Abstract
Free full text

Endoscopic diagnosis of extrahepatic bile duct carcinoma: Advances and current limitations
Abstract
The accurate diagnosis of extrahepatic bile duct carcinoma is difficult, even now. When ultrasonography (US) shows dilatation of the bile duct, magnetic resonance cholangiopancreatography followed by endoscopic US (EUS) is the next step. When US or EUS shows localized bile duct wall thickening, endoscopic retrograde cholangiopancreatography should be conducted with intraductal US (IDUS) and forceps biopsy. Fluorescence in situ hybridization increases the sensitivity of brush cytology with similar specificity. In patients with papillary type bile duct carcinoma, three biopsies are sufficient. In patients with nodular or infiltrating-type bile duct carcinoma, multiple biopsies are warranted, and IDUS can compensate for the limitations of biopsies. In preoperative staging, the combination of dynamic multi-detector low computed tomography (MDCT) and IDUS is useful for evaluating vascular invasion and cancer depth infiltration. However, assessment of lymph nodes metastases is difficult. In resectable cases, assessment of longitudinal cancer spread is important. The combination of IDUS and MDCT is useful for revealing submucosal cancer extension, which is common in hilar cholangiocarcinoma. To estimate the mucosal extension, which is common in extrahepatic bile duct carcinoma, the combination of IDUS and cholangioscopy is required. The utility of current peroral cholangioscopy is limited by the maneuverability of the “baby scope”. A new baby scope (10 Fr), called “SpyGlass” has potential, if the image quality can be improved. Since extrahepatic bile duct carcinoma is common in the Far East, many researchers in Japan and Korea contributed these studies, especially, in the evaluation of longitudinal cancer extension.
INTRODUCTION
Most patients with bile duct cancer are diagnosed in an advanced stage[1-3]. From 1998 to 2004, the 5-year survival rate after surgical resection was 33.1% for bile duct cancer in Japan[3]. To improve the therapeutic effect of bile duct carcinoma, efforts have been focused on diverse areas: early detection of the lesions, accurate differentiation of benign and malignant biliary stenosis, assessment of locoregional tumor extension, development of surgical methods, biliary stenting, and chemoradiotherapy for unresectable bile duct cancer.
In the diagnosis of intrahepatic cholangiocarcinoma, non-invasive, cross-sectional imaging tests including computed tomography (CT) and magnetic resonanace imaging (MRI) are useful. MRI in the form of magnetic resonance cholangiopancreatography (MRCP) and multi-detector low CT (MDCT) are the most commonly performed imaging tests in these patients. In contrast, for the diagnosis of extrahepatic bile duct cancer, an endoscopic approach is essential. Endoscopic techniques are more invasive and include the use of endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasonography (EUS). In this review, we describe the advances and current limitations of our professional area: endoscopic procedures in the diagnosis of extrahepatic bile duct carcinoma.
EARLY DETECTION OF EXTRAHEPATIC BILE DUCT LESIONS
Tompkins et al[1] reported that 91% of patients with bile duct cancer who underwent surgery had serum bilirubin levels greater than 2.0 mg/dL. Tio et al[2] reported that almost all patients were diagnosed after developing obstructive jaundice, and only two of 76 patients showed stage T1 disease. In contrast, Sugiyama et al[4] reported that 18 of 103 patients showed no jaundice, and eight of 103 patients had stage T1 disease. When a patient complained of abdominal discomfort or showed an elevation of serum biliary enzymes, they routinely performed ultrasonography (US) to screen for pancreatobiliary ductal diseases, which resulted in the early detection of lesions with good prognosis.
US
In the middle and distal bile duct, US cannot assess tumors sufficiently due to disturbance by gastrointestinal gas[5]. Even now, the sensitivities of US in demonstrating hilar tumor, middle bile duct tumor, and distal bile duct tumor are 85.6%, 59.1%, and 33.3%, respectively[5]. Therefore, bile duct dilatation on US findings is an important sign for the early diagnosis of bile duct cancer[5]. Our group has reported an asymptomatic unicteric patient with bile duct carcinoma, in whom US at the time of health examination showed dilatation of the bile duct[6]. In most countries, routine US examination for these patients might be difficult due to cost-effectiveness. In Japan, US equipment has become popular even in small clinics in the past two decades, and screening of the biliary tract using US is increasingly performed.
MRCP
Until recently, when US showed dilatation of the bile duct, ERCP was the next step to obtain cholangiography. Recently, MRCP has become an alternative to ERCP as it is a non-invasive modality[7,8]. When US shows intraductal tumor, initial ERCP rather than MRCP should be carried out for cost-effectiveness, even if the patient shows no jaundice. However, when US shows only dilatation of the extrahepatic bile duct, MRCP is a safe modality to obtain a clear cholangiogram.
A recent excellent prospective study by Sai et al[7] demonstrated that MRCP showed a sensitivity of 90% in evaluating extrahepatic bile duct carcinoma in the nonicteric stage. They investigated nonicteric patients who had abnormal concentrations of serum biliary enzymes and whose common hepatic duct was more than 8 mm in diameter on abdominal US due to unknown reasons. In this study, 10 patients were diagnosed with extrahepatic carcinoma including 5 patients in T1 stage during a 7-year period.
EUS
By using intraduodenal scanning, in the extrahepatic bile duct, EUS can provide high resolution power without echo attenuation and without the influence of gastrointestinal gas. When evaluating extrahepatic bile duct carcinoma in the nonicteric stage, the sensitivity and specificity of MRCP followed by EUS were 90% and 98%, respectively[7]. In another study, in 32 patients with normal serum liver enzymes and whose common bile duct was dilated on US findings, EUS did not show biliary malignancy[9]. Therefore, the patients who had abnormal concentrations of serum biliary enzymes and whose common hepatic duct was dilated on abdominal US will be good targets for EUS. EUS was also useful in diagnosing distal biliary strictures without a mass on CT[10].
Fernández-Esparrach et al[8] also performed a prospective study of MRCP and EUS in the evaluation of 63 patients with unexplained common bile duct dilation on standard US, although most of these patients had jaundice. The sensitivity and specificity of MRCP in diagnosing malignancy in these patients were 95% and 98%, respectively. The sensitivity and specificity of EUS were 100% and 100%, respectively[8].
ACCURATE DIFFERENTIATION OF BENIGN AND MALIGNANT BILIARY STRICTURES
ERCP
An ERCP image of a patient with extrahepatic bile duct carcinoma in the nonicteric stage is shown in Figure Figure11.
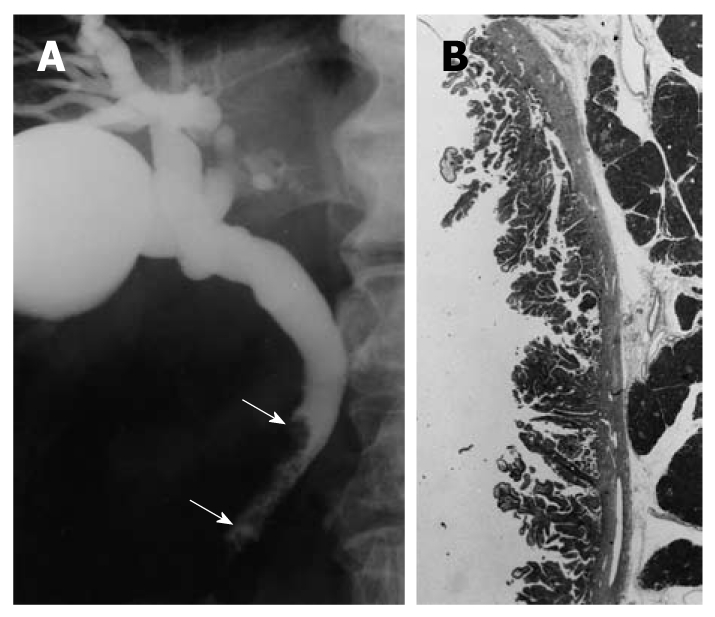
Cholangiographic finding of extrahepatic bile duct carcinoma in the nonicteric stage. A: Cholangiography shows a papillary tumor at the distal common bile duct (arrows); B: The histologic findings of the resected specimen showed papillary adenocarcinoma confined to the mucosal layer (hematoxylin and eosin, × 1).
On ERCP or MRCP findings, benign diseases including post-operative stenosis, chronic pancreatitis, primary sclerosing cholangitis, or autoimmune pancreatitis show bile duct stenosis as well as malignant disease[11-13]. Cholangiography shows filling defects at the common bile duct in patients with adenomyoma[11] or inflammatory strictures[12]. Therefore, accurate distinction between benign and malignant biliary structures is essential to avoid unnecessary surgery.
The accuracy of MRCP is comparable with that of ERCP[13]. Malignancy is suggested when cholangiography shows long (greater than 10 mm), asymmetric, and irregular strictures. Benign disease is suggested when cholangiography shows short, regular, and symmetric strictures. Using these criteria, the diagnostic sensitivity and specificity for ERCP were 74% and 70%, respectively. The diagnostic sensitivity and specificity for MRCP were 70% and 72%, respectively[13].
Although ERCP is conducted for the purpose of biliary drainage to release obstructive jaundice, the utility of preoperative biliary drainage is controversial. Some reports indicate that preoperative biliary drainage increased infectious complications after hepatectomy for proximal bile duct tumor[14]. In preoperative biliary drainage for cholangiocarcinoma, endoscopic nasobiliary drainage (NBD) is preferable to endoscopic biliary stenting, because secondary cholangitis due to the retrograde flow of duodenal fluid into the biliary tree does not occur[15-17]. NBD is also useful to obtain a clear cholangiogram to evaluate longitudinal cancer extension along the bile duct, which is common in cholangiocarcinoma[15-17]. In contrast, a clear cholangiogram is unnecessary in patients with pancreatic cancer, since longitudinal cancer extension is rare in these patients. One randomized controlled trial of preoperative biliary drainage for cancer of the head of the pancreas has been reported[18]. This report concluded that routine preoperative biliary drainage in patients undergoing surgery for cancer of the pancreatic head, with obstructive jaundice and a bilirubin level less than 14.6 mg/dL, increases the rate of complications. Therefore, we agree that routine ERCP is not required in patients with pancreatic cancer.
Bile cytology during ERCP
In some prospective studies, bile exfoliative cytology aspirated after insertion of an external biliary catheter showed disappointing results (sensitivity 6%-24%)[19,20]. Brush cytology has a specificity of nearly 100%[21-38]. When its specificity is 100%, its sensitivity for cholangiocarcinoma ranges from 23% to 80%[21-36] as shown in Table Table1.1. Its sensitivity for pancreatic cancer is low, ranging from 0% to 66%[21-36], even now. The low sensitivity is related to low cellularity of these tumors and the demoplastic reaction. A new long brush did not improve results[35]. Repeated brushing improved the sensitivity from 35% to 44% (P = 0.01), although dilation of the stenosis did not improve its sensitivity[38] (Table (Table11).
Table 1
Sensitivity rates for detection of malignancy by endoscopic brush cytology of a biliary stricture
| Authors and year | Country | Panc cancer | Bile duct cancer | Specificity |
| Venu et al[21], 1990 | USA | 60% (3/5) | 80% (20/25) | 100% (88/88) |
| Rupp et al[22], 1990 | USA | 91% (21/23) | 100% (6/6) | 88% (7/8) |
| Foutch et al[23], 1991 | USA | 0% (0/6) | 100% (5/5) | 100% (3/3) |
| Ryan et al[24], 1991 | USA | 30% (6/20) | 44% (4/9) | 100% (17/17) |
| Howell et al[25], 1992 | USA | 0% (0/18) | 50% (2/4) | 100% (5/5) |
| Kurzawinski et al[26], 1993 | Great Britain | 65% (15/23) | 60% (6/10) | 100% (7/7) |
| Ferrari Júnior et al[27], 1994 | USA | 66% (16/29) | 20% (2/10) | 100% (22/22) |
| Ponchon et al[28], 1995 | France | 15% (3/20) | 44% (12/25) | 97% (64/66) |
| Sugiyama et al[29], 1996 | Japan | 36% (5/14) | 59% (10/17) | 100% (12/12) |
| Mansfield et al[30], 1997 | Great Britain | 38% (10/28) | 63% (10/16) | 100% (2/2) |
| Vandervoort et al[31], 1999 | USA | 11% (5/46) | 30% (3/10) | 100% (37/37) |
| Glasbrenner et al[32], 1999 | Germany | 35% (11/31) | 80% (16/20) | 90% (19/21) |
| Jailwala et al[33], 2000 | USA | 24% (11/46) | 23% (7/30) | 100% (29/29) |
| Farrell et al[34], 2001 | USA | 78% (14/18) | 60% (6/10) | 83% (10/12) |
| Fogel et al[35], 2006 | USA | 36% (32/88) | 26% (10/38) | 100% (8/8) |
| Kitajima et al[36], 2007 | Japan | 60% (9/15) | 71% (15/21) | 100% (7/7) |
Percutaneous transhepatic cholangioscopy
Percutaneous transhepatic cholangioscopy (PTCS) is predominantly performed in Asian countries such as Japan, Korea, and Taiwan, where there is a high prevalence of intrahepatic stones and cholangiocarcinoma[39-53]. Even in patients with non-dilated intrahepatic bile duct, percutaneous transhepatic biliary drainage (PTBD) can be performed with the assistance of cholangiography via NBD[52,53]. Once the PTBD tract is established, insertion of the cholangioscope from the percutaneous tract is relatively easy. On cholangioscopic findings, irregularly dilated and tortuous vessels, so called “tumor vessels”, are good targets for biopsy[39-50]. Although PTCS is an excellent procedure to obtain the target biopsy with a sensitivity of 93%-96%[39-41], it requires an invasive technique compared to the transpapillary approach. The sensitivities of the target biopsy for bile duct carcinoma, pancreatic carcinoma, and gallbladder carcinoma are 95.7% (135/141), 67.2% (45/67), and 76.2% (48/63), respectively[39,41].
The numbers and locations of the biopsies required to make a diagnosis of carcinoma depend on the origin and cholangioscopic appearance of the tumor[45]. A diagnosis of carcinoma was made in all patients (n = 4) with a tumor of the papilla of Vater and in all patients (n = 15) with a polypoid bile duct tumor, with two biopsies from the tip of the polypoid mass. In patients with bile duct cancer of the stenotic type (n = 19), cancer was diagnosed in 95% of cases when three biopsies were taken from the margin of the stenotic area rather than within the area of the stenosis. When cholangioscopy showed a tortuous, dilated vessel (n = 10), the diagnosis of cancer was made with two biopsies taken from the margin of the stenosis rather than inside the stenosis. In the patients with metastatic bile duct cancer (n = 14), the diagnosis was made in only 43% of cases when three biopsies were taken from the margin of the area of stenosis. When combined with results from the three biopsies taken from within the area of stenosis, the sensitivity for diagnosing pancreatic cancer improved from 20% to 60%[45].
Transpapillary biopsy
The sensitivity of transpapillary bile duct biopsy is reported to be 52%-81%[28,29,34,54-62] as shown in Table Table2.2. In these series, various biliary diseases, including pancreatic cancer and gallbladder cancer are included. When the tumor is located outside the bile duct (pancreatic or gallbladder cancer), the sensitivity of the biopsy is low (50%-71%). However, in patients with bile duct carcinoma, the sensitivity of transpapillary bile duct biopsy is 84%-89%[29,54,56,57,59]. Sugiyama et al[29] designed new biopsy forceps which could be introduced into the bile duct without sphincterotomy. Once the guidewire is introduced into the bile duct, the forceps can be inserted into the bile duct along the placed guidewire[56-59]. In the patient with cholangiocarcinoma, the diagnostic results of this clamshell type forceps with a soft outer Teflon sheath (Olympus Optimal. Co. Ltd.)[29,54,59] is superior to that of triple tissue sampling using the Howell system[33,55]. Ropeway-type biopsy forceps are also commercially available now[57]. Selective biopsy of both hepatic ducts is also possible[58]. Dumonceau et al[62] used a giant basket to grasp the tissue, and reported a sensitivity of 80%.
Table 2
Sensitivity rates for detection of malignancy by endoscopic forceps biopsy of a biliary stricture
| Authors and year | Country | Panc cancer | Bile duct cancer | Specificity |
| Kubota et al[54], 1993 | Japan | 50% (2/4) | 89% (16/18) | 100% (5/5) |
| Ponchon et al[28], 1995 | France | 46% (6/13) | 44% (7/16) | 97% (35/36) |
| Sugiyama et al[29], 1996 | Japan | 71% (10/14) | 88% (15/17) | 100% (12/12) |
| Jailwala et al[33], 2000 | USA | 33% (15/46) | 30% (9/30) | 100% (10/10) |
| Tamada et al[59], 2002 | Japan | 50% (6/12) | 84% (21/25) | 100% (18/18) |
| Kitajima et al[36], 2007 | Japan | 60% (9/15) | 57% (12/21) | 100% (7/7) |
The required number and the location of biopsy should be selected according to the type of tumor. In patients with papillary (polypoid) type bile duct carcinoma, three biopsies from the tip of the polypoid lesion were sufficient for the diagnosis with a sensitivity of 100%[59,63]. In patients with nodular or infiltrating type cholangiocarcinoma, multiple biopsies from the margin of the stenosis and within the stenosis improve sensitivity[59,63]. Since endoscopic skill is an art, endoscopists must sufficiently manipulate the tip of the forceps by free hand according to the gross finding of the tumor to improve the sensitivity of the method. The passion to improve the results is important as well as objective comparison with published data. In patients with pancreatic cancer, this modality has limitations, and other methods should be selected if ERCP tissue sampling shows negative results[29,54,59].
Percutaneous transhepatic intraluminal biopsy
Via the PTBD route, multiple intraluminal biliary ductal biopsies using a sheath with a side port show good results[63]. In patients with polypoid-type cholangiocarcinoma, the sensitivities of a single biopsy and 2 biopsies were 67% (4/6) and 100% (6/6), respectively. In patients with nodular-type cholangiocarcinoma, the sensitivities of a single biopsy, 3 biopsies, 6 biopsies, and 9 biopsies were 40% (4/6), 80% (16/20), 90% (18/20), and 95% (19/20), respectively. These results suggest that repeated biopsies may improve the sensitivity of transpapillary biopsy in patients with nodular-type cholangiocarcinoma.
Advanced techniques in cytology
Advanced cytologic techniques, including digitized image analysis (DIA) and fluorescence in situ hybridization (FISH), have been used to increase the sensitivity of bile cytology[64-67]. The DIA technique quantitates nuclear DNA via special stains to assess the presence of aneuploidy, whereas FISH analysis detects chromosomal polysomy by using fluorescent probes.
In a prospective study, when routine cytology was negative, FISH had an increased sensitivity (35%-60%) compared to routine cytology, however, the sensitivity and specificity of DIA was intermediate as compared to routine cytology[64]. In another prospective study, DIA increased the sensitivity from 15% to 43%, but decreased the specificity from 100% to 92%[65]. FISH increased the sensitivity from 15% to 44%, with similar specificities (98% for FISH and 100% for routine cytology)[65]. In patients with negative brush cytology and forceps biopsy, DIA, FISH, and composite DIA/FISH were able to predict malignant diagnoses in 14%, 62%, and 67%, respectively[66]. In another comparative study, FISH increased the sensitivity from 20.1% to 42.9% compared to routine cytology, with similar specificities (99.8% for FISH and 100% for routine cytology). DIA was not a significant independent predictor of malignancy[67]. These results show that FISH is a useful technique to improve the diagnostic ability of cytology.
“Mother-baby” system - peroral cholangioscopy
A small caliber (3.2-4.1 mm) “baby” cholangioscope is inserted into the common bile duct through the channel of a large caliber “mother” duodenoscope[68-75]. To date, there have been only a few large studies on diagnostic peroral cholangioscopy (POCS) in biliary-tract diseases, despite many reports on therapeutic POCS and diagnostic PTCS. The utility of POCS is further limited by the fragility of the cholangioscopes and insufficient optical resolution. Biopsies can be performed through the cholangioscope, but adequate sampling remains challenging due to the small size of the working channel (1.2 mm) and the limited maneuverability of the long baby scope.
Fukuda et al[68] reported that the diagnostic criteria of PTCS[39-41] was useful in POCS, and that the addition of POCS improved diagnostic ability compared with endoscopic retrograde cholangiography/tissue sampling (accuracy 78%-93.4%, sensitivity 57.9%-100%). In recent years, the development of video cholangioscopy has largely overcome the issue of poor image quality[71-75]. This improvement due to video cholangioscopy can provide better quality images, resulting in the ability to observe each lesion clearly and to perform the correct target biopsy. Furthermore, video cholangioscopy makes it possible to use the narrow-banding imaging (NBI) system[71,73]. Itoi et al[71] reported that POCS combined with NBI could clarify the fine surface structure of lesions and mucosal vessels compared with conventional white-light observation in all cases. These results suggest that POCS combined with NBI may lead to higher detectability of biliary-tract lesions, even minute lesions. One problem of NBI cholangioscopy is that bile is recognized as an incoming reddish fluid similar to blood[71]. This is a significant issue that requires improvement, because it leads to poor images, and it is time consuming to clean the bile duct.
Carbon dioxide insufflation is a tactic for obtaining clear images of the bile duct during POCS[75].
Direct cholangioscopy
In one of the first reports of POCS in 1977, a straight-view fiberscope of 8.8 mm diameter could be directly inserted through the mouth, into the biliary system after an endoscopic sphincterotomy, without the need for a mother scope[76]. Our research group also reported the utility of direct POCS in 1982, using a previously placed balloon catheter in the intrahepatic bile duct as the anchor system[77]. Since this system has the limitation of poor insertion rates of cholangioscopy, the modality was replaced by the “mother baby” system. However, recently a thin-caliber gastroscope made it possible to perform this method again[78-80]. Only one endoscopist is necessary, and the larger working channel (2.0 mm) of the endoscope allows for large biopsies[78-80]. This modality may be useful for the evaluation of intraductal papillary mucinous neoplasms of the bile duct[81].
SpyGlass
Recently, the SpyGlass peroral cholangio-pancreatoscopy system (Boston Scientific Co., Natick, MA, USA) has been introduced[82-84]. This system uses a reusable optical probe, a disposable access and delivery catheter (SpyScope), and disposable biopsy forceps. The outer diameter of the SpyScope is 10 French. This system offers several advantages over previous cholangioscopes. It allows for single-operator control of both the duodenoscope and the SpyScope because the SpyScope catheter is mounted on the duodenoscope by a silastic belt. The endoscopist can sequentially manipulate both the duodenoscope and the SpyScope with one hand; thus, two endoscopists are not needed. This system also uses 4-way tip deflection, which allows for improved access of tertiary ducts. Furthermore, the irrigation channel (0.6 mm) is separate from the working channel (1.2 mm), which allows for sustained continuous irrigation even if the working channel is in use. Therefore, the SpyGlass system can be used for cholangioscopic-guided target biopsy[84]. The sensitivity and specificity of SpyGlass-directed biopsy was 71% and 100%, respectively, in an evaluation of intraductal lesions in 20 patients[84].
Intraductal US
When the findings of ERCP or MRCP are equivocal, we have been performing transpapillary intra-bile ductal US to detect small tumors or localized wall thickening[6,59]. Ultrasound imaging of the intra-bile duct using a thin (2.0 to 2.4 mm in diameter), high-frequency (15 to 20 MHz) ultrasonic probe, called “intraductal ultrasonography”, is capable of producing high quality cross-sectional images of the bile duct, and is used for the differential diagnosis of biliary strictures[6,44,59,60,66,85-89].
Many investigators have reported that intraductal US (IDUS) could compensate for the false negative results of ERCP tissue diagnosis[59,60,66,85-89]. Multiple regression analysis showed that the presence of a sessile tumor (intraductal or outside the bile duct: P < 0.05), tumor size greater than 10.0 mm (P < 0.001), and interrupted wall structure (P < 0.05) were independent variables that predicted malignancy[59]. We must bear in mind, however, that asymmetric localized bile duct wall thickening with normal bile duct structure on IDUS images occurs in primary sclerosing cholangitis and other inflammatory changes as well as in bile duct carcinoma[44,59].
Optimal coherence tomography
Optimal coherence tomography (OCT) is a new technique that produces cross-sectional images using infrared light. OCT has an axial resolution which is 10-fold better than that of high-frequency ultrasound. However, its depth penetration is limited to approximately 1 mm vs 10 mm for a 20 MHz ultrasound probe.
Preliminary studies have demonstrated the ability of OCT to generate high resolution images of the biliary tree that correlate with histological findings[90,91]. OCT has the potential to identify small bile duct lesions, however, it is not widely available except in a few centers. Therefore the role of OCT in the diagnostic workup of bile duct carcinoma is not yet established.
EUS-fine-needle aspiration
In patients with extrahepatic bile duct carcinoma, percutaneous US-guided fine-needle aspiration (FNA) is difficult, since these tumors are too small. On the other hand, EUS has high-resolution power imaging and can puncture lesions of 3 mm or greater[92-98].
In one study, sensitivity was better for ERCP-based techniques (brush cytology and forceps biopsy) in biliary tumors (ERCP 75% vs EUS 25%), whereas EUS-guided biopsy was superior for pancreatic masses (EUS 60% vs ERCP 38%)[93]. Therefore, pancreatic cancer is a good target for EUS-FNA. In recent studies of EUS-FNA in patients with hilar strictures with negative brush cytology, the diagnostic sensitivity, specificity, and accuracy were 77%-89%, 100%, and 79%-91%, respectively[94,96]. A potential risk of this modality is intraperitonal seeding. In patients with unresectable bile duct cancer, EUS-FNA may be conducted after negative ERCP results[97,98].
ASSESSMENT OF CANCER DEPTH INFILTRATION
Accurate diagnosis of the extent of the cancer is essential to enable selection of the appropriate medical and surgical therapy. On dynamic CT findings, extrahepatic cholangiocarcinoma may be seen as a focal thickening of the ductal wall with various enhancement patterns. Until now, in many cases of extrahepatic cholangiocarcinoma, visualization of the tumor was not definitive because they were too small to be detected, as previously reported[3-10,59,60,66,86-89]. More recent studies, however, have shown the utility of modern contrast-enhanced MDCT in the preoperative staging of hilar cholangiocarcinoma[99-105]. The diagnostic ability of 16-channel MDCT is excellent[99,104]. The diagnostic ability of dynamic MRI combined with MRCP using a 1.5 T MR system is comparable with MDCT[105-107]. The reports which clarified the utility of MDCT for the staging of extrahepatic cholangioma are limited compared to hilar cholangiocarcinoma[101,108].
IDUS is utilized to compensate for dynamic MDCT to demonstrate the tumor extension in the hepatoduodenal ligament. IDUS has become a promising modality in assessing the depth of cancer infiltration in the bile duct[109-118]. In recent years, three-dimensional IDUS has become an excellent modality for assessing tumor staging[114,116]. However, IDUS could not assess tumor invasion outside the hepatoduodenal ligament, for example, in the superior mesenteric vein, proper hepatic artery or left hepatic artery due to echo attenuation. In addition, IDUS can not assess distant metastases. Therefore, the combination of dynamic CT and IDUS is essential for accurate preoperative staging.
Vascular invasion
MDCT correctly revealed hepatic artery invasion with 75%-100% sensitivity and 90%-100% specificity and portal vein invasion with 92.3%-100% sensitivity and 80%-100% specificity[99-105,108].
On IDUS, when the high-echoic layer between the tumor and a vessel disappeared, it was diagnosed as positive for vascular invasion. Using this criterion, the accuracies of IDUS in assessing tumor invasion to the right hepatic artery and portal vein were 86%-100% and 92%-100%, respectively[87,109-118]. However, visualization of the left hepatic artery and proper hepatic artery was poor (14%/18%) due to anatomical reasons which caused ultrasound attenuation[112,113]. Some investigators reported that 3D-IDUS may improve diagnostic accuracy for the detection of tumor invasion into the portal vein and the hepatic artery[114,116].
Lymph node metastases
Dynamic CT detected lymph node swelling, however, it was not effective in differentiating whether the swelling indicated an inflammatory or a malignant change[108,119-121]. The accuracy of MDCT in evaluating lymph node metastases was 57%-69%[108,119-121]. The accuracy of CT for the characterization of paraaortic nodes was not different from that of MRI[119]. A short axis-diameter over 5.3 mm, irregular margin, and the presence of central necrosis were suggestive morphologic features of malignant nodes[119]. Another report also showed that a round node with a short-axis diameter exceeding 18 mm showed high positive predictive values of malignancy (67%). However, CT was not useful since nodes of this size and character were rare[120].
On IDUS, although high-resolution US might improve detection of small epicholedochal lymph nodes, due to the limited depth of ultrasonic penetration, IDUS was inferior to conventional EUS with respect to detection of lymph node metastases[110,117,118]. A hypo-echoic, clear margin, and round shaped lymph node was judged as malignant swelling. An irregular or angle shaped lymph node was judged as inflammatory swelling. Using these criteria, the accuracy of IDUS in assessing lymph node metastases was 75%-78%[110,117,118,122]. As these results show, the assessment of lymph node metastases using CT and IDUS is difficult.
Distinction of T1 and T2 biliary tumors
The inside hypo-echoic layer on IDUS images corresponded not only to the fibromuscular layer but also to a part of the peri-muscular connective tissue. Therefore, even if the tumor was limited to the inside low-echoic layer, it suggests a T2 tumor (the tumor invaded the peri-muscular connective tissue) as well as a T1 tumor (the tumor which is confined to the fibromuscular layer). Therefore, accurate distinction of T1 and T2 tumors by IDUS as well as CT is difficult[110,123].
Invasion of the serosa
When the outside hyper-echoic layer was interrupted, IDUS assessed it as positive serosal invasion. Using these criteria, the accuracy of IDUS in assessing tumor invasion to the serosa was 86%-93%[109,110].
Invasion of the pancreatic parenchyma
Sonographic detection of a bile duct tumor protruding into the pancreatic parenchyma or disruption of the outer bile duct layer were diagnosed as positive for invasion of the pancreatic parenchyma. Using these criteria, the accuracy of IDUS in assessing tumor invasion to the pancreas was 93%-100%[109,110,124].
ASSESSMENT OF LONGITUDINAL CANCER EXTENSION
Cholangiography
Extrahepatic bile duct carcinoma shows longitudinal spread along the bile duct, often resulting in residual tumor at the surgical margin. Conventional cholangiography can inadequately assess this as previously reported[115-144].
Longitudinal extension of cholangiocarcinoma consists of mucosal (superficial) or submucosal (invasive) infiltration depending on the tumor growth pattern. Mucosal extension is predominantly seen with papillary (intraductal) and nodular (mass-forming) tumors, while submucosal extension is mainly seen with infiltrating (sclerosing) and nodular-infiltrating tumors[125-128]. The length of longitudinal extension is determined by the type of invasion, with a mean length of 6-10 mm for the submucosal spread and 10-20 mm for the mucosal spread[125-128]. Therefore, a gross surgical margin of more than 1 cm in the infiltrating type and more than 2 cm in the papillary and nodular types is recommended to achieve negative microscopic resection margins[127,128].
Dynamic MRI
The addition of contrast-enhanced dynamic images to unenhanced and MRCP images did not significantly improve the diagnostic accuracy for assessment of the longitudinal extent of bile duct cancer[133].
CT
MDCT correctly revealed longitudinal extension of hilar cholangiocarcinoma in 77.8%-87% of patients[99,100,103,134], and extrahepatic cholangiocarcinoma in 62.5%-78.6% of patients[134,135]. MDCT revealed wall thickening of the bile duct accompanied by submucosal cancer extension, which is common in hilar cholangiocarcinoma[99,100,103,134]. However, CT has a strong tendency to underestimate longitudinal mucosal spread, which is common in extrahepatic cholangiocarcinoma[125-132,135]. In these patients, at the hepatic margin of the mucosal spread, the width of the mucosa is too thin to be demonstrated by CT or MRI[133-135].
PTCS
Preoperative assessment of longitudinal spread of bile duct cancer has been conducted by mapping biopsy using PTCS[39-42,44,45,47,110,136-138]. With the combination of PTCS and cholangiography, its accuracy improved to 80%-92%[42,110,138]. Observation of the fine mucosal structure is essential to compensate for the false-negative study of mapping biopsy. Nodular, finely reticulogranular and highly papillary forms of papillogranular mucosa were characteristic of superficial spreading carcinoma[39-42,44,45,47,136-138]. Regular papillogranular mucosa was seen even in the non-cancerous area, and methylene blue satin was useful, since the mucosa that did not stain was characteristic of mucosal spread[42,136]. The presence of irregularly dilated and tortuous vessels, so-called tumor vessels, and the patterns of luminal narrowing, suggested intramural cancer extension[39-42,44-46,136-139]. Regular non-dilated vessels were seen even in the non-cancerous area[39-42,44,45,136-138]. Lee et al[137] reported that PTCS was essential to evaluate longitudinal cancer extension in patients with polypoid-type cholangiocarcinoma, however, MRCP was sufficient for stenotic-type cholangiocarcinoma. Kim et al[138] reported the utility of the combination of PTCS and IDUS in evaluating longitudinal cancer extension of extrahepatic bile duct carcinoma. Since PTCS requires an invasive procedure and may lead to seeding along the PTCS tract, this information should be utilized by POCS from now on.
IDUS
The assessment of longitudinal cancer extension along the bile duct is a promising aspect of IDUS[138-145]. However, to establish the diagnostic system of longitudinal spread by IDUS, some problems have been solved. Bile duct wall thickening occurs by inflammatory change due to mechanical stimulation of the drainage catheter as well as intra-wall extension of the cancer which shows asymmetric thickening, as previously reported[140-144].
A possible solution to this problem might be accurate assessment of the appearance and the internal echo of the wall thickening. When IDUS shows a papillary pattern of the bile duct mucosal surface, heterogeneous bile duct wall thickening (width ≥ 1.8 mm) with irregular outer marginal, or asymmetric bile duct wall thickening (width ≥ 1.8 mm) with rigid inner edge, it may be judged as a sign of longitudinal spread of the cancer. However, asymmetric bile duct wall thickening without a rigid inner edge without an irregular outer marginal border should be judged as a sign of inflammation[143].
On IDUS findings, Inoue evaluated the asymmetry of the thickened bile duct wall by measureing the maximum thickening of the medial hypoechoic layer and the minimum thickening of this layer. The maximum/minimum thickening rate of the cancer spread site and the non-spread site were 2.7 (1.1-4.5) and 1.9 (1.3-3.3), respectively[144].
Our research group has already reported that in patients who had not undergone biliary drainage, 95% did not show bile duct wall thickening over 1.8 mm at the common hepatic duct on IDUS images via the transpapillary route, when they did not have primary sclerosing cholangitis or longitudinal cancer extension along the bile duct[139]. Once a biliary catheter was inserted, accuracy of IDUS in assessing longitudinal cancer extension was 71%-72%[110,142,143].
Transpapillary IDUS prior to biliary drainage is useful to reduce artifacts associated with bile duct drainage tubes. When employing this technique any asymmetrically bile duct wall detected with IDUS was judged to be a phenomenon of longitudinal tumor spread allowing for an accuracy of 85%[145]. In the remaining 15% of patients, at the border of the longitudinal cancer extension, the thickening of the mucosal spread was too thin to be visualized on IDUS. The results of IDUS in this area are listed in Table Table33.
Table 3
Intraductal ulytrasonography for the evaluation of longitudinal cancer extension of extrahepatic bile duct carcinoma
| Authors and year | Country | Route | Accuracy of IDUS |
| Tamada et al[110], 1995 | Japan | PTBD/ERCP | 68% (13/19) |
| Inui et al[115], 1998 | Japan | PTBD/ERCP | 85% (11/13) |
| Fujita et al[116], 1998 | Japan | ERCP | 80% (12/15) |
| Menzel et al[118], 2000 | Germany | ERCP | 80% (24/30) |
| Tamada et al[143], 2001 | Japan | PTBD/ERCP | 71% (25/35) |
| Tamada et al[145], 2001 | Japan | ERCP | 84% (16/19) |
| Kim et al[138], 2010 | Korea | PTBD | 92% (18/19) |
PTBD: Percuteneous transhepatic biliary drainage; ERCP: Endoscopic retrograde cholangiopancreatography.
POCS
Itoi et al[71] suggested that NBI cholangioscopy is expected to make it possible to detect not only polypoid lesions but also flat superficial cancerous lesions. They also suggested some limitations of this method. First, observation of the proximal portion of the biliary tumor was possible only in limited cases, since the cholangioscope could not easily be passed through. Secondly, submucosal cancerous progression with non-neoplastic bile-duct epithelium could not be identified even by NBI. These data suggest that POCS by using NBI is limited in cases that show surface structure changes at this stage. IDUS should be conducted to compensate for this limitation[137,139]. Until now, only one report has described the utility of POCS in evaluating the longitudinal cancer extension of extrahepatic bile duct carcinoma in contrast to PTCS (Table (Table44).
Table 4
Cholangioscopy for the evaluation of longitudinal cancer extension of extrahepatic bile duct carcinoma
| Authors and year | Country | Modality | Accuracy |
| Tamada et al[110], 1995 | Japan | PTCS + mapping biopsy; PTCS + mapping biopsy + IDUS | 80% (12/15); 93% (14/15) |
| Sato et al[42], 1998 | Japan | PTCS | 81% (13/16) |
| Kawakami et al[72], 2009 | Japan | POCS; POCS + mapping biopsy | 77% (10/13); 100% (13/13) |
| Kim et al[138], 2010 | Korea | PTCS + mapping biopy; PTCS + mapping biopy + IDUS | 90% (18/20); 95% (18/19) |
IDUS: Intraductal ultrasonography; PTCS: Percuteneous transhepatic cholangioscopy; POCS: Peroral cholangioscopy.
The development of a baby scope the size of “SpyGlass” and with excellent image quality is warranted.
OCT
Since OCT has an axial resolution 10-fold better than that of high-frequency ultrasound, and its depth penetration is limited to approximately 1 mm vs 10 mm for a 20 MHz ultrasound probe[90,91], this modality is expected to be utilized for the diagnosis of longitudinal cancer extension. Unfortunately, there is no previous report of OCT in this area.
CONCLUSION
When US shows dilatation of the bile duct, MRCP followed by EUS is the next step to diagnose bile duct carcinoma. When US or EUS shows localized bile duct wall thickening, ERCP should be conducted with IDUS and forceps biopsy (Figure (Figure2).2). FISH increases the sensitivity of brush cytology with similar specificity. In patients with papillary (polypoid) type bile duct carcinoma, three biopsies are sufficient for the diagnosis. In patients with nodular-type bile duct carcinoma, multiple biopsies are warranted, and IDUS can compensate for the limitations of biopsies. In patients with pancreatic cancer, the sensitivities of forceps biopsy and brush cytology are low. In patients with hilar cholangiocarcinoma, dynamic MDCT provides excellent information for the detection of vascular invasion. In patients with extrahepatic bile duct carcinoma, the combination of MDCT and IDUS is useful to evaluate vascular invasion and cancer depth infiltration (Figure (Figure3).3). However, assessment of lymph node metastases is difficult. In cholangiocarcinoma, assessment of longitudinal cancer spread is important. Its extension consists of mucosal (superficial) or submucosal (invasive) infiltration depending on the tumor growth pattern. Mucosal extension is predominantly seen with papillary and nodular tumors, while submucosal extension is mainly seen with infiltrating and nodular-infiltrating tumors. The length of longitudinal extension is determined by the type of invasion, with a mean length of 6-10 mm for submucosal spread and 10-20 mm for mucosal spread. The combination of IDUS and MDCT is useful for revealing submucosal cancer extension, which is common in hilar cholangiocarcinoma. To estimate mucosal extension, which is common in extrahepatic bile duct carcinoma, the combination of IDUS and cholangioscopy is required. The utility of current POCS is limited by the maneuverability of the “baby scope”. The new thin baby scope (10 Fr), called “SpyGlass”, has potential, if the image quality can be improved. In patients with unresectable cholangiocarcinoma, EUS-FNA is useful to compensate for the negative results of ERCP tissue sampling.
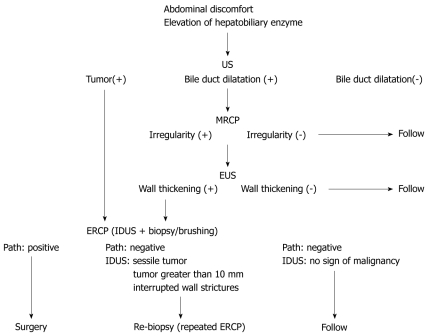
Diagnostic methods for extrahepatic bile duct carcinoma without jaundice. US: Ultrasonography; MRCP: Magnetic resonance cholangiopancreatography; EUS: Endoscopic ultrasonography; ERCP: Endoscopic retrograde cholangiopancreatography; IDUS: Intraductal ultrasonography.
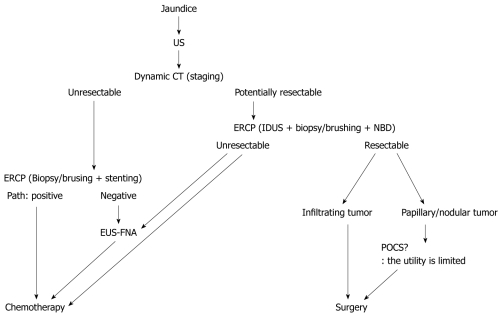
Diagnostic methods for extrahepatic bile duct carcinoma with jaundice. US: Ultrasonography; CT: Computed tomography; NBD: Naso-biliary drainage; EUS-FNA: Endoscopic ultrasonography-guided fine needle aspiration; ERCP: Endoscopic retrograde cholangiopancreatography; IDUS: Intraductal ultrasonography; POCS: Peroral cholangioscopy.
Since extrahepatic bile duct carcinoma is common in the Far East, many researchers (histopathologists, surgeons, radiologists, and endoscopists) in Japan and Korea contributed these studies, especially, the evaluation of longitudinal cancer extension, as shown in Table Table33 and references.
Footnotes
Peer reviewer: Hiroki Yamaue, MD, Second Department of Surgery, Wakayama Medical University, School of Medicine, 811-1 Kimiidera, Wakayama 641, Japan
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
Articles from World Journal of Clinical Oncology are provided here courtesy of Baishideng Publishing Group Inc
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/127303078
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.5306/wjco.v2.i5.203
Article citations
Advanced ultrasound diagnosis of extrahepatic bile duct lesions.
J Med Ultrason (2001), 21 Oct 2024
Cited by: 0 articles | PMID: 39432029
Review
Practice guidelines for managing extrahepatic biliary tract cancers.
Ann Hepatobiliary Pancreat Surg, 28(2):161-202, 29 Apr 2024
Cited by: 1 article | PMID: 38679456 | PMCID: PMC11128785
Subserosal Layer and/or Pancreatic Invasion Based on Anatomical Features as a Novel Prognostic Indicator in Patients with Distal Cholangiocarcinoma.
Diagnostics (Basel), 13(22):3406, 09 Nov 2023
Cited by: 0 articles | PMID: 37998542 | PMCID: PMC10670817
Clinical Outcomes of Robotic Resection for Perihilar Cholangiocarcinoma: A First, Multicenter, Trans-Atlantic, Expert-Center, Collaborative Study.
Ann Surg Oncol, 31(1):81-89, 18 Sep 2023
Cited by: 4 articles | PMID: 37718337
Endoscopic Ultrasound in the Diagnosis of Extrahepatic Cholangiocarcinoma: What Do We Know in 2023?
Diagnostics (Basel), 13(6):1023, 08 Mar 2023
Cited by: 3 articles | PMID: 36980331 | PMCID: PMC10047764
Review Free full text in Europe PMC
Go to all (45) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Limitations of three-dimensional intraductal ultrasonography in the assessment of longitudinal spread of extrahepatic bile duct carcinoma.
J Gastroenterol, 35(12):919-923, 01 Dec 2000
Cited by: 8 articles | PMID: 11573728
[Intraductal ultrasonography for the selection of self expandable metal stent in extrahepatic bile duct carcinoma].
Korean J Gastroenterol, 48(6):415-420, 01 Dec 2006
Cited by: 0 articles | PMID: 17189925
Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology.
World J Gastroenterol, 19(6):874-881, 01 Feb 2013
Cited by: 39 articles | PMID: 23430958 | PMCID: PMC3574884
[Endoscopic staging of hilar cholangiocarcinoma].
Korean J Gastroenterol, 46(1):16-19, 01 Jul 2005
Cited by: 2 articles | PMID: 16030399
Review



