Abstract
Background & aims
Liver transplantation is the primary treatment for various end-stage hepatic diseases but is hindered by the lack of donor organs and by complications associated with rejection and immunosuppression. There is increasing evidence to suggest the bone marrow is a transplantable source of hepatic progenitors. We previously reported that multipotent bone marrow-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells with almost 100% induction frequency under defined conditions, suggesting the potential for clinical applications. The aim of this study was to critically analyze the various parameters governing the success of bone marrow-derived mesenchymal stem cell-based therapy for treatment of liver diseases.Methods
Lethal fulminant hepatic failure in nonobese diabetic severe combined immunodeficient mice was induced by carbon tetrachloride gavage. Mesenchymal stem cell-derived hepatocytes and mesenchymal stem cells were then intrasplenically or intravenously transplanted at different doses.Results
Both mesenchymal stem cell-derived hepatocytes and mesenchymal stem cells, transplanted by either intrasplenic or intravenous route, engrafted recipient liver, differentiated into functional hepatocytes, and rescued liver failure. Intravenous transplantation was more effective in rescuing liver failure than intrasplenic transplantation. Moreover, mesenchymal stem cells were more resistant to reactive oxygen species in vitro, reduced oxidative stress in recipient mice, and accelerated repopulation of hepatocytes after liver damage, suggesting a possible role for paracrine effects.Conclusions
Bone marrow-derived mesenchymal stem cells can effectively rescue experimental liver failure and contribute to liver regeneration and offer a potentially alternative therapy to organ transplantation for treatment of liver diseases.Free full text

Stem Cell Therapy for Liver Disease: Parameters Governing the Success of Using Bone Marrow Mesenchymal Stem Cells
Abstract
Background & Aims
Liver transplantation is the primary treatment for various end-stage hepatic diseases but is hindered by the lack of donor organs and by complications associated with rejection and immunosuppression. There is increasing evidence to suggest the bone marrow is a transplantable source of hepatic progenitors. We previously reported that multipotent bone marrow–derived mesenchymal stem cells differentiate into functional hepatocyte-like cells with almost 100% induction frequency under defined conditions, suggesting the potential for clinical applications. The aim of this study was to critically analyze the various parameters governing the success of bone marrow–derived mesenchymal stem cell–based therapy for treatment of liver diseases.
Methods
Lethal fulminant hepatic failure in nonobese diabetic severe combined immunodeficient mice was induced by carbon tetrachloride gavage. Mesenchymal stem cell–derived hepatocytes and mesenchymal stem cells were then intrasplenically or intravenously transplanted at different doses.
Results
Both mesenchymal stem cell–derived hepatocytes and mesenchymal stem cells, transplanted by either intrasplenic or intravenous route, engrafted recipient liver, differentiated into functional hepatocytes, and rescued liver failure. Intravenous transplantation was more effective in rescuing liver failure than intrasplenic transplantation. Moreover, mesenchymal stem cells were more resistant to reactive oxygen species in vitro, reduced oxidative stress in recipient mice, and accelerated repopulation of hepatocytes after liver damage, suggesting a possible role for paracrine effects.
Conclusions
Bone marrow–derived mesenchymal stem cells can effectively rescue experimental liver failure and contribute to liver regeneration and offer a potentially alternative therapy to organ transplantation for treatment of liver diseases.
A wide variety of liver diseases lead to the impairment of liver function and require medical intervention. Liver transplantation is the primary treatment for end-stage hepatic diseases, with a 4-year survival rate of 70% or greater for most clinical indications.1,2 Although effective, extensive clinical application is limited by the lack of availability of donor organs. Other adverse factors such as rejection, problems associated with the long-term use of immunosuppressants, and perioperative morbidity and mortality contribute to additional complications.3 In view of these shortfalls, cell-based hepatocyte transplantation is of particular interest and believed to hold great promise because of the simpler and less invasive procedure. A single donor could serve multiple recipients, and excess cells could be cryopreserved for future use.4 However, studies have shown that less than 20%–30% of transplanted hepatocytes survive upon transplantation and that multiple transplantation procedures are required to achieve meaningful liver repopulation.5 Furthermore, the procurement of transplantable hepatocytes is hampered by the paucity of cadaveric liver, the limited replicative potential, the concomitant loss of characteristic hepatic functions upon in vitro culture, and reduced numbers of viable and functional cells upon cryopreservation.6,7
There is increasing evidence in the literature suggesting bone marrow as a transplantable source of hepatic progenitors.8–10 However, which cell populations within the bone marrow hold clinical promise remains controversial. Initial reports of the hepatic potential of hematopoietic stem cells were later shown to have resulted from fusion between transplanted donor cells and the resident recipient hepatocytes.11–13 Mesenchymal stem cells (MSCs) are a stem cell population within the bone marrow that has been shown to have increasing therapeutic potentials in a wide range of diseases.14–18 MSCs are defined as plate-adhering, fibroblast-like cells possessing self-renewal ability with the capacity to differentiate into multiple mesenchymal cell lineages such as osteoblasts, chondrocytes, and adipocytes. MSCs are readily available from a variety of tissues such as bone marrow, umbilical cord blood, trabecular bone, synovial membrane, and adipose tissue.19–23 In our previous studies, we showed that clonally derived human MSCs, under chemically defined conditions, differentiate into hepatocyte-like cells that not only express liver-specific genes but possess functions of adult hepatocytes.20,24 We further showed that in utero transplantation of human MSCs in mice contributed to numerous tissues, including the liver.25 Subsequently, others have shown the hepatic engraftment of transplanted MSCs using sublethal animal models of liver injury, further signifying the clinical potential of MSCs in the treatment of liver diseases.26,27 In this study, we critically analyzed the parameters governing the success of using bone marrow–derived MSCs for treatment of liver disease, including the cell type and cell dose for transplantation and the route of administration and compared their effects on functional recovery.
Materials and Methods
Details of the materials and methods used are described in the supplementary material (see supplementary material online at www.gastrojournal.org). A brief summary of the animal model and cells used for transplantation is given in the following text.
Cells
The isolation and characterization of MSCs from bone marrow was as reported previously24,28 and was approved by the institutional review board of the Taipei Veterans General Hospital. MSCs used in this study were clonally derived, and their surface immune phenotype and multilineage differentiation potentials into osteoblasts, adipocytes, chondrocytes, and hepatocytes were previously characterized.24,28 For generation of mesenchymal stem cell–derived hepatocytes (MDHs), hepatic induction was performed for 3 weeks using the 2-step protocol that we previously reported.24
Animal Model
Nonobese diabetic severe combined immunodeficient (NOD-SCID) mice were purchased from Tzu Chi University Laboratory Animal Center (Hualien, Taiwan). All animal experiments were performed with the approval of the Animal Care Committee of the Taipei Veterans General Hospital. Carbon tetrachloride was dissolved in mineral oil at 10% concentration and administered to animals by gavage. Dosages between 0.2 and 0.4 mL CCl4/kg body wt were tested for hepatotoxicity and lethality. Transplants were performed at 24 hours after administration of CCl4.
Preparation of Liver Cell Suspension
Liver tissues were minced in collagenase buffer containing 3 mg/mL collagenase for 40 minutes at 37°C under gentle agitation. Cells were collected by centrifugation, resuspended in fluorescence-activated cell sorting (FACS) buffer, and successively passed through 23- and 26-gauge needles and a 40-μm cell strainer to yield a single cell suspension. Red blood cells were removed by ACK lysing buffer. Cell viabilities were >93% by trypan blue dye exclusion, and total cells produced from each liver was consistently greater than 5 × 107.
Statistical Analysis
Data are presented as mean ± SD of n determinations as indicated in the figure legends. Animal survival was analyzed by log-rank tests, and P values are as shown. All other data were analyzed by paired t test, where P < .05 was considered significant.
Results
MSCs and MDHs Rescue Lethal Fulminant Hepatic Failure
The lethality of CCl4 on NOD-SCID mice was tested by gavage. We determined that doses at ≤0.24 mL/kg body wt could not induce sufficient lethality, while doses at ≥0.32 mL/kg body wt elicited hyperacute injuries leading to rapid death. A dose of 0.28 mL/kg body wt was deemed optimal, resulting in lethality in all animals at 6 days after administration of CCl4 (Figure 1A). All subsequent experiments were therefore performed at this dose. Biochemical assays showed a dramatic elevation in the serum levels of marker proteins such as serum glutamyl oxaloacetic transaminase, serum glutamyl pyruvic transaminase, albumin, total bilirubin, and lactate dehydrogenase, confirming the infliction of acute extensive liver injury by CCl4 (Table 1). Postmortem analysis showed that the presence of centrilobular sub-massive necrosis of the liver (Supplementary Figure 1; see supplementary material online at www.gastrojournal.org) led to hepatic failure in animals treated with a dose of 0.28 mL/kg body wt.

MDHs and MSCs rescue lethal FHF. (A) Effect of CCl4 on NOD-SCID mice survival at 0.2–0.4 mL/kg administered by gavage. Survival curves of NOD-SCID mice that underwent intrasplenic transplantation with 1.4 × 106 to 4.2 × 107/kg of (B) MDHs and (C) MSCs and intravenous transplantation with 1.4 × 106 to 4.2 × 107/kg of (D) MDHs and (E) MSCs at 24 hours after administration of 0.28 ml/kg CCl4. Statistical analysis was performed by log-rank test (intrasplenic MSC vs intrasplenic MDH: 4.2 × 107/kg, P = .0001; 1.4 × 107/kg, P = .0018; 4.2 × 106/kg, P = .0190; 1.4 × 106/kg, P = .0190; intravenous MSC vs intravenous MDH: 4.2 × 107/kg, P = .1380; 1.4 × 107/kg, P = .0190; 4.2 × 106/kg, P = .0047; 1.4 × 106/kg, P = .0009).
Table 1
Liver Function Tests of NOD-SCID Mice That Were Administered 0.28 mL/kg CCl4 and Underwent Transplantation With MSCs, MDHs, or Placebo Control
| Serum glutamyl pyruvic transaminase (IU/L) | Serum glutamyl oxaloacetic transaminase (IU/L) | Albumin (g/dL) | Total bilirubin (mg/dL) | Lactate dehydrogenase (IU/L) | |
|---|---|---|---|---|---|
| Normal NOD-SCID | 15 ± 2.5 | 63 ± 2.3 | 2.5 ± 0.35 | 0.53 ± 0.058 | 608 ± 42.7 |
| Post CCl4 day 1 | 6490 ± 453.7 | 390 ± 22.5 | 5.9 ± 0.25 | 4.3 ± 0.058 | 2746 ± 145.9 |
| Post CCl4 day 3 | |||||
 Placebo Placebo | 497 ± 35.5 | 387 ± 20.5 | 1.4 ± 0.17 | 1.6 ± 0.25 | 1367 ± 46.72 |
 IS-MSC IS-MSC | 125 ± 8.62 | 446 ± 18.9 | 1.3 ± 0.32 | 1.4 ± 0.15 | 1806 ± 53.05 |
 IS-MDH IS-MDH | 104 ± 7.64 | 667 ± 19.1 | 0.97 ± 0.21 | 1.0 ± 0.15 | 2074 ± 128.0 |
| Post CCl4 day 5 | |||||
 Placebo Placebo | 430 ± 25.2 | 346 ± 8.50 | 1.2 ± 0.058 | 1.3 ± 0.25 | 1159 ± 103.4 |
 IS-MSC IS-MSC | 19 ± 2.5 | 146 ± 15.3 | 1.9 ± 0.31 | 0.33 ± 0.058 | 914 ± 77.1 |
 IS-MDH IS-MDH | 21 ± 3.5 | 178 ± 15.1 | 1.6 ± 0.25 | 0.40 ± 0.10 | 1814 ± 55.18 |
| Post CCl4 day 7 | |||||
 Placebo Placebo | ND | ND | ND | ND | ND |
 IS-MSC IS-MSC | 17 ± 3.5 | 27 ± 4.0 | 2.0 ± 0.29 | 0.23 ± 0.058 | 444 ± 23.9 |
 IS-MDH IS-MDH | ND | ND | ND | ND | ND |
| Post CCl4 day 14 | |||||
 Placebo Placebo | ND | ND | ND | ND | ND |
 IS-MSC IS-MSC | 32 ± 6.4 | 60 ± 5.7 | 2.5 ± 0.25 | 0.73 ± 0.058 | 535 ± 50.7 |
 IS-MDH IS-MDH | ND | ND | ND | ND | ND |
| Post CCl4 day 30 | |||||
 Placebo Placebo | ND | ND | ND | ND | ND |
 IS-MSC IS-MSC | 22 ± 8.5 | 67 ± 8.3 | 2.7 ± 0.40 | 0.53 ± 0.058 | 547 ± 22.3 |
 IS-MDH IS-MDH | ND | ND | ND | ND | ND |
NOTE. Data are presented as mean ± SD of 3 determinations.
IS, intrasplenic transplantation; ND, not done.
Given the clinical shortage of hepatocytes, the chemically defined hepatic differentiation system we previously reported24 meant that large quantities of MDHs could be generated for transplantation. To assess the therapeutic potential of MDHs, 4 different cell doses ranging between 1.4 × 106 and 4.2 × 107 cells/kg body wt were intrasplenically transplanted at 24 hours after administration of CCl4. Transplantation of 1.4 × 106 and 4.2 × 106 MDHs/kg body wt failed to rescue recipient animals from fulminant hepatic failure (FHF), while 2 of 6 and 4 of 12 mice recovered from doses of 1.4 × 107 and 4.2 × 107 MDHs/kg body wt, respectively (Figure 1B). Although only one third of the recipients were successfully rescued by transplantation of 1.4 × 107 and 4.2 × 107 MDHs/kg body wt, the results suggest that MDHs may serve as alternative sources for cell transplantation because the cell doses administered are comparable to those currently used for hepatocyte transplantation into patients with FHF.29,30
To compare the therapeutic potential of MSCs with that of MDHs, 1.4 × 106 to 4.2 × 107 MSCs/kg body wt were transplanted under identical conditions. Two thirds of the animals recovered from transplantation of 1.4 × 106 and 4.2 × 106 MSCs/kg body wt (4/6 mice in each group), and all recipients were rescued from FHF by transplantation of 1.4 × 107 and 4.2 × 107 MSCs/kg body wt (12/12 and 16/16 mice, respectively) (Figure 1C). Surprisingly, transplantation of MSCs showed superior rescuing potentials than MDHs at all 4 doses (P < .05 in all cases). To attribute the rescuing effect to MSCs, recipient mice underwent transplantation with a fibroblast cell line FS-5 at 1.4 × 107 cells/kg body wt, but all animals died by 6 days after CCl4 administration (n = 3) (results not shown).
To determine whether the route of administration plays an important role in the therapeutic regimen, we compared the effectiveness of intravenous with that of intrasplenic transplantation. A cell dose-dependent effect was observed from intravenous transplantation of MDHs; all animals infused with 1.4 × 106 cells/kg body wt died of hepatic failure, 1 of 6 mice survived following transplantation of 4.2 × 106 cells/kg body wt, 2 of 6 mice were rescued by transplantation of 1.4 × 107 cells/kg body wt, and 4 of 6 mice recovered from transplantation of 4.2 × 107 cells/kg body wt (Figure 1D). Remarkably, intravenous transplantation of MSCs effectively rescued all recipient animals from hepatic failure at all 4 cell doses investigated (Figure 1E). Although the superior rescuing potential of MSCs over MDHs (4.2 × 107 cells/kg, not significant; P < .05 for other cell doses) was similar to the findings for intrasplenic transplantations, it is noteworthy that recipient survival was apparently enhanced by intravenous compared with intrasplenic infusions regardless of the cell type transplanted, with the exception of 4.2 × 107 and 1.4 × 107 MSCs/kg.
Transplanted Donor Cells Engraft in the Liver and Can Differentiate Into Functional Hepatocytes
We next investigated whether transplanted cells can differentiate and engraft in the liver parenchyma of recipient animals. Recipient animals that were rescued by intrasplenic transplantation of 4.2 × 107 cells/kg body wt of either MDHs or MSCs were killed at 4 weeks post-transplantation, and histologic evaluations showed complete regeneration of the liver (Supplementary Figure 2A; see supplementary material online at www.gastrojournal.org). An antibody against human albumin that does not cross-react with murine albumin (Supplementary Figure 2B; see supplementary material online at www.gastrojournal.org) was used to stain liver sections of rescued mice at 4 weeks posttransplantation. Albeit rare, a small number of clusters of human albumin-positive cells were found in the perivenous region of the liver of both MDH-rescued and MSC-rescued recipients (Supplementary Figure 2C; see supplementary material online at www.gastrojournal.org). To verify that transplanted donor cells had differentiated into hepatocytes, we used FACS to isolate cells that expressed human β2-microglobulin (B2M) from the liver of recipient mice at 4 weeks posttransplantation (Figure 2A) and analyzed them for expression of liver marker genes (Figure 2B). From 3 independent FACS, 4.2% ± 0.88% of cells in the liver were positive for human B2M (a representative plot is shown in Figure 2A). As seen, albumin, cytokeratin 18, hepatocyte nuclear factor 4, α1-antitrypsin, cytochrome P450, and glutamine synthetase were detected by Western blotting (Figure 2B).
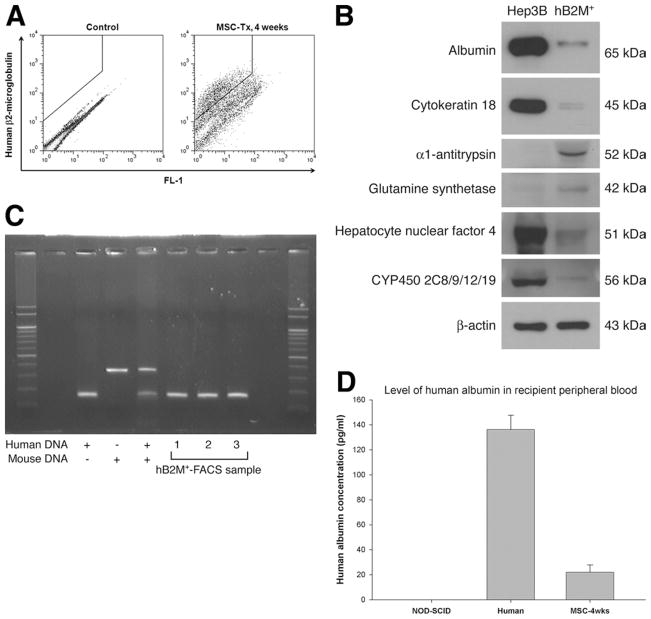
Transplanted donor cells engraft recipient liver and differentiate into hepatocytes. (A) FACS for human B2M-expressing cells at 4 weeks posttransplantation of MSCs (MSC-Tx). Human B2M-sorted cells (hB2M+) were (B) analyzed for expression of liver marker genes by Western blot and (C) verified for their human origin by genomic DNA polymerase chain reaction. (D) Detection of human albumin in the peripheral blood of recipients rescued by MSC transplantation (enzyme-linked immunosorbent assay, mean ± SD of 3 determinations).
Previous studies have shown that transplanted stem cells may acquire hepatic cell fate through cell fusion rather than differentiation, leading to different interpretations.11–13 To determine whether differentiation or fusion of transplanted cells with recipient hepatocytes was responsible for the human albumin-positive cells, a highly sensitive genomic DNA multiplex polymerase chain reaction capable of detecting a single copy of mouse B2M gene among 100,000 copies of human B2M gene25 was set up, and human B2M-sorted cells were amplified by polymerase chain reaction to detect the presence of human and mouse genomic DNA. In all 3 independent FACS samples, the presence of human DNA was consistently found in the absence of mouse DNA (Figure 2C), indicating that the cells were human in origin. While our data do not definitively exclude the occurrence of cell fusion events, they nevertheless show that human MSCs can differentiate into hepatocytes independent of cell fusion. To further show the functionality of differentiated cells, sera fractionated from the peripheral blood of rescued animals were quantified for the concentration of human albumin by enzyme-linked immunosorbent assay (Figure 2D). The results show that donor-derived hepatocytes were capable of secreting human albumin into the serum.
To evaluate the degree of recovery of the recipient liver, we performed 60% partial hepatectomy on recipient animals that were rescued from FHF at 4 weeks after transplantation of MSCs. Animals were killed at 3 weeks postoperation, and approximately two thirds of the tissue mass removed was regained through compensatory growth (not shown), indicating that the livers of MSC-rescued mice were extensively regenerated.
Engraftment frequencies of transplanted donor cells in recipient liver at 4 weeks posttransplantation (4.2% ± 0.88%) is in line with reports in the literature that only 1%–3% of the host liver is readily repopulated by donor cells following a single session of cell transplantation.31 By 3 months posttransplantation, human B2M-positive cells were below the sensitivity of detection, and thus we adapted a real-time genomic DNA polymerase chain reaction–based approach previously reported in the literature.32 Although human cells were detectable by this approach, frequencies were estimated to be 0.001%–0.01% in the liver at both 3 and 6 months posttransplantation, conceivably due to the dilution of donor-derived cells as a result of normal tissue turnover.
MSCs Possess Greater Resistance to Oxidative Stress
We initially hypothesized that MDHs may offer spontaneous functional support and expedite recovery of recipient animals but, on the contrary, rescuing frequencies were vastly enhanced by transplantation of MSCs. The ability to rescue recipient animals in such a short time, together with the reduced cell dose requirement, suggests that rescue of FHF by MSCs was not primarily mediated by differentiation of transplanted cells but involved other mechanisms.
The hepatotoxic effects of CCl4 are dependent on its metabolic activation in the liver by the cytochrome P450–dependent monooxygenase system to reactive free radicals.33,34 Transplanted cells must withstand the unfavorable microenvironment to endow their therapeutic effects. Given that MSCs do not exhibit cytochrome P450 activity, the supplementation of CCl4 under in vitro culture would not recapitulate the in vivo oxidative effects. We assessed the effect of oxidative stress on the survival of MDHs and MSCs using a potent oxidant, paraquat. While the viability of MDHs was seemingly less affected by paraquat-induced oxidative stress under short-term treatment, MSCs exhibited greater resistance under prolonged treatment and the difference in viability became increasingly evident with increasing concentrations of paraquat (Figure 3A). The differential resistance to oxidative stress was further confirmed by treatment with hydrogen peroxide (Figure 3B) and by directly generating superoxide anions under in vitro culture (Supplementary Figure 3A; see supplementary material online at www.gastrojournal.org).

MSCs are more resistant to oxidative stress than MDHs. Effect of exogenous oxidant (A) paraquat and (B) hydrogen peroxide on the viability of MSCs and MDHs (mean ± SD of 3 determinations). (C) Basal expression of antioxidative enzymes in MSCs and MDHs by Western blot. CuZnSOD, copper/zinc superoxide dismutase; MnSOD, manganese superoxide dismutase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. >Change in expression of antiapoptotic genes (D) Bcl-2 and (E) Bcl-xL in MSCs and MDHs in the presence of exogenous oxidant paraquat (mean ± SD of 3 determinations). (F) Changes in reduced glutathione/oxidized glutathione ratio in mice that underwent transplantation with MSCs and MDHs at 24 hours after administration of 0.28 mL CCl4/kg (mean ± SD of 3 determinations). Statistical analysis was performed by paired t test; P < .05 was considered significant. *Statistical significance (D–F).
The prominent resistance to oxidative stress was believed to be attributed to enhanced reactive oxygen species (ROS)-scavenging potentials of MSCs over MDHs, and we investigated the expression of antioxidative enzymes in these cells. While basal expression of mitochondrial-specific manganese superoxide dismutase was significantly higher in MDHs, the cytosolic copper/zinc superoxide dismutase was found to be higher in MSCs (Figure 3C), suggesting that MSCs have an inherently stronger potential to scavenge ROS entering the cytosolic space from the microenvironment. Another possible mechanism conferring resistance to environmental stress is mediated through antiapoptotic programs. Indeed, results in Figure 3D and E show that expression of anti-apoptotic genes Bcl-2 and Bcl-xL, respectively, was more dramatically up-regulated in MSCs than in MDHs. Furthermore, expression of the downstream target Akt was also up-regulated in MSCs but down-regulated in MDHs (Supplementary Figure 3B; see supplementary material online at www.gastrojournal.org).
The greater resistance to oxidative stress and the superior scavenging potentials of MSCs suggest a possible faster clearance of ROS in the recipient animal. Therefore, we investigated the change in oxidative stress in vivo by measuring the ratio of reduced glutathione to oxidized glutathione, where a decrease in reduced glutathione/oxidized glutathione ratio indicates an increase in oxidative stress. Indeed, mice that underwent transplantation with MSCs showed a faster reduction of oxidative stress in vivo than mice that underwent transplantation with MDHs (Figure 3F).
MSC Transplantation Enhances Repopulation of Endogenous Liver Cells
To further explore whether transplantation of MSCs protected endogenous hepatocytes from necrosis or promoted regeneration of the liver, we compared liver sections from mice that underwent transplantation with either cell type and were killed at different times after CCl4 administration. Histologic evaluations of MDH-recipient livers at 3 days (60% ± 4.8% necrosis; Figure 4A and Supplementary Figure 4A; see supplementary material online at www.gastrojournal.org) and 6 days after administration of CCl4 (63% ± 2.7% necrosis; Figure 4A and Supplementary Figure 4B; see supplementary material online at www.gastrojournal.org) showed little signs of regeneration of the necrotized regions. On the other hand, necrosis of the MSC recipient liver was comparable to that of MDH recipient liver at 3 days after administration of CCl4 (63% ± 4.3% necrosis; Figure 4A and Supplementary Figure 4C; see supplementary material online at www.gastrojournal.org). Significant restoration of the necrotized tissue was observed by day 6 (31% ± 4.1% necrosis; Figure 4A and Supplementary Figure 4D; see supplementary material online at www.gastrojournal.org), suggesting that MSCs enhanced repopulation by endogenous cells.

Transplantation of MSCs rescues endogenous hepatocytes. (A) Mean percentage of necrosis in the liver of recipient mice that underwent transplantation with MSCs and MDHs at 3 and 6 days after CCl4 administration (mean ± SD of 10 determinations). (B) Change in the number of donor-derived cells in the liver of recipient mice that underwent transplantation with MSCs and MDHs at 3, 5, and 7 days after CCl4 administration (mean ± SD of 3 determinations). Bromodeoxyuridine incorporation in the liver of (C) MSC recipients and (D) MDH recipients at 3 and 5 days after CCl4 administration (red arrowheads, QuantumDot-labeled donor cells; blue, nuclei staining; brown nuclei, bromodeoxyuridine-labeled cells). (E) Effect of MSC and MDH coculture on the proliferation of hepatocytes after paraquat treatment (mean ± SD of 3 determinations). Statistical analysis was performed by paired t test; P < .05 was considered significant. *Statistical significance (A,B,E).
To further explore the fate of transplanted cells, we labeled MSCs and MDHs with fluorescent nanocrystals (QuantumDot; Invitrogen, Carlsbad, CA) and compared the absolute number of label-positive cells in the liver at 3, 5, and 7 days after administration of CCl4 by FACS. Results showed that approximately 10.5 × 103 MSCs were present within the recipient liver on day 3 and increased to 24.5 × 103 and 67.0 × 103 by day 5 and day 7, respectively (Figure 4B). In comparison, only 5.5 × 103 and 5.4 × 103 label-positive MDHs were detected in the liver at day 3 and day 5, respectively, and were reduced to <500 cells by day 7 (Figure 4B). These findings correlated well with the differential resistance of MSCs and MDHs to oxidative stress (Figure 3A and E and Supplementary Figure 3A and B; see supplementary material online at www.gastrojournal.org). Also consistent with the enhanced animal survival, intravenously transplanted MSCs showed a greater number of label-positive cells within the recipient liver (Supplementary Figure 4E; see supplementary material online at www.gastrojournal.org) than intrasplenic transplantation (Figure 4B). Immunostaining for bromodeoxyuridine incorporation in these mice showed that numerous proliferating hepatocytes were present in the liver of MSC recipients by day 3 and further increased by day 5 (Figure 4C, brown nuclei). In contrast, there was a paucity of proliferating hepatocytes in MDH recipients (Figure 4D, brown nuclei). Transplanted QuantumDot-labeled MSCs (Figure 4C, red arrowheads) were found localized among the proliferating hepatocytes (Figure 4C). Also consistent with the above, biochemical tests showed improved recovery of liver functions by transplantation of MSCs over MDHs and placebo controls, and complete recovery was achieved by 2 weeks posttransplantation (Table 1).
To explore the possible role of a paracrine effect, NOD-SCID mice–derived hepatocytes were subjected to oxidative stress followed by the addition of MDHs and MSCs as coculture at 24 hours after treatment. Consistent with the above, viability and proliferation of primary hepatocytes were significantly increased by coculturing with MSCs compared with MDHs (Figure 4E).
Discussion
Using a murine model of lethal chemically induced FHF, we investigated various parameters governing the success and efficacy of using MSCs as an alternative cell source to hepatocyte transplantation for treatment of liver disease. Our data show that MDHs are a potential alternative transplantable cell source for the treatment of FHF. However, transplantation of MSCs improved the rescuing efficiency compared with MDHs. Further enhancements were accomplished by systemic transplantation compared with the intrasplenic approach commonly used clinically. Although donor-derived hepatocytes were detected in the recipients, the reduced cell requirement and the rapidity of rescue from FHF suggest that MSC-mediated therapeutic effects involved mechanisms other than functional complementation from direct differentiation. MSCs were found to be more resistant to ROS and resulted in faster reduction of oxidative stress in recipient mice. In addition, MSCs prominently stimulated hepatocyte proliferation after oxidative insult, suggesting a possible role for paracrine effects. The significance of adopting a lethal hepatic failure model is that it parallels more closely the clinical demand for alternative or bridging therapies while waiting for donor organs. In addition, transplantation of MSCs after establishment of extensive liver injury in this study mimics the realistic time frame for medical intervention, such as in the case of acetaminophen overdose where patients are typically admitted to the hospital within 24 hours.
To date, only a few clinical studies of hepatocyte transplantation for treatment of FHF have been reported in the literature with limited degrees of success.29,30,35,36 In one study, only 3 of 7 patients recovered from transplantation of fetal hepatocytes29 and, in another, survival was prolonged between 12 and 52 days in 3 of 5 patients who underwent transplantation with hepatocytes.30 In these studies, 109 to 1010 total cells were used. In our study, it was found that a single systemic transplantation of as few as 1.4 × 106 MSCs/kg body wt, approximating 1 × 108 cells for an average adult body weight of 70 kg, effectively rescued all recipient mice from FHF. Although not investigated in this study, it is conceivable that the therapeutic effects can be further enhanced by multiple sessions of cell infusion, which may be required for treating patients who are delayed in reporting to the hospital and have sustained prolonged liver injury.
Following a single session of cell transplantation, only 1%–3% of the normal rodent liver is repopulated readily by donor cells.31 Our finding of donor cell engraftment frequencies at 4 weeks post-transplantation was in line with those previously reported. Although differentiation of some of the transplanted donor cells into hepatocytes was detected, consistent with those reported by Sato et al26 and Aurich et al,27 the combined findings of predominant repopulation by murine cells, the superior rescuing potential of MSCs over MDHs, as well as the ability to rescue FHF in such a short period suggest that differentiation of donor cells into hepatocytes was not the primary attribute.
A number of factors may be conceived to confer therapeutic advantages on MSCs over predifferentiated hepatocytes, but survival of transplanted cells is a prerequisite to the endowment of any regenerative effects that the cells may possess. Like various other hepatotoxins, hepatotoxicity of CCl4 is mediated by oxidative damage through metabolic activation, producing free radicals and leading to lipid peroxidation, DNA damage, and cellular death.33,34,37,38 The generation of high levels of ROS by CCl4 not only results in tissue damages but continues to exert effects on the transplanted cells. Our data showing superior resistance of MSCs to oxidative stress in vitro and in vivo seems to find support from studies reporting the persistence of MSCs in patients after myeloablation39 and is coherent with the recipient survival curves. Although conferment of resistance was found, principally, through the up-regulation of anti-apoptotic and cell survival signals, the higher basal expression of cytosolic superoxide dismutases suggests a role of MSCs in protecting against oxidative insults.
Our findings that MSCs enhanced repopulation of necrotized tissue by endogenous cells rather than protected cells from necrosis, as well as that in vitro coculture with MSCs more significantly promoted the proliferation and regeneration of murine hepatocytes after oxidative injury, suggest a role for paracrine effects in the rescue of FHF. All cells are known to secrete various bioactive molecules, and it has been documented that multipotent MSCs synthesize a wide variety of growth factors and cytokines, exerting a paracrine effect on local cellular dynamics.40,41 Such trophic effects include stimulation of revascularization, leading to measurable therapeutic benefits in animal models of stroke, myocardial infarction, and renal failure, irrespective of direct differentiation of transplanted cells into lineages of the respective tissues.42,43 Such trophic effects are also conceived to enhance endogenous cell proliferation and revascularization of the liver in our model. As cells proceed toward a specific lineage phenotype, the quantity and array of secreted bioactive factors change,41 and it is likely that MDHs have reduced production of stimulatory factors and increased production of molecules primarily associated with liver functioning. The contribution of paracrine effects is further supported by a recent study reporting the therapeutic benefit of using MSC-derived biomolecules for treatment of acute liver injury.44
Earlier studies have reported that transplanted hematopoietic stem cells are capable of repopulating the liver.45 Although functional recovery of the liver was achieved in selected animal models of inherited metabolic liver disease, it was later shown that the effect was mediated by fusion between the transplanted donor cells and the recipient hepatocytes,12,13 and it remains undetermined whether the frequency of such events is sufficient for realistic clinical use. In addition, diseases resulting from extensive damage to the liver, such as chemical- or viral-induced hepatitis, may lack viable cells for fusion events to take place. On the other hand, MSCs are extrahepatic stem cells that are easily accessible from a variety of postnatal tissues and can contribute to liver regeneration, which makes MSCs an outstanding source for transplantation and offers a novel therapy for acute liver diseases. However, it remains to be seen whether MSC transplantation will be effective for treatment of chronic liver diseases of different etiologies. It could be anticipated that the paracrine effects of MSCs may also apply to chronic conditions that are induced by drugs or alcohol, but less could be speculated of its application in hepatitis arising from viral infections. Although it has been reported by Oyagi et al that liver fibrosis could be reduced by MSC transplantation,46 suggesting potential applications of MSCs in the treatment of chronic liver diseases. Further efforts are required to explore the effect of various parameters in different disease models.
Acknowledgments
Supported by the National Science Council, Taiwan (grants NSC 95-2745-B-075-002-MY3 and NSC 95-2314-B-075-014-MY2), by the intramural grant from the Taipei Veterans General Hospital, Taiwan (grant V96S4-026), and by the Liver Disease Prevention and Treatment Research Foundation, Taiwan.
Abbreviations used in this paper
| B2M | β2-microglobulin |
| FACS | fluorescence-activated cell sorting |
| FHF | fulminant hepatic failure |
| MDH | mesenchymal stem cell– derived hepatocyte |
| MSC | mesenchymal stem cell |
| NOD-SCID | nonobese diabetic severe combined immunodeficient |
| ROS | reactive oxygen species |
Footnotes
The authors report that no conflicts of interest exist.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at 10. 1053/j.gastro.2008.03.015.
References
Full text links
Read article at publisher's site: https://doi.org/10.1053/j.gastro.2008.03.015
Read article for free, from open access legal sources, via Unpaywall:
http://www.gastrojournal.org/article/S0016508508004514/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1053/j.gastro.2008.03.015
Article citations
Bone Marrow Mesenchymal Stem Cells Promote Ovarian Cancer Cell Proliferation via Cytokine Interactions.
Int J Mol Sci, 25(12):6746, 19 Jun 2024
Cited by: 0 articles | PMID: 38928452
Adipose-Derived Stem Cells to Treat Ischemic Diseases: The Case of Peripheral Artery Disease.
Int J Mol Sci, 24(23):16752, 25 Nov 2023
Cited by: 1 article | PMID: 38069074 | PMCID: PMC10706341
Review Free full text in Europe PMC
Efficacy and safety of mesenchymal stem cell therapy in liver cirrhosis: a systematic review and meta-analysis.
Stem Cell Res Ther, 14(1):301, 20 Oct 2023
Cited by: 5 articles | PMID: 37864199 | PMCID: PMC10590028
Review Free full text in Europe PMC
Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases.
Stem Cell Res Ther, 14(1):235, 04 Sep 2023
Cited by: 11 articles | PMID: 37667383 | PMCID: PMC10478247
Review Free full text in Europe PMC
Mesenchymal stromal/stem cells and their extracellular vesicles in liver diseases: insights on their immunomodulatory roles and clinical applications.
Cell Biosci, 13(1):162, 05 Sep 2023
Cited by: 3 articles | PMID: 37670393 | PMCID: PMC10478279
Review Free full text in Europe PMC
Go to all (277) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure.
J Gastroenterol Hepatol, 24(1):70-77, 25 Jun 2008
Cited by: 160 articles | PMID: 18624899
Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation.
Tissue Eng Part A, 18(13-14):1352-1364, 05 Jun 2012
Cited by: 45 articles | PMID: 22519429 | PMCID: PMC3397120
Useful properties of undifferentiated mesenchymal stromal cells and adipose tissue as the source in liver-regenerative therapy studied in an animal model of severe acute fulminant hepatitis.
Cytotherapy, 17(8):1052-1065, 01 Aug 2015
Cited by: 19 articles | PMID: 26139545
[Application of mesenchymal stem cells to liver regenerative medicine].
Yakugaku Zasshi, 128(1):3-9, 01 Jan 2008
Cited by: 9 articles | PMID: 18176050
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: R01 CA084197
Grant ID: R01 CA084197-14
NIDDK NIH HHS (4)
Grant ID: R01 DK052230-14
Grant ID: R24 DK064399
Grant ID: R24 DK064399-09
Grant ID: R01 DK052230





