Abstract
Free full text

Transient Regenerative Potential of the Neonatal Mouse Heart
Abstract
Certain fish and amphibians retain a robust capacity for cardiac regeneration throughout life, but the same is not true of the adult mammalian heart. Whether the capacity for cardiac regeneration is absent in mammals or whether it exists and is switched off early after birth has been unclear. We found that the hearts of 1-day-old neonatal mice can regenerate after partial surgical resection, but this capacity is lost by 7 days of age. This regenerative response in 1-day-old mice was characterized by cardiomyocyte proliferation with minimal hypertrophy or fibrosis, thereby distinguishing it from repair processes. Genetic fate mapping indicated that the majority of cardiomyocytes within the regenerated tissue originated from preexisting cardiomyocytes. Echocardiography performed 2 months after surgery revealed that the regenerated ventricular apex had normal systolic function. Thus, for a brief period after birth, the mammalian heart appears to have the capacity to regenerate.
Urodele amphibians and teleost fish retain a remarkable capacity for cardiac regeneration throughout life (1, 2). Adult zebrafish, for example, can undergo complete cardiac regeneration without scar formation after resection of up to 20% of the ventricle (2), a response thought to occur primarily through cardiomyocyte proliferation (3, 4). Similarly, a recent study demonstrated that the embryonic mammalian heart is capable of mounting a hyperplastic response to injury (5), which suggests that the mammalian heart may retain regenerative capacity during early life. A number of studies have shown that the adult mammalian heart also possesses a measurable capacity for cardiomyocyte renewal (6–9), albeit not sufficient to restore contractile function after substantial cardiac injury.
There are numerous fundamental differences between mammalian and fish cardiac biology. The four-chambered, double-circulation mammalian heart works at high pressure and is anatomically distinct from the two-chambered, single-circulation fish heart. In this regard, the adult zebrafish heart is anatomically similar to the embryonic mammalian heart before septation. In contrast to mammalian cardiomyocytes, which withdraw from the cell cycle and become bi-nucleate shortly after birth (10, 11), zebrafish cardiomyocytes are small, are mononucleated, and retain proliferative potential throughout life (12).
Given the similarities between the adult zebrafish heart and the immature mammalian heart, we hypothesized that the mechanisms underlying cardiac regeneration in fish might be conserved in the neonatal mammalian heart. To examine at a histological level how the heart of neonatal mice responds to injury, we surgically resected the left ventricular apex (~15% of the ventricular myocardium) from 1-day-old mice (13). Serial histological analysis revealed progressive regeneration of the apex, with full restoration of the resected myocardium within 21 days (Fig. 1, A to H and M). At 1 day post-resection (dpr), the resection plane was characterized by a large blood clot that sealed the entire apex (Fig. 1, E and F) and, similar to the zebrafish model, was associated with a robust inflammatory response (fig. S1). Later time points revealed gradual resorption of the apical blood clot and its replacement by normal myocardial tissue (Fig. 1, E to H). By 21 dpr, the entire apical defect was replaced by cardiomyocytes, as detected by morphological analysis (Fig. 1H) and cardiac troponin T staining (fig. S5). In addition, many blood vessels were observed in the regenerated ventricular apex at 21 dpr (fig. S2, A to D). Thus, at least histologically, injury-induced heart regeneration in zebrafish (2) and in neonatal mice appears to be similar.

Regeneration of the ventricular myocardium of neonatal mice (A to D) Hematoxylin and eosin (H&E) staining of the mouse heart at 1, 2, 7, and 21 days post-resection (dpr). Arrow denotes injury site. (E to H) H&E-stained sections at higher magnification. The asterisk marks a large blood clot adjacent to the left ventricular chamber (arrow) at 1 and 2 dpr. Dashed line indicates the resection plane. Scale bars, 200 μm. (I to L) Trichrome-stained serial sections showing early deposition of epicardial extracellular matrix (blue staining) at 7 dpr, with minimal evidence of cardiac fibrosis by day 21. Scale bars, 200 μm. (M) Quantification of surgical reproducibility and regeneration. Ventricle weights and sagittal section surface area are presented as percentages of sham-operated controls. Numbers of samples analyzed are indicated within the bars. Values are presented as means ± SEM; *P < 0.05. (N) Images of 2D echocardiogram in the parasternal long axis view. Yellow line indicates motion (M) mode plane. Lower panel shows left ventricular (LV) dimensions and function of sham-operated and resected hearts at 60 dpr. Left ventricular internal diameter at end systole (LVIDs) and end diastole (LVIDd) were used for calculating fractional shortening (FS) and ejection fraction (EF). Values are presented as means ± SEM; n = 3 per group.
Restoration of normal contractile function is a crucial aspect of myocardial regeneration. Therefore, we assessed left ventricular ejection fraction by echocardiography 2 months after apical resection and confirmed that the newly formed apex had normal systolic function, similar to sham-operated hearts (Fig. 1N).
Given the capacity of neonatal mouse cardiomyocytes to divide (14), we examined whether apical resection stimulates cardiomyocyte proliferation. Cardiomyocyte mitosis and cytokinesis were assessed by colocalization of phosphohistone H3 (pH3) and aurora B kinase, respectively, with cardiac troponin T. We found a significantly higher number of pH3-positive, and aurora B kinase–positive, cardiomyocytes in resected hearts relative to sham-operated hearts at 7 dpr (Fig. 2, A to C, fig. S3, F, H, and J, and fig. S4).
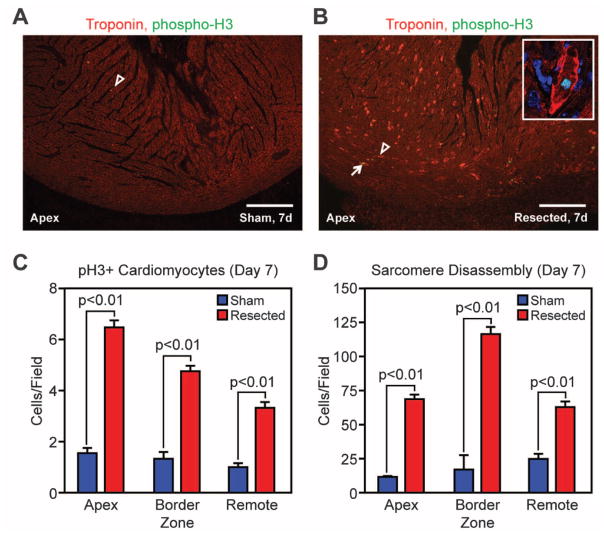
Cardiomyocyte proliferation accompanies regeneration of the neonatal mouse heart. (A and B) Cardiomyocyte mitoses were identified by staining for pH3 (green), troponin T (red), and nuclei (blue) in sham-operated (A) and resected (B) hearts. Arrowheads denote cardiomyocytes with disassembled sarcomeres; arrows show cardiomyocytes positive for pH3. Scale bars, 200 μm. The inset is a high-magnification image of a cardiomyocyte with disassembled sarcomeres positively stained for pH3. (C and D) Quantification of pH3 staining (C) and sarcomere disassembly (D) at 7 dpr. Quantitative analysis represents counts per field from four independent samples per group.
Cardiomyocyte proliferation is also characterized by sarcomere disassembly—specifically, by marginalization of sarcomeric structures to the periphery of the cells during mitosis (15, 16). A significantly higher number of cardiomyocytes in resected hearts displayed disorganized sarcomeric structures relative to sham-operated hearts at 7 dpr (Fig. 2, A, B, and D, and fig. S3, E, G, and I). This proliferative response peaked at 7 dpr and was activated in the entire heart, not just at the regenerating apex (fig. S3). These findings are consistent with the idea that the regenerative potential of heart tissue in neonatal mice involves widespread dedifferentiation and proliferation of cardiomyocytes.
To establish whether the regenerated apex contains newly formed cardiomyocytes, we used 5-bromo-2-deoxyuridine (BrdU) in a pulse-chase experiment after apical resection. BrdU-positive cardiomyocytes were identified within the newly formed apex at 21 dpr after three BrDU pulses (at 1, 7, and 14 dpr) (Fig. 3A and fig. S5). Relative to sham-operated controls, the number of BrdU-positive cardiomyocyte nuclei was higher in the apex and border zone regions of resected hearts by a factor of ~7 (Fig. 3A). These data suggest that these myocytes are newly formed and had undergone at least one round of DNA replication.
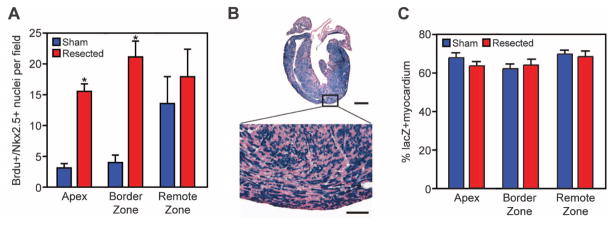
The majority of newly formed cardiomyocytes within the regenerated apex are derived from preexisting cardiomyocytes. (A) Quantitative analysis of BrdU/Nkx2.5+ nuclei at 21 dpr in sham-operated and resected hearts, after three BrdU pulses (at 1, 7, and 14 dpr). Quantitative analysis represents counting of multiple fields from three independent samples per group (~12 fields per group). Values are presented as means ± SEM; *P < 0.05. (B) β-Galactosidase enzymatic staining of αMHC-MerCreMer; Rosa26-lacZ reporter mouse heart at 21 dpr after a single dose of tamoxifen at birth. Scale bars, 1 mm (upper panel), 100 μm (lower panel). (C) Quantification of the percentage of lacZ-positive myocardium showing no difference between sham-operated and resected hearts at 21 dpr. Quantitative analysis represents counting of multiple fields from three independent samples per group (~18 fields per group). Values are presented as means ± SEM.
We next used a genetic fate mapping approach to determine the lineage of origin of cardiomyocytes within the regenerated apex. Rosa26-lacZ reporter mice were crossed with αMHC-MerCreMer (tamoxifen-inducible Cre recombinase under control of αMHC promoter) mice, and neonates were given a single subcutaneous dose of tamoxifen at birth to label the cardiomyocyte population (fig. S6). Histological analysis revealed no differences in the percentage of lacZ-positive myocardium between sham and resected hearts. The majority of cardiomyocytes within the newly formed apex at 21 dpr stained positive for lacZ, indicating that these myocytes arose from an αMHC-positive cardiac lineage (Fig. 3, B and C) rather than from an unspecified stem cell population.
Apical resection was not associated with induction of myocyte hypertrophy (fig. S7, A to C), or a robust fibrotic response (Fig. 1, I to L, and fig. S7D), thereby distinguishing the regenerative response from the repair process in the adult heart. One of the hallmarks of the zebrafish cardiac regenerative response is epicardial activation, characterized by up-regulation of epicardial-enriched genes (17). Apical resection of the neonatal mouse heart was also associated with epicardial activation (fig. S8).
To determine whether the regenerative potential of the neonatal mouse heart is lost when cardiomyocytes withdraw from the cell cycle, we performed apical resection on 7-day-old (P7) mice, a time point coinciding with cardiomyocyte proliferative arrest in rodents (10). Because of higher rates of surgical mortality in P7 pups relative to P1 pups, a smaller proportion of the apex was resected, with minimal chamber exposition (Fig. 4A). In contrast to 1-day-old mice, 7-day-old mice failed to regenerate their myocardium after apical resection (Fig. 4, A to C) and developed significant fibrosis (Fig. 4, D to F). No evidence of sarcomere disassembly or cardiomyocyte proliferation was noted at 7 dpr (fig. S9). Collectively, these results indicate that the regenerative capacity of the neonatal mouse heart is lost within the first week of postnatal life.
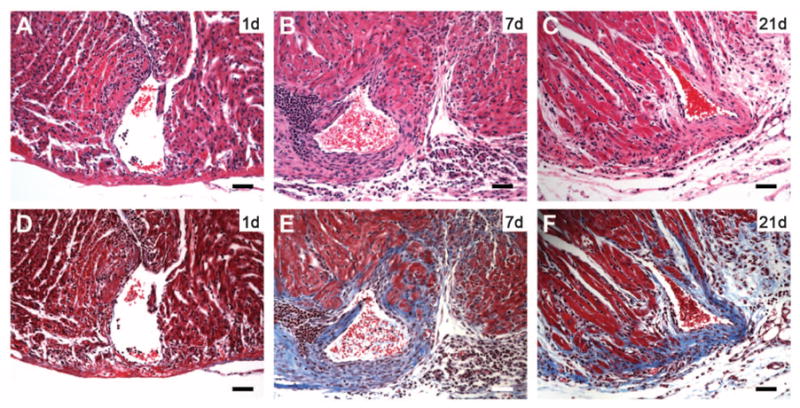
Lack of regeneration after apical resection of 7-day-old mice. (A to C) H&E staining at 1, 7, and 21 dpr, respectively. (D to F) Trichrome staining at 1, 7, and 21 dpr. Note fibrotic scar (blue staining) surrounding resected ventricular chamber at 7 and 21 dpr [(E) and (F)]. Scale bars, 200 μm.
Our results show that surgical resection of the ventricular apex in 1-day-old mice stimulates a regenerative response that appears to restore the damaged heart to its normal anatomy and function. The mouse heart loses this regenerative potential within the first week of postnatal life, which coincides with the developmental window when cardiomyocytes become binucleate and withdraw from the cell cycle (10). It is unclear whether the loss of this regenerative potential in the adult heart is due to an intrinsic cell cycle block in adult cardiomyocytes, or to loss of mitogenic stimuli as the heart ages (or both). Our lineage-tracing data support a role for myocyte proliferation in the neonatal heart’s regenerative potential, but we cannot exclude the possibility that stem or progenitor cells also contribute to this process. It will be important to determine the mechanisms by which the mammalian heart switches off this regenerative capacity in the week after birth.
Acknowledgments
We thank T. Simsek, J. Shelton, and the UT Southwestern Histology Core for help with histology and immunostaining, and J. Cabrera for graphical assistance. Supported by an overseas postdoctoral fellowship from the National Health and Medical Research Council and National Heart Foundation of Australia (E.R.P.), a cardiovascular research scholar award from Gilead Sciences and a fellow-to-faculty award from the American Heart Association (H.A.S.), and NIH grant HL100401-01, the Donald W. Reynolds Center for Clinical Cardiovascular Research, the American Heart Association, the Jon Holden DeHaan Foundation, the Leducq Foundation, and the Robert A. Welch Foundation (E.N.O.).
References and Notes
Full text links
Read article at publisher's site: https://doi.org/10.1126/science.1200708
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3099478?pdf=render
Free to read at www.sciencemag.org
http://www.sciencemag.org/cgi/content/abstract/331/6020/1078
Subscription required at www.sciencemag.org
http://www.sciencemag.org/cgi/content/full/331/6020/1078
Subscription required at www.sciencemag.org
http://www.sciencemag.org/cgi/reprint/331/6020/1078.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102372343
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1126/science.1200708
Article citations
Turning back time: the promise of cardiac regeneration.
Nat Rev Cardiol, 21(12):846, 01 Dec 2024
Cited by: 0 articles | PMID: 39317841
Harnessing the regenerative potential of interleukin11 to enhance heart repair.
Nat Commun, 15(1):9666, 08 Nov 2024
Cited by: 0 articles | PMID: 39516197 | PMCID: PMC11549343
Cauterization of the root of the left coronary artery as a straightforward, large and reproducible ischemic injury model in neonatal mice.
Lab Anim (NY), 53(11):308-326, 22 Oct 2024
Cited by: 0 articles | PMID: 39438662 | PMCID: PMC11518978
Review Free full text in Europe PMC
Cell cycle visualization tools to study cardiomyocyte proliferation in real-time.
Open Biol, 14(10):240167, 09 Oct 2024
Cited by: 0 articles | PMID: 39378987 | PMCID: PMC11461051
Review Free full text in Europe PMC
The Multifaceted Roles of Hippo-YAP in Cardiovascular Diseases.
Cardiovasc Toxicol, 24(12):1410-1427, 04 Oct 2024
Cited by: 0 articles | PMID: 39365552
Review
Go to all (1,471) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Early Regenerative Capacity in the Porcine Heart.
Circulation, 138(24):2798-2808, 01 Dec 2018
Cited by: 146 articles | PMID: 30030417
Extending the time window of mammalian heart regeneration by thymosin beta 4.
J Cell Mol Med, 18(12):2417-2424, 06 Oct 2014
Cited by: 24 articles | PMID: 25284727 | PMCID: PMC4302647
Prolonged Myocardial Regenerative Capacity in Neonatal Opossum.
Circulation, 146(2):125-139, 26 May 2022
Cited by: 8 articles | PMID: 35616010
Turning back the cardiac regenerative clock: lessons from the neonate.
Trends Cardiovasc Med, 22(5):128-133, 01 Jul 2012
Cited by: 20 articles | PMID: 22902092
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (18)
Grant ID: R01 HL077439-06
Grant ID: R01 HL093039
Grant ID: R01 HL093039-02
Grant ID: R01 HL077439-08
Grant ID: R01 HL077439-07
Grant ID: R01 HL077439-06W1
Grant ID: R01 HL093039-03
Grant ID: R01 HL115275
Grant ID: R37 HL053351-15
Grant ID: R37 HL053351
Grant ID: R01 HL093039-01A1W1
Grant ID: R37 HL053351-13
Grant ID: R37 HL053351-14
Grant ID: HL100401-01
Grant ID: R01 HL077439
Grant ID: R01 HL093039-01A1
Grant ID: R37 HL053351-12
Grant ID: U01 HL100401
National Institutes of Health (1)
Grant ID: HL100401-01




