheumatologic etiology for the musculoskeletal symptoms. Only a small fraction of patients had elevated levels of any of the following laboratory tests: TSH (5.3%), ESR (7.9%), CRP (18.4%), CK (10.5%), RF (5.3%), and ANA (16.2%) (Table 3). No baseline laboratory values were available for comparison. Four patients had low levels of ANA positivity (titer 1:160), and one patient had a positive ANA (1:5,120). This latter patient’s laboratory finding was not accompanied by clinical signs of an autoimmune disease and she had a normal ESR, but her CK level was elevated at 331. Of note, her symptoms were present prior to study drug initiation. Three additional patients also had elevated CK values, ranging from 297 to 1,893, without clear etiology.
Table 3
Laboratory values for patients referred to rheumatology (n = 38)
| Test | Median | Range | No. of abnormal (%) |
|---|---|---|---|
| Thyroid stimulating hormone | 1.45 mU/L | 0.22–6.36 | 2 (5.3%) |
| Erythroid sedimentation rate | 13.5 mm/h | 4–87 | 3 (7.9%) |
| C-reactive protein | 0.55 mg/dl | < 0.1–2.7 | 7 (18.4%) |
| Creatine kinase | 108.5 IU/L | 44–1893 | 4 (10.5%) |
| Rheumatoid factora | < 20 IU/mL | < 20–40 | 2 (5.3%) |
| Anti-nuclear antibodyb | Negative | Negative–1:5,120 | 6 (16.2%)c |
Diagnoses
At the time of evaluation, the majority of patients were judged by the evaluating rheumatologists as having moderate-intensity, non-inflammatory regional musculoskeletal disorders. Seven patients were considered to have mild pain not interfering with function, whereas 31 patients were considered to have moderate to severe pain that interfered with function or activities of daily living. The change in HAQ score between AI initiation and rheumatology referral was statistically significantly higher in subjects diagnosed with moderate/severe pain compared to those subjects with mild pain (p = 0.0285). There was no statistically significant correlation between degree of pain and functional status impairment on clinical assessment and HAQ or VAS scores at baseline or at time of referral, or absolute change in VAS between AI initiation and time of referral.
The most frequent diagnoses based on clinical evaluation were tendonitis or tenosynovitis, osteoarthritis, and carpal tunnel syndrome (CTS) (Table 4). Multiple rheumatologic conditions were diagnosed in 37% of patients. A few cases represented exacerbations of previously existing abnormalities, but most patients reported new symptoms since initiation of therapy. Three of the nine patients with clinical symptoms consistent with CTS were tested with electroneuromyography, and all three had changes consistent with CTS. In 73% of subjects, musculoskeletal symptoms were judged as being definitely (18%) or possibly (55%) attributable to AI therapy.
Table 4
Clinical rheumatologic diagnoses for patients referred to rheumatology (n = 38)
| Diagnosis | Number of patients (%) |
|---|---|
| Bursitis | 8 (21.1%) |
 Trochanteric Trochanteric | 6 (15.8%) |
| Carpal tunnel syndrome | 8 (21.1%) |
| Osteoarthritis | 11 (28.9%) |
 Knee Knee | 3 (7.9%) |
 Hand Hand | 2 (5.3%) |
| Tendonitis | 14 (36.8%) |
 Rotator cuff/shoulder Rotator cuff/shoulder | 8 (21.2%) |
 Wrist Wrist | 3 (7.9%) |
 Elbow Elbow | 2 (5.3%) |
| Patellofemoral syndrome | 7 (18.4%) |
Some subjects had more than one diagnosis
Discussion
Aromatase inhibitors are increasingly used for adjuvant treatment of postmenopausal women with breast cancer. In this prospectively followed and evaluated, highly annotated cohort, 45.4% of subjects met pre-determined criteria for rheumatologic evaluation, 39.2% underwent a complete rheumatologic evaluation, and 13% discontinued therapy because of intolerable musculoskeletal toxicity. We were unable to identify any baseline patient characteristics, including pre-existing co-morbid conditions or specific breast cancer therapies, that were statistically significantly associated with the development of symptoms. It remains unclear whether these symptoms are due to a class effect or a specific medication. Therefore, we have recently amended our protocol to allow patients who develop intolerable symptoms to switch from one drug to the other, provided their initial symptoms completely resolve.
Overall, the clinical presentation of rheumatologic side effects was most consistent with AIs inducing a non-inflammatory musculoskeletal syndrome or the development of inflammation localized to the tenosynovial structures, including the carpal tunnel structures. Since there was no clear correlation between presence of symptoms and laboratory test abnormalities, we do not recommend routine clinical evaluation of the laboratory studies that were assessed in this trial. Because the distribution of reasons for referral were evenly divided between increased pain and worsened function, and there was a correlation between severity of pain and functional limitation on clinical assessment and increase in HAQ score with therapy, both measures for assessment of patients will continue to be utilized for the remaining patients accrued to this and other COBRA-conducted clinical studies.
The incidence of arthralgias and musculoskeletal symptoms in this study is similar to that reported in prior clinical trials of AIs in the adjuvant setting [1–7, 16, 17], although the drug discontinuation rate is higher in our study than previously reported. An increased incidence of CTS has been reported for both steroidal and nonsteroidal AIs compared to tamoxifen (IES trial: exemestane 2.8%, tamoxifen 0.3%; ATAC trial: anastrozole 3%, tamoxifen 1%) [7, 16]. A recent study using ultrasound and magnetic resonance imaging to characterize the wrists of women with severe wrist symptoms during AI therapy revealed flexor tenosynovitis. However, this study was quite small and all evaluated patients were symptomatic at the time of assessment [18]. These results suggest that AI therapy may lead to localized inflammation around tendons and nerves that may result in development of symptoms. Whether these changes are due to AI therapy, whether they only occur at the wrist, and how best to manage them remains to be determined.
The effects of other breast cancer therapies, such as surgery, radiation therapy, chemotherapy, and tamoxifen, might also play a role in the development of AI-related musculoskeletal symptoms. Other authors have suggested that treatment with chemotherapy alone might lead to myalgias and arthralgias [19–23]. Another study demonstrated an inverse association between prior tamoxifen therapy and development of AI-related joint stiffness [8]. Development of musculoskeletal symptoms in patients treated with chemotherapy and/or tamoxifen prior to an AI could therefore be influenced by either treatment regimen [8, 24]. In our study, no correlation was noted between surgery, radiation therapy, chemotherapy, or tamoxifen and the development of symptoms. This lack of demonstration of a statistically significant association may be due to insufficient power, since only a subset of women received each treatment modality.
The mechanism underlying the development of AI-related musculoskeletal symptoms is unknown, although estrogen deprivation has been proposed as one etiology. Arthralgias increase in incidence as women gradually progress through the menopausal transition, and are more common in women who undergo surgical menopause [25, 26]. Arthralgias and myalgias have also been noted in 25% of premenopausal women treated with gonadotropin-releasing hormone agonists[27]. However, a prior study showed no correlation between estrone level and development of joint pain in menopausal women [26]. The association between estrogen deprivation and development of musculoskeletal symptoms in this patient population therefore remains unclear.
Autoimmunity has also been proposed as a possible underlying mechanism. Researchers found that aromatase-knockout mice develop a Sjögren’s syndrome-like autoimmune lymphoproliferative disease[28]. However, in a small study, women who developed AI-induced musculoskeletal symptoms had no evidence of anti-doublestranded DNA antibodies to suggest an autoimmune etiology [29]. Similarly, in our study population, there was no evidence of autoimmunity based on clinical criteria from history and physical examination, and the majority of patients did not demonstrate auto-antibody production of RF or ANA.
In summary, the musculoskeletal symptoms that occur with AI therapy are clinically important as they lead to discontinuation of therapy in more than 10% of patients. In this study, no baseline characteristics predicted this toxicity, and these symptoms do not appear to be consistent with any defined clinical or serologic rheumatologic syndrome. These important negative findings support the need for more research into the mechanisms underlying this significant toxicity and its management.
Acknowledgments
Supported in part by: Pharmacogenetics Research Network Grant Number U-01 GM61373 (DAF) and Clinical Pharmacology training grants 5T32-GM08425 (DAF) from the National Institute of General Medical Sciences, National Institutes of Health, Bethesda, MD. Grant Numbers M01-RR000042 (UM), M01-RR00750 (IU), and M01-RR00052 (JHU) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. Grants from Pfizer, Inc. (DFH), Novartis Pharma AG (DFH), and Fashion Footwear Association of New York/QVC Presents Shoes on Sale™ (DFH) ClinicalTrials.gov number: NCT00 228956.
Footnotes
Disclaimers: Consultant—LabCorp (DAF), Roche Molecular Diagnostics (DAF), Wyeth (VS), Pfizer (DFH), Honoraria—Pfizer (AMS), Pfizer (DFH), Research Funding—Pfizer (DAF, DFH, VS, DA, AMS), Novartis (DAF, DFH, VS, DA), AstraZeneca (DAF), GlaxoSmithKline (VS)
Contributor Information
N. Lynn Henry, Breast Oncology Program, University of Michigan, Comprehensive Cancer Center, 1500 East Medical Center Drive, 6312 CCGC, Ann Arbor, MI 48109-0942, USA.
Jon T. Giles, Division of Rheumatology, Johns Hopkins University, Baltimore, MD, USA.
Dennis Ang, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
Monika Mohan, Division of Rheumatology, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA.
Dina Dadabhoy, Division of Rheumatology, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA.
Jason Robarge, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
Jill Hayden, Breast Oncology Program, University of Michigan, Comprehensive Cancer Center, 1500 East Medical Center Drive, 6312 CCGC, Ann Arbor, MI 48109-0942, USA.
Suzanne Lemler, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
Karineh Shahverdi, Breast Cancer Program, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, MD, USA.
Penny Powers, Breast Cancer Program, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, MD, USA.
Lang Li, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
David Flockhart, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
Vered Stearns, Breast Cancer Program, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, MD, USA.
Daniel F. Hayes, Breast Oncology Program, University of Michigan, Comprehensive Cancer Center, 1500 East Medical Center Drive, 6312 CCGC, Ann Arbor, MI 48109-0942, USA.
Anna Maria Storniolo, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
Daniel J. Clauw, Division of Rheumatology, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s10549-007-9774-6
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3081690?pdf=render
Citations & impact
Impact metrics
Article citations
Local Synthesis of Estradiol in the Rostral Ventromedial Medulla Protects against Widespread Muscle Pain in Male Mice.
eNeuro, 11(8):ENEURO.0332-24.2024, 28 Aug 2024
Cited by: 0 articles | PMID: 39111835 | PMCID: PMC11360981
Common Non-Rheumatic Medical Conditions Mimicking Fibromyalgia: A Simple Framework for Differential Diagnosis.
Diagnostics (Basel), 14(16):1758, 13 Aug 2024
Cited by: 1 article | PMID: 39202246 | PMCID: PMC11354086
Review Free full text in Europe PMC
A Cohort Study to Evaluate Genetic Predictors of Aromatase Inhibitor Musculoskeletal Symptoms: Results from ECOG-ACRIN E1Z11.
Clin Cancer Res, 30(13):2709-2718, 01 Jul 2024
Cited by: 1 article | PMID: 38640040
Association of Aromatase Inhibitor-Induced Musculoskeletal Symptoms with Central Sensitization-Related Symptoms: A Cross-Sectional Study.
Breast Care (Basel), 19(4):207-214, 18 Jun 2024
Cited by: 0 articles | PMID: 39185132
Aromatase inhibitor-induced arthralgia ameliorated by Mediterranean diet and active lifestyle guided by continuous glucose monitoring: a case report and review of the literature.
Front Oncol, 14:1189287, 01 Feb 2024
Cited by: 0 articles | PMID: 38361780 | PMCID: PMC10867103
Go to all (139) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A prospective study of aromatase inhibitor-associated musculoskeletal symptoms and abnormalities on serial high-resolution wrist ultrasonography.
Cancer, 116(18):4360-4367, 01 Sep 2010
Cited by: 29 articles | PMID: 20549827 | PMCID: PMC2936686
The CIRAS study: a case control study to define the clinical, immunologic, and radiographic features of aromatase inhibitor-induced musculoskeletal symptoms.
Breast Cancer Res Treat, 131(2):699-708, 11 Nov 2011
Cited by: 10 articles | PMID: 22076476 | PMCID: PMC3664236
Patient-Reported Outcomes and Early Discontinuation in Aromatase Inhibitor-Treated Postmenopausal Women With Early Stage Breast Cancer.
Oncologist, 21(5):539-546, 23 Mar 2016
Cited by: 40 articles | PMID: 27009936 | PMCID: PMC4861358
Reducing the risk for breast cancer recurrence after completion of tamoxifen treatment in postmenopausal women.
Clin Ther, 29(8):1535-1547, 01 Aug 2007
Cited by: 8 articles | PMID: 17919537
Review
Funding
Funders who supported this work.
NCRR NIH HHS (6)
Grant ID: M01 RR000042
Grant ID: M01-RR000042
Grant ID: M01 RR000052
Grant ID: M01 RR000750
Grant ID: M01-RR00750
Grant ID: M01-RR00052
NIGMS NIH HHS (6)
Grant ID: T32 GM008425-11
Grant ID: U01 GM061373
Grant ID: U01 GM061373-01
Grant ID: 5T32-GM08425
Grant ID: T32 GM008425
Grant ID: U-01 GM61373