Abstract
Free full text

Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke
Abstract
Transplantation of human mesenchymal stem cells has been shown to reduce infarct size and improve functional outcome in animal models of stroke. Here, we report a study designed to assess feasibility and safety of transplantation of autologous human mesenchymal stem cells expanded in autologous human serum in stroke patients. We report an unblinded study on 12 patients with ischaemic grey matter, white matter and mixed lesions, in contrast to a prior study on autologous mesenchymal stem cells expanded in foetal calf serum that focused on grey matter lesions. Cells cultured in human serum expanded more rapidly than in foetal calf serum, reducing cell preparation time and risk of transmissible disorders such as bovine spongiform encephalomyelitis. Autologous mesenchymal stem cells were delivered intravenously 36–133 days post-stroke. All patients had magnetic resonance angiography to identify vascular lesions, and magnetic resonance imaging prior to cell infusion and at intervals up to 1 year after. Magnetic resonance perfusion-imaging and 3D-tractography were carried out in some patients. Neurological status was scored using the National Institutes of Health Stroke Scale and modified Rankin scores. We did not observe any central nervous system tumours, abnormal cell growths or neurological deterioration, and there was no evidence for venous thromboembolism, systemic malignancy or systemic infection in any of the patients following stem cell infusion. The median daily rate of National Institutes of Health Stroke Scale change was 0.36 during the first week post-infusion, compared with a median daily rate of change of 0.04 from the first day of testing to immediately before infusion. Daily rates of change in National Institutes of Health Stroke Scale scores during longer post-infusion intervals that more closely matched the interval between initial scoring and cell infusion also showed an increase following cell infusion. Mean lesion volume as assessed by magnetic resonance imaging was reduced by >20% at 1 week post-cell infusion. While we would emphasize that the current study was unblinded, did not assess overall function or relative functional importance of different types of deficits, and does not exclude placebo effects or a contribution of recovery as a result of the natural history of stroke, our observations provide evidence supporting the feasibility and safety of delivery of a relatively large dose of autologous mesenchymal human stem cells, cultured in autologous human serum, into human subjects with stroke and support the need for additional blinded, placebo-controlled studies on autologous mesenchymal human stem cell infusion in stroke.
Introduction
Transplantation of mesenchymal stem cells (MSCs), derived from bone marrow, into rodent cerebral ischaemia models can reduce infarct size and improve functional outcome (Chen et al., 2001; Li et al., 2002; Nomura et al., 2005; Honma et al., 2006; Horita et al., 2006; Liu et al., 2006; Onda et al., 2007; Ukai et al., 2007; Omori et al., 2008; Toyama et al., 2009; Komatsu et al., 2010). MSCs can differentiate into cells of neuronal and glial lineage under appropriate cell culture conditions in vitro (Kobune et al., 2003; Kim et al., 2006) as well as in vivo (Prockop et al., 1997; Woodbury et al., 2000; Nomura et al., 2005; Honma et al., 2006). The beneficial effects of MSCs early after transplantation are thought to result from multiple mechanisms that include neuroprotective (Chen et al., 2001; Nomura et al., 2005; Honma et al., 2006; Horita et al., 2006; Liu et al., 2006), anti-inflammatory (Ohtaki et al., 2008) and/or angiogenic (Chen et al., 2003; Liu et al., 2006; Onda et al., 2007; Prockop, 2007; Ukai et al., 2007; Omori et al., 2008; Toyama et al., 2009; Komatsu et al., 2010) effects. Human MSCs genetically modified to produce trophic (Nomura et al., 2005; Horita et al., 2006; Liu et al., 2006) or angiogenic (Liu et al., 2006; Onda et al., 2007; Toyama et al., 2009) factors can markedly improve function and reduce lesion volume in rodent brain ischaemia models.
Bang et al. (2005), in a study primarily aimed at assessing safety, infused freshly prepared autologous bone marrow-derived MSCs expanded in foetal calf serum into five severely affected stroke patients 1–2 months after stroke onset. They report safety, feasibility and a suggestion of efficacy. Human MSC infusion has been studied in cancer (Koc et al., 2000; Lazarus et al., 2005) and neurological disorders other than stroke (Koc et al., 2002) and it appears that in these disorders the use of autologous cells can minimize immune reactions. These considerations, and experimental data showing that human MSC infusion leads to improved outcome and reduced lesion volume in rodent ischaemia models, provide the rationale for the present study.
MSCs constitute a small fraction of the bone marrow non-haematopoietic cell fraction and culture conditions are critical in enriching cultures with human MSCs. Human MSCs can be expanded rapidly with more stable gene expression and maintained in the undifferentiated stem cell state in autologous human serum compared with those in foetal calf serum (Kobayashi et al., 2005; Shahdadfar et al., 2005). In the present study, we assessed autologous human MSCs expanded using autologous human serum under good manufacturing practice conditions. The autologous cells used in this study were well-defined as an immature stem cell population and the molecular phenotype was consistent between patients. Moreover, the use of autologous human serum rather than foetal calf serum resulted in more rapid expansion of cells, which reduces cell preparation time, a factor that may be important for autologous transplantation studies and minimizes potential risk of transmitting viruses, prions [e.g. bovine spongiform encephalomyelitis; see Scolding et al. (2008)] and/or proteins that may cause xenogeneic immunogenicity (delayed hypersensitivity reaction) (Drach et al., 1977). In the present study, we examined feasibility and safety of cell therapy using autoserum-expanded autologous human MSCs in stroke patients. Patients with both grey and white matter ischaemic lesions were studied. A single infusion of well-characterized autologous human MSCs was delivered in the subacute or chronic phase of the stroke. Neurological and neuroradiological analysis was carried out for 1 year. Early results of this study have been reported in an abstract (Honmou et al., 2008).
Materials and methods
Patient enrolment
This study reports observations on 12 patients enrolled in a protocol designed to assess safety and feasibility of autologous human MSC infusion, carried out at Sapporo Medical University and approved by the Institutional Review Board at Sapporo Medical University. Written informed consent from patients or, if aphasic, from their families was obtained before enrolling each patient. Enrolment criteria included the following: (i) age between 20 and 75 years old; (ii) stroke onset within the past 6 months; (iii) relevant ischaemic supratentorial lesion(s) as assessed by MRI; (iv) severely disabling deficits (modified Rankin Scale score of 3 or worse at enrolment); and (v) impairments of consciousness were not severe (Japan coma scale between 0 and 100; Ohta et al., 1986). We excluded patients who met one or more of the following criteria: (i) severe haemorrhagic transformation of ischaemic lesion; (ii) severe consciousness disturbance (Japan Coma scale between 200 and 300); (iii) pregnant or possibly pregnant; (iv) severe medical complications such as kidney dysfunction, liver dysfunction, gastrointestinal, etc; (v) malignant diseases; (vi) severe ischaemic diseases such as ischaemic heart disease, systemic atherosclerotic changes; (vii) severe anaemia; (viii) strokes that were infratentorial; or (ix) other severe comorbidity. All patients received formal rehabilitation (physical therapy, occupational therapy and speech therapy) as appropriate prior to autologous human MSC infusion and these were continued after infusion. There were no additional drug treatments (e.g. sedatives, anti-depressants, anti-spasticity agents) after cell infusion.
Cell preparation
Autologous human MSCs were prepared using a previously described method with minor modification (Kim et al., 2006) after informed consent was obtained; the subject’s or, if aphasic, the family’s consent was obtained according to the Declaration of Helsinki. All cell culture procedures were carried out in good manufacturing practice conditions by personnel who had received formal training in good manufacturing practice within a facility with highly controlled temperature, room air, pressure, etc.
Autologous human MSCs were prepared from each patient in a good manufacturing practice cell processing centre and cryopreserved until use. A previous clinical stroke study infused human MSCs that were prepared with foetal calf serum (Bang et al., 2005). In the present study, we cultured the autologous human MSCs in autologous human serum. Briefly, bone marrow (30–73 ml) was obtained from the posterior iliac crest of patients under local anaesthesia, and was diluted with Dulbecco’s modified Eagle’s medium (Mediatech Inc.) supplemented with 10% autologous human serum, 2
ml) was obtained from the posterior iliac crest of patients under local anaesthesia, and was diluted with Dulbecco’s modified Eagle’s medium (Mediatech Inc.) supplemented with 10% autologous human serum, 2 mM l-glutamine (Sigma-Aldrich), 100
mM l-glutamine (Sigma-Aldrich), 100 U/ml penicillin–streptomycin (Sigma-Aldrich), was plated on 150
U/ml penicillin–streptomycin (Sigma-Aldrich), was plated on 150 mm tissue culture dish (IWAKI) and incubated in a humidified atmosphere of 5% CO2 at 37°C for several days. Foetal calf serum (Life Technologies) was used only for growth rate comparison and was not used for clinical use. Autologous human MSCs, when selected by plastic adhesion, require the elimination of non-adherent cells by replacing the medium. When cultures almost reached confluence, the adherent cells were detached with trypsin–EDTA solution (Mediatech Inc.) and subcultured at 1
mm tissue culture dish (IWAKI) and incubated in a humidified atmosphere of 5% CO2 at 37°C for several days. Foetal calf serum (Life Technologies) was used only for growth rate comparison and was not used for clinical use. Autologous human MSCs, when selected by plastic adhesion, require the elimination of non-adherent cells by replacing the medium. When cultures almost reached confluence, the adherent cells were detached with trypsin–EDTA solution (Mediatech Inc.) and subcultured at 1 ×
× 104
104 cells/ml. Thus, autologous human MSCs were expanded up to ~
cells/ml. Thus, autologous human MSCs were expanded up to ~ 1
1 ×
× 108
108 cells (1.05
cells (1.05 ±
± 0.33
0.33 ×
× 108) (Table 2) within a relatively short culture period (20.3
108) (Table 2) within a relatively short culture period (20.3 ±
± 7.1 days). The expanded autologous human MSCs were dissociated, diluted in 51
7.1 days). The expanded autologous human MSCs were dissociated, diluted in 51 ml of storage solution [20.5
ml of storage solution [20.5 ml of RPMI (Life Technologies), 20.5
ml of RPMI (Life Technologies), 20.5 ml of autoserum, 5
ml of autoserum, 5 ml of low molecular dextran L (Otsuka), 5
ml of low molecular dextran L (Otsuka), 5 ml dimethyl sulphoxide (Sigma-Aldrich)], frozen with the programme freezer (Planer PLC) and stored in a deep freezer (−150°C) (Sanyo) until use. Cell passages were limited to three or less, targeting a cell number of 1.0
ml dimethyl sulphoxide (Sigma-Aldrich)], frozen with the programme freezer (Planer PLC) and stored in a deep freezer (−150°C) (Sanyo) until use. Cell passages were limited to three or less, targeting a cell number of 1.0 ×
× 108
108 cells. Cryopreservation permitted detailed characterization of cells and pathogens, which required several days prior to cell infusion and resulted in higher cell viability (>95.2%). On the day of infusion, cryopreserved units were thawed at the bedside in a 37°C water bath. Extrapolation from our previous experimental studies in rodents, where 1
cells. Cryopreservation permitted detailed characterization of cells and pathogens, which required several days prior to cell infusion and resulted in higher cell viability (>95.2%). On the day of infusion, cryopreserved units were thawed at the bedside in a 37°C water bath. Extrapolation from our previous experimental studies in rodents, where 1 ×
× 106
106 cells showed efficacy (Honma et al., 2006), suggested a target cell range scaled to body weight of 0.5
cells showed efficacy (Honma et al., 2006), suggested a target cell range scaled to body weight of 0.5 ×
× 108 to 5
108 to 5 ×
× 108 per patient. We administered 0.6 to 1.6
108 per patient. We administered 0.6 to 1.6 ×
× 108
108 cells to each patient over 30
cells to each patient over 30 min.
min.
Table 2
Characteristics of processed MSCs
| Patient no. | Number of injected MSCs | CD34+ (%) | CD45+ (%) | CD105+ (%) |
|---|---|---|---|---|
| 1 | 0.8 × × 108 108 | 0.5 | 0.2 | 100.0 |
| 2 | 0.6 × × 108 108 | 0.8 | 4.8 | 100.0 |
| 3 | 1.6 × × 108 108 | 1.2 | 1.5 | 99.9 |
| 4 | 1.2 × × 108 108 | 1.0 | 7.6 | 99.9 |
| 5 | 1.1 × × 108 108 | 0.3 | 0.5 | 99.8 |
| 6 | 1.1 × × 108 108 | 1.8 | 6.5 | 98.6 |
| 7 | 1.5 × × 108 108 | 2.1 | 2.8 | 99.8 |
| 8 | 1.3 × × 108 108 | 0.4 | 0.7 | 99.9 |
| 9 | 0.6 × × 108 108 | 1.8 | 3.6 | 99.8 |
| 10 | 1.2 × × 108 108 | 1.9 | 3.1 | 99.9 |
| 11 | 1.5 × × 108 108 | 1.0 | 2.7 | 100.0 |
| 12 | 1.2 × × 108 108 | 0.8 | 5.2 | 100.0 |
Cell characterization and pathogen screening
Flow cytometric analysis of autologous human MSCs was performed as previously described (Honma et al., 2006; Liu et al., 2006). Briefly, cell suspensions were washed twice with phosphate-buffered saline containing 0.1% bovine serum albumin. For direct assays, aliquots of cells at a concentration of 1 ×
× 106
106 cells/ml were immunolabelled at 4°C for 30
cells/ml were immunolabelled at 4°C for 30 min with the following antihuman antibodies: fluorescein isothiocyanate-conjugated CD24 (Dako), phycoerythrin-conjugated CD34 (Beckman Coulter), phycoerythrin-conjugated CD45, (Life Technologies) and phycoerythrin-conjugated CD105 (BioLegend). As an isotype-matched control, mouse immunoglobulin G1 (Becton Coulter) was used. Labelled cells were analysed by a FACSCalibur flow cytometer (Becton Dickinson Bioscience). Dead cells were gated out with forward-versus side-scatter window and propidium iodide staining. The expanded cells were tested for sterility [bacteria, Treponema pallidum haemagglutination fungi, viral (hepatitis B, hepatitis C, adult T cell leukaemia virus, HIV, Parvovirus B19, mycoplasma)] and endotoxin level.
min with the following antihuman antibodies: fluorescein isothiocyanate-conjugated CD24 (Dako), phycoerythrin-conjugated CD34 (Beckman Coulter), phycoerythrin-conjugated CD45, (Life Technologies) and phycoerythrin-conjugated CD105 (BioLegend). As an isotype-matched control, mouse immunoglobulin G1 (Becton Coulter) was used. Labelled cells were analysed by a FACSCalibur flow cytometer (Becton Dickinson Bioscience). Dead cells were gated out with forward-versus side-scatter window and propidium iodide staining. The expanded cells were tested for sterility [bacteria, Treponema pallidum haemagglutination fungi, viral (hepatitis B, hepatitis C, adult T cell leukaemia virus, HIV, Parvovirus B19, mycoplasma)] and endotoxin level.
Patient evaluation
All enrolled patients were evaluated based upon a protocol that included general laboratory data, neuroradiological findings and stroke scales, with a primary outcome of safety (adverse events, neurological worsening and evidence of tumour or abnormal cell growth on MRI). Neurological scores [National Institutes of Health Stroke Scale (NIHSS)] were assessed on admission, just prior to cell infusion, immediately after cell infusion, 1, 2, 4, 7 and 14 days, 1, 3 and 6 months and 1 year post-infusion by neurosurgeons and neurologists who were not blinded. Modified Rankin scores were also recorded and are presented in parentheses after NIHSS scores, so that a patient with an NIHSS score of 5 and a modified Rankin score of 3 on a given day is presented as having scores of 5(3). Brain MRI and magnetic resonance angiography (MRA) (1.5 Tesla, GE) were performed in all patients before and after infusion, and brain 3D CT angiography (Toshiba) was carried out in some patients. MRIs were targeted for admission, 1–2 days prior to cell infusion, immediately after cell infusion and 1 and 2 days, 1 and 2 weeks, 1, 3 and 6 months and 1 year post-infusion and were interpreted by unblinded radiologists. However, some patients had less frequent testing.
All MRI measurements were performed using 1.5 Tesla, GE SIGNA. Fluid attenuated inversion recovery images were obtained from a 4-mm thick axial section using a 20 ×
× 20
20 mm field of view, repetition time
mm field of view, repetition time =
= 10
10 000
000 ms, echo time
ms, echo time =
= 120
120 ms, inversion time
ms, inversion time =
= 2300
2300 ms and reconstructed using a 256
ms and reconstructed using a 256 ×
× 192 image matrix. The ischaemic lesion area was calculated from fluid attenuated inversion recovery images using imaging software (Image-Pro, PLUS, Media Cybernetics, Inc.), based on the method of Neumann-Haefelin et al. (2000). For each slice, the higher intensity lesions in fluid attenuated inversion recovery images where the signal intensity was twice as high were marked as the ischaemic lesion area, and infarct volume was calculated taking slice thickness into account.
192 image matrix. The ischaemic lesion area was calculated from fluid attenuated inversion recovery images using imaging software (Image-Pro, PLUS, Media Cybernetics, Inc.), based on the method of Neumann-Haefelin et al. (2000). For each slice, the higher intensity lesions in fluid attenuated inversion recovery images where the signal intensity was twice as high were marked as the ischaemic lesion area, and infarct volume was calculated taking slice thickness into account.
All patients were monitored closely during and within 24 h of autologous human MSC injections. Oxygen saturation, body temperature, electrocardiogram, blood pressure, pulse and respiratory rate were carefully monitored before and after injection. Patients also had chest films before and after autologous human MSC injection. All patients were followed for 12 months.
h of autologous human MSC injections. Oxygen saturation, body temperature, electrocardiogram, blood pressure, pulse and respiratory rate were carefully monitored before and after injection. Patients also had chest films before and after autologous human MSC injection. All patients were followed for 12 months.
Data analysis
Data are presented as mean values ±
± SEM. Differences between cell numbers for autoserum versus foetal calf serum were assessed by t-test to identify individual group differences. Significance of median incremental daily changes in NIHSS was assessed using a non-parametric Mood’s median test. Differences in mean % change in lesion volume were assessed with the Kruskal–Wallis test. Differences were deemed statistically significant at P
SEM. Differences between cell numbers for autoserum versus foetal calf serum were assessed by t-test to identify individual group differences. Significance of median incremental daily changes in NIHSS was assessed using a non-parametric Mood’s median test. Differences in mean % change in lesion volume were assessed with the Kruskal–Wallis test. Differences were deemed statistically significant at P <
< 0.05.
0.05.
Results
Cell preparation
Cells cultured in human autologous serum were compared with those cultured in foetal calf serum. The growth rate of the human MSCs was greater when the cells were grown in autologous human serum. At 7 days in culture, the density of cells grown in human serum was more than double that of those grown in foetal calf serum (93.6 ×
× 104
104 ±
± 9.4
9.4 ×
× 104 versus 35.6
104 versus 35.6 ×
× 104
104 ±
± 4.2
4.2 ×
× 104; n
104; n =
= 5, P
5, P <
< 0.0001). The expanded autologous human MSCs were tested for sterility; there was no evidence of bacterial, T. pallidum haemagglutination, fungal, viral (hepatitis B, hepatitis C, adult T cell leukaemia virus, HIV, Parvovirus B19) or mycoplasmal contamination, and endotoxin levels were non-pathogenic in all samples.
0.0001). The expanded autologous human MSCs were tested for sterility; there was no evidence of bacterial, T. pallidum haemagglutination, fungal, viral (hepatitis B, hepatitis C, adult T cell leukaemia virus, HIV, Parvovirus B19) or mycoplasmal contamination, and endotoxin levels were non-pathogenic in all samples.
Case presentations
Twelve patients ranging in age from 41 to 73 (59.2 ±
± 8.2) years and of both genders (Table 1) were studied, from an initial group of 16 patients who were referred as candidates for this study. All 16 patients matched the inclusion criteria; four of the 16 patients were excluded and did not receive autologous human MSCs because they were found to meet exclusion criteria (deep vein thrombosis, strong allergic reaction to anti-platelet drug or anti-epileptic drug, thyroid cancer, nephritic syndrome). No patients refused to participate and no patients withdrew from the study. Results of all of the 12 patients were analysed.
8.2) years and of both genders (Table 1) were studied, from an initial group of 16 patients who were referred as candidates for this study. All 16 patients matched the inclusion criteria; four of the 16 patients were excluded and did not receive autologous human MSCs because they were found to meet exclusion criteria (deep vein thrombosis, strong allergic reaction to anti-platelet drug or anti-epileptic drug, thyroid cancer, nephritic syndrome). No patients refused to participate and no patients withdrew from the study. Results of all of the 12 patients were analysed.
Table 1
Patient characteristics
| Patient no. | Sex/age | Symptoms | Time onset to MSC injection (days) | Aetiology | Primary ischaemic lesion | NIHSS at MSC injection |
|---|---|---|---|---|---|---|
| 1 | Female/51 | Hemiparesis | 61 | Moyamoya | Mixed | 10 |
| 2 | Male/62 | Hemiparesis | 133 | Atherosclerosis | White matter | 5 |
| 3 | Male/52 | Hemiparesis | 43 | Atherosclerosis | White matter | 5 |
| 4 | Male/62 | Hemiparesis | 36 | Atherosclerosis | White matter | 4 |
| 5 | Male/73 | Hemiparesis | 59 | Lacunar | White matter | 7 |
| 6 | Male/58 | Hemiparesis and aphasia | 80 | Atherosclerosis | Grey matter | 6 |
| 7 | Male/60 | Hemiparesis | 61 | Lacunar | White matter | 5 |
| 8 | Female/67 | Hemiparesis and aphasia | 59 | Intra-operative | Mixed | 5 |
| 9 | Female/63 | Hemiparesis | 88 | Lacunar | White matter | 2 |
| 10 | Male/60 | Hemiparesis and aphasia | 74 | Atherosclerosis | Mixed | 15 |
| 11 | Male/41 | Hemiparesis and aphasia | 66 | Embolic | Mixed | 15 |
| 12 | Male/61 | Hemiparesis and aphasia | 56 | Atherosclerosis | Mixed | 20 |
All patients studied displayed hemiparesis and five were aphasic. Patients received standard treatment before enrolment in the study, and were assessed by MRI analysis and neurological testing and scored using the NIHSS and modified Rankin scores. Table 1 indicates, for each patient, whether the lesion was in predominantly white matter or grey matter, or was mixed. The autologous human MSCs were intravenously infused into the patients between 36 and 133 days post-stroke; the day of infusion is designated as ‘Day 0’ and subsequent days designated ‘1’, ‘2’, ‘3’, etc. in Figs 1–12 with minus signs designating days pre-infusion. The number and antigenic profiles of autologous human MSCs infused into the patients are presented in Table 2. Case descriptions for each of the 12 patients are described below.
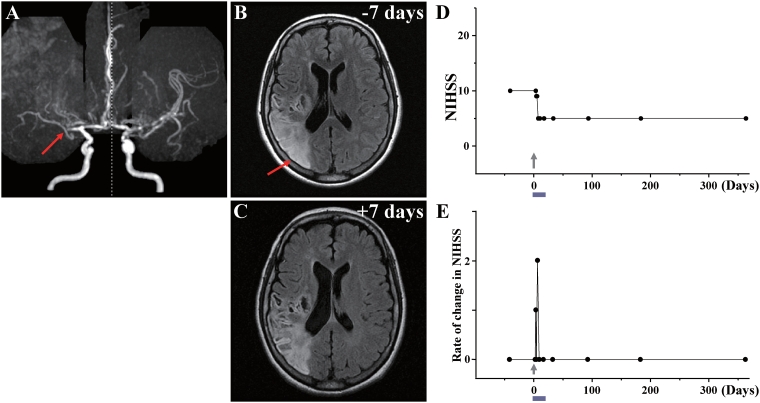
Case 1: (A) MRA showed occlusion of the right middle cerebral artery (red arrow). (B) MRI 7 days before cell injection and (C) 7 days after cell injection are shown. Red arrow in B indicates the infarcted lesion. (D) NIHSS scores for 1 year. (E) Rate of change in NIHSS for each day plotted. For example, the rate of change in NIHSS score at Day X was calculated as (NIHSS score on previous examination—NIHSS score on Day X)/number of days between examinations, to give an estimate of the daily rate of change.
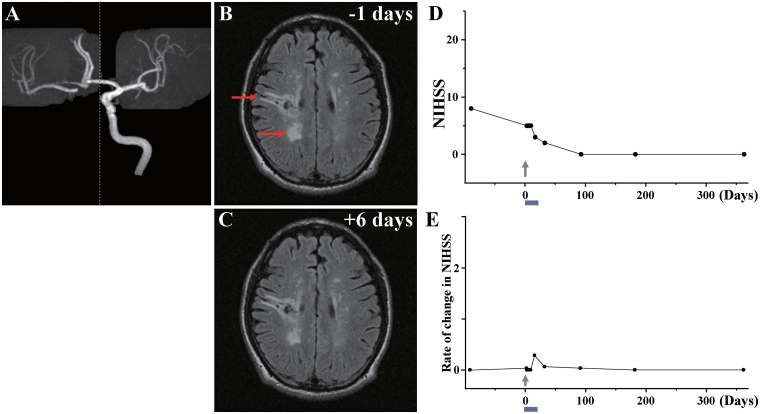
Case 2: (A) MRA showed occlusion of the right internal carotid artery. (B) MRI 1 day before cell injection and (C) 6 days after cell injection. Red arrows in B indicate the infarcted lesions. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
day before cell injection and (C) 6 days after cell injection. Red arrows in B indicate the infarcted lesions. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
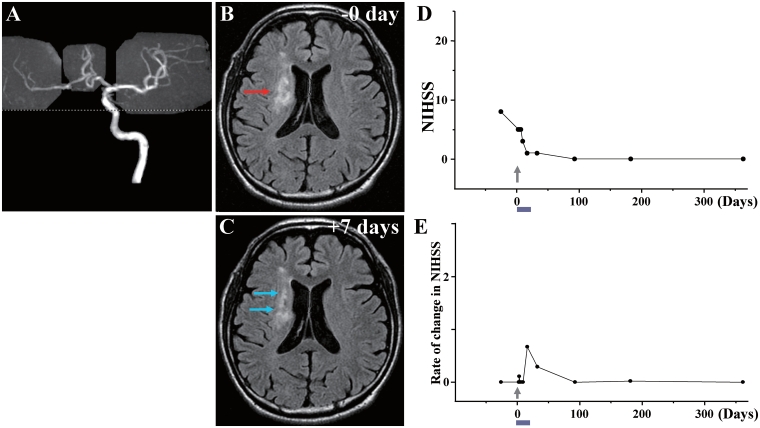
Case 3: (A) MRA showed occlusion of the right internal carotid artery. (B) MRI just before cell injection and (C) 7 days after cell injection. Red arrow in B indicates the infarcted lesion before cell injection, and blue arrows in C show the reduced lesion volume and lower signal intensity after cell injection. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
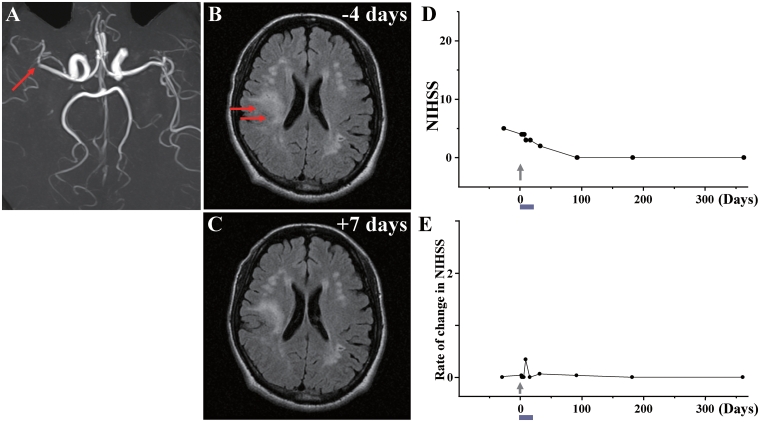
Case 4: (A) MRA showed occlusion of a branch of the right middle cerebral artery (red arrow). (B) MRI 4 days before cell injection and (C) 7 days after cell injection. Red arrows in B indicate the infarcted lesion. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
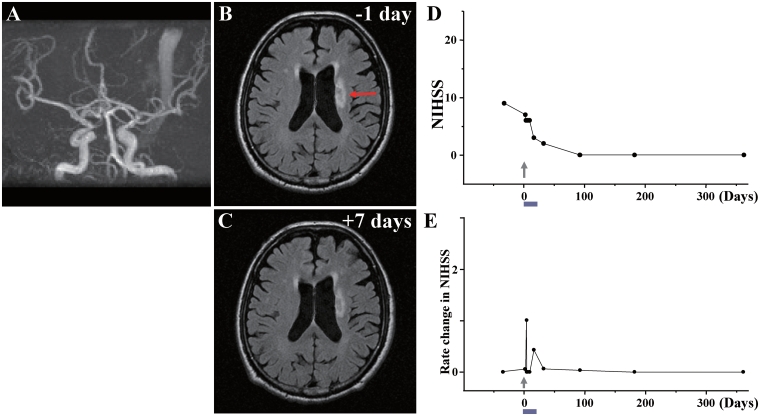
Case 5: (A) MRA showed that major cerebral arteries were intact. (B) MRI 1 day before cell injection and (C) 7 days after cell injection. Red arrow in B indicates the infarcted lesion. (D) NIHSS scores 1 year and (E) rate of change in NIHSS.
day before cell injection and (C) 7 days after cell injection. Red arrow in B indicates the infarcted lesion. (D) NIHSS scores 1 year and (E) rate of change in NIHSS.
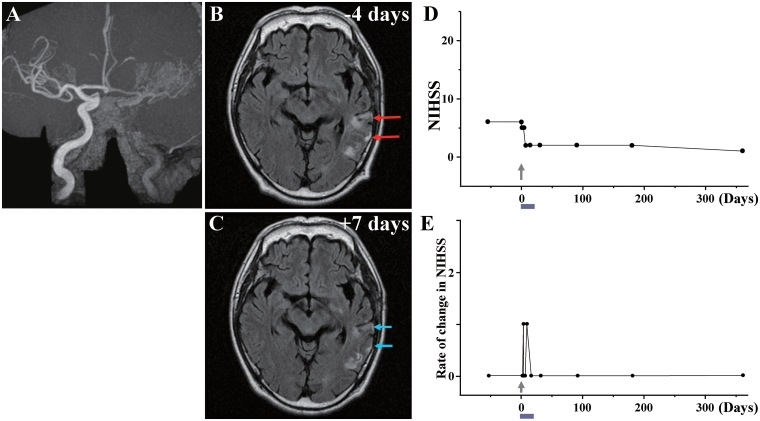
Case 6: (A) MRA showed occlusion of the left internal carotid artery. (B) MRI 4 days before cell injection and (C) 7 days after cell injection. Red arrows in B indicate the infarcted lesions before cell injection, and blue arrows in C show the reduced lesion volume and lower signal intensity after cell injection. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
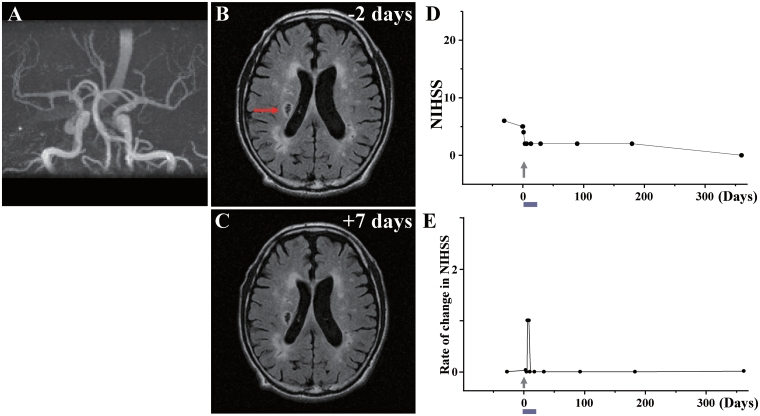
Case 7: (A) MRA showed that major cerebral arteries were intact. (B) MRI 2 days before cell injection and (C) 7 days after cell injection. Red arrow in B indicates the infarcted lesion. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
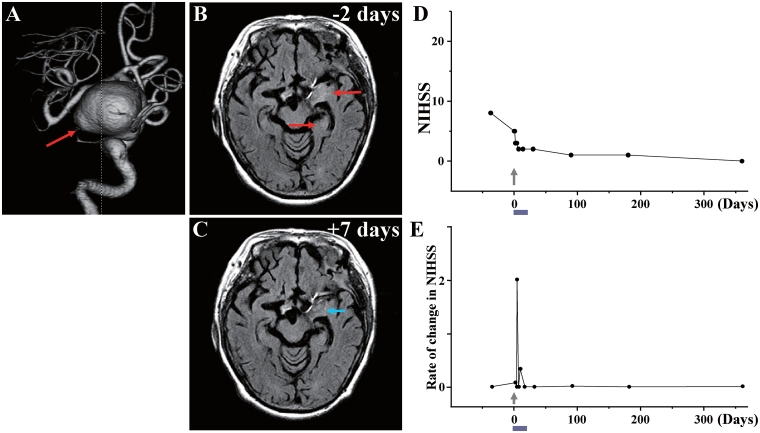
Case 8: (A) 3D-CT angiography showed the large aneurysm (red arrow) in the left internal carotid artery. (B) MRI 2 days before cell injection and (C) 7 days after cell injection. Red arrows in B indicate the infarcted lesions before cell injection, and blue arrow in C shows the reduced lesion volume and lower signal intensity after cell injection. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
Case 9: (A) MRA showed that major cerebral arteries were intact. (B) MRI 5 days before cell injection and (C) 7 days after cell injection. Red arrows in B indicate the infarcted lesions. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
Case 10: (A) MRA showed occlusion of the left internal carotid artery. (B) MRI 3 days before cell injection and (C) 7 days after cell injection. Red arrows in B indicate the infarcted lesions before cell injection, and blue arrows in C show the reduced lesion volume and lower signal intensity after cell injection. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
Case 11: (A) MRA showed occlusion of the left middle cerebral artery (red arrow). (B) MRI 6 days before cell injection and (C) 7 days after cell injection. Red arrows in B indicate the infarcted lesions. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
Case 12: (A) MRA showed occlusion of the left internal carotid artery. (B) MRI 1 day before cell injection and (C) 7 days after cell injection. Red arrows in B indicate the infarcted lesion. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
day before cell injection and (C) 7 days after cell injection. Red arrows in B indicate the infarcted lesion. (D) NIHSS scores for 1 year and (E) rate of change in NIHSS.
Case 1
This 51-year-old female with a history of hyperlipidaemia/hypertension sustained a stroke due to Moyamoya disease with occlusion of the right middle cerebral artery (Fig. 1A). MRI demonstrated infarction of grey matter and white matter in the right frontal, parietal and occipital lobes (Fig. 1B). The patient displayed severe left hemiparesis and left visual field deficits. The NIHSS/Rankin scores were 10(4) on Day −44 (i.e. 44 days prior to autologous human MSC infusion) and were 10(4) on Day 0 immediately before autologous human MSC infusion. Sixty-one days after the stroke, the patient received an intravenous infusion of 0.8 ×
× 108 autologous human MSCs. The NIHSS/Rankin scores were 9(4) on Day 1 and 2 and 5(4) on Days 4, 7, 14 and 30 (Fig. 1D). NIHSS/Rankin scores were 5(3) on Days 90, 180 and 360. Incremental changes per day in NIHSS after autologous human MSC infusion are plotted in Fig. 1E. There were no major cell injection-related adverse events except slight itching at the injection site, which resolved within days. MRI demonstrated lesion volume 174.98
108 autologous human MSCs. The NIHSS/Rankin scores were 9(4) on Day 1 and 2 and 5(4) on Days 4, 7, 14 and 30 (Fig. 1D). NIHSS/Rankin scores were 5(3) on Days 90, 180 and 360. Incremental changes per day in NIHSS after autologous human MSC infusion are plotted in Fig. 1E. There were no major cell injection-related adverse events except slight itching at the injection site, which resolved within days. MRI demonstrated lesion volume 174.98 cm3 on Day −7 prior to cell infusion, and 148.22
cm3 on Day −7 prior to cell infusion, and 148.22 cm3 on Day 7 post-infusion, without new lesions (Fig. 1C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 1C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
Case 2
This 62-year-old male, with a history of gastric ulcer/polycystic kidney/mild coronary artery stenosis/hypertension, sustained an atherosclerotic stroke due to right internal carotid artery occlusion (Fig. 2A). MRI demonstrated infarction primarily affecting right frontal and parietal subcortical white matter (Fig. 2B). The patient displayed left hemiparesis with spastic rigidity, with NIHSS/Rankin scores of 8(4) on Day −92, and NIHSS/Rankin scores of 5(3) on Day 0, immediately prior to autologous human MSC infusion. The patient received an intravenous infusion of 0.6 ×
× 108 autologous human MSCs 133 days after the stroke. NIHSS/Rankin scores were 5(3) on Days 1–7, 3(3) on Day 14, 2(3) on Day 30 and 0(2) thereafter (Fig. 2D and E). There were no major cell injection-related adverse events. MRI demonstrated lesion volume 24.52
108 autologous human MSCs 133 days after the stroke. NIHSS/Rankin scores were 5(3) on Days 1–7, 3(3) on Day 14, 2(3) on Day 30 and 0(2) thereafter (Fig. 2D and E). There were no major cell injection-related adverse events. MRI demonstrated lesion volume 24.52 cm3 on Day −1 prior to cell infusion, and 20.87
cm3 on Day −1 prior to cell infusion, and 20.87 cm3 6 days post-infusion, without new lesions (Fig. 2C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 6 days post-infusion, without new lesions (Fig. 2C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
Case 3
This 52-year-old male, with a history of premature ventricular contractions/diabetes mellitus/hypertension, sustained an atherosclerotic stroke due to right internal carotid artery occlusion (Fig. 3A). MRI demonstrated infarction of white matter in the right frontal lobe and adjacent basal ganglia (Fig. 3B). The patient displayed left hemiparesis. NIHSS/Rankin scores were 8(4) on Day −28 and 5(4) on Day 0, immediately prior to cell infusion. Forty-three days after the stroke, the patient received an intravenous infusion of 1.6 ×
× 108 autologous human MSCs. NIHSS/Rankin scores were 3(3) on Day 7, 1(2) on Day 14, 1(2) on Day 30 and 0(1) on Day 90 and thereafter (Fig. 3D). There were no major cell injection-related adverse events except slight itching on the hand, face and head. MRI demonstrated lesion volume 21.67
108 autologous human MSCs. NIHSS/Rankin scores were 3(3) on Day 7, 1(2) on Day 14, 1(2) on Day 30 and 0(1) on Day 90 and thereafter (Fig. 3D). There were no major cell injection-related adverse events except slight itching on the hand, face and head. MRI demonstrated lesion volume 21.67 cm3 on Day 0 immediately prior to cell infusion, and 14.61
cm3 on Day 0 immediately prior to cell infusion, and 14.61 cm3 on Day 7 post-infusion, without new lesions (Fig. 3C). MRI perfusion analysis showed increased cerebral blood flow 7 days after injection (Fig. 13E and F). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 3C). MRI perfusion analysis showed increased cerebral blood flow 7 days after injection (Fig. 13E and F). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
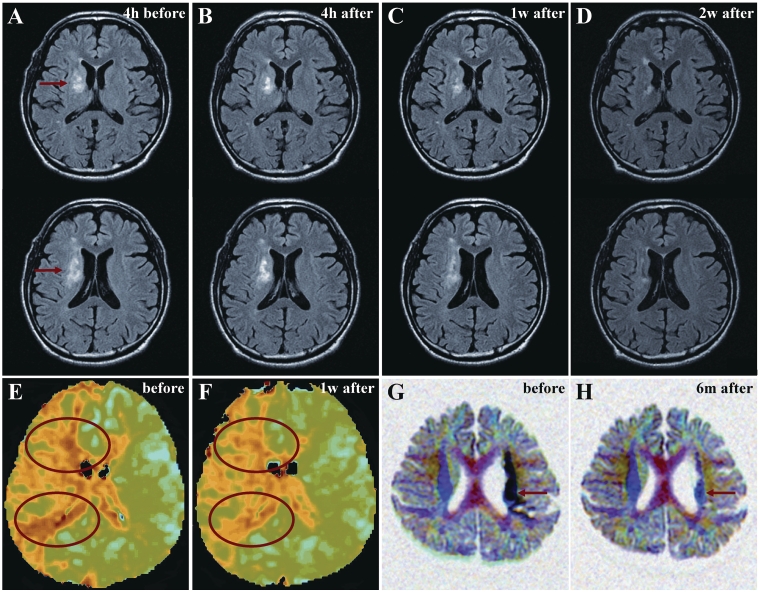
(A) MRIs from Case 3 4 h before cell injection and (B) 4
h before cell injection and (B) 4 h, (C) 1 week and (D) 2 weeks after cell injection. Red arrows in A indicate the infarcted lesion. (E) Perfusion images from Case 3 before and (F) 1 week after cell injection. Red ovals show regions of interest. (G) 3D diffusion tensor axonography from Case 11 before and (H) 6 months after cell injection. The black signal in internal capsule (red arrows in G) reverted toward normal signal (red arrows in H).
h, (C) 1 week and (D) 2 weeks after cell injection. Red arrows in A indicate the infarcted lesion. (E) Perfusion images from Case 3 before and (F) 1 week after cell injection. Red ovals show regions of interest. (G) 3D diffusion tensor axonography from Case 11 before and (H) 6 months after cell injection. The black signal in internal capsule (red arrows in G) reverted toward normal signal (red arrows in H).
Case 4
This 62-year-old male, with a history of hypertension/asymptomatic cerebral infarction, sustained an atherosclerotic stroke due to occlusion of a branch of right middle cerebral artery (Fig. 4A). MRI demonstrated sub-cortical infarcts in the right frontal and parietal lobes (Fig. 4B). The patient displayed left hemiparesis and spastic rigidity. NIHSS/Rankin scores were 5(4) on Day −29 and 4(4) on Day 0, immediately prior to cell infusion. Thirty-six days after the stroke, the patient received an intravenous infusion of 1.2 ×
× 108 autologous human MSCs. NIHSS/Rankin scores were 3(3) on Days 7 and 14, 2(3) on Day 30 and 0(2) thereafter (Fig. 4D and E). There were no major cell injection-related adverse events. MRI demonstrated lesion volume 18.92
108 autologous human MSCs. NIHSS/Rankin scores were 3(3) on Days 7 and 14, 2(3) on Day 30 and 0(2) thereafter (Fig. 4D and E). There were no major cell injection-related adverse events. MRI demonstrated lesion volume 18.92 cm3 on Day −4 prior to cell infusion; and 15.45
cm3 on Day −4 prior to cell infusion; and 15.45 cm3 on day 7 post-infusion, without new lesions (Fig. 4C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on day 7 post-infusion, without new lesions (Fig. 4C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
Case 5
This 73-year-old male, with a history of hypertension/diabetes mellitus/dilated myocarditis, sustained a giant lacunar stroke (atherosclerotic branch disease). MRA showed that major cerebral arteries were intact (Fig. 5A). MRI demonstrated infarction of deep white matter and basal ganglia in the left frontal lobe (Fig. 5B). The patient displayed right hemiparesis and spastic rigidity. NIHSS/Rankin scores were 9(4) on Day −35 and 7(4) on Day 0 immediately prior to cell infusion. Fifty-nine days after the stroke, the patient received an intravenous infusion of 1.1 ×
× 108 autologous human MSCs. NIHSS/Rankin scores were 6(4) on Day 7 after autologous human MSC infusion, 3(3) on Day 14, 2(3) on Day 30, 0(3) on Day 90 and 0(2) thereafter (Fig. 5D and E). The patient developed a slight fever and nausea immediately after cell injection, which resolved within 6
108 autologous human MSCs. NIHSS/Rankin scores were 6(4) on Day 7 after autologous human MSC infusion, 3(3) on Day 14, 2(3) on Day 30, 0(3) on Day 90 and 0(2) thereafter (Fig. 5D and E). The patient developed a slight fever and nausea immediately after cell injection, which resolved within 6 h. No other major adverse events were observed. MRI demonstrated lesion volume 7.58
h. No other major adverse events were observed. MRI demonstrated lesion volume 7.58 cm3 on Day −1 prior to cell infusion; and 4.94
cm3 on Day −1 prior to cell infusion; and 4.94 cm3 on Day 7 post-infusion, without new lesions (Fig. 5C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 5C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
Case 6
This 58-year-old male, with a history of hypertension/prostate hypertrophy, sustained an atherosclerotic stroke due to left internal carotid artery occlusion (Fig. 6A). MRI demonstrated infarction of grey and white matter in the left frontal, temporal and parietal lobes (Fig. 6B). The patient displayed aphasia and right hemiparesis. NIHSS/Rankin scores were 6(3) on Day −54 and 6(3) on Day 0, immediately prior to cell infusion. Eighty days after the stroke, the patient received an intravenous infusion of 1.1 ×
× 108 autologous human MSCs. NIHSS/Rankin scores were 5(3) on Days 1–4 and 2(2) on Days 7, 14 and 30. NIHSS/Rankin scores were 2(1) on Days 90 and 180, and were 1(1) at 1 year (Fig. 6D and E). There were no major cell injection-related adverse events. MRI lesion volume was 5.77
108 autologous human MSCs. NIHSS/Rankin scores were 5(3) on Days 1–4 and 2(2) on Days 7, 14 and 30. NIHSS/Rankin scores were 2(1) on Days 90 and 180, and were 1(1) at 1 year (Fig. 6D and E). There were no major cell injection-related adverse events. MRI lesion volume was 5.77 cm3 on Day −4 prior to cell infusion, and 4.12
cm3 on Day −4 prior to cell infusion, and 4.12 cm3 on Day 7 post-infusion, without new lesions (Fig. 6C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 6C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
Case 7
This 60-year-old male, with a history of paroxysmal atrial fibrillation/asymptomatic cerebral infarction/hypertension/prostate hypertrophy, sustained a lacunar stroke due to embolism caused by paroxysmal atrial fibrillation. MRA showed that major cerebral arteries were intact (Fig. 7A). MRI demonstrated a new lacunar lesion in the corona radiata of the right hemisphere and other old embolic lacunar lesions. The patient displayed left hemiparesis. NIHSS/Rankin scores of 6(5) on Day −30 and 5(4) on Day 0 immediately prior to cell infusion. Sixty-one days after the stroke, the patient received an intravenous infusion of 1.5 ×
× 108 autologous human MSCs. NIHSS/Rankin scores were 4(4) on 2 days after autologous human MSC infusion and were 2(4) on 4 days, and 2(3) on 7–180 days. NIHSS/Rankin scores was 0(3) at 1 year (Fig. 7D and E). There were no major symptomatic cell injection-related adverse events. MRI lesion volume was 2.17
108 autologous human MSCs. NIHSS/Rankin scores were 4(4) on 2 days after autologous human MSC infusion and were 2(4) on 4 days, and 2(3) on 7–180 days. NIHSS/Rankin scores was 0(3) at 1 year (Fig. 7D and E). There were no major symptomatic cell injection-related adverse events. MRI lesion volume was 2.17 cm3 on Day −2 prior to cell infusion; and 1.78
cm3 on Day −2 prior to cell infusion; and 1.78 cm3 on Day 7 post-infusion, without new lesions (Fig. 7C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 7C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
Case 8
This 67-year-old female, with a history of hypertension/diabetes mellitus/hyperlipidaemia/unruptured large cerebral aneurysm in the left internal carotid artery (Fig. 8A), sustained an intra-operative stroke due to temporary occlusion of the left anterior choroidal artery. MRI demonstrated infarction of the left amygdala, hippocampus, thalamus and internal capsule (Fig. 8B). The patient displayed right hemiparesis and thalamic aphasia. NIHSS/Rankin scores were 8(5) on Day −37 and 5(4) on Day 0 immediately prior to cell infusion. Fifty-nine days after the stroke, the patient received an intravenous infusion of 1.3 ×
× 108 autologous human MSCs. NIHSS/Rankin scores were 3(4) on Days 2 and 4, 2(3) on Day 7, 2(2) on Day 14 and 2(2) on Day 30. NIHSS/Rankin scores were 1(1) on Days 90 and 180, and 0(1) at 1 year (Fig. 8D and E). There were no major cell injection-related adverse events. MRI lesion volume was 11.79
108 autologous human MSCs. NIHSS/Rankin scores were 3(4) on Days 2 and 4, 2(3) on Day 7, 2(2) on Day 14 and 2(2) on Day 30. NIHSS/Rankin scores were 1(1) on Days 90 and 180, and 0(1) at 1 year (Fig. 8D and E). There were no major cell injection-related adverse events. MRI lesion volume was 11.79 cm3 on Day −2 prior to cell infusion; and 6.01
cm3 on Day −2 prior to cell infusion; and 6.01 cm3 on Day 7 post-infusion, without new lesions (Fig. 8C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 8C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
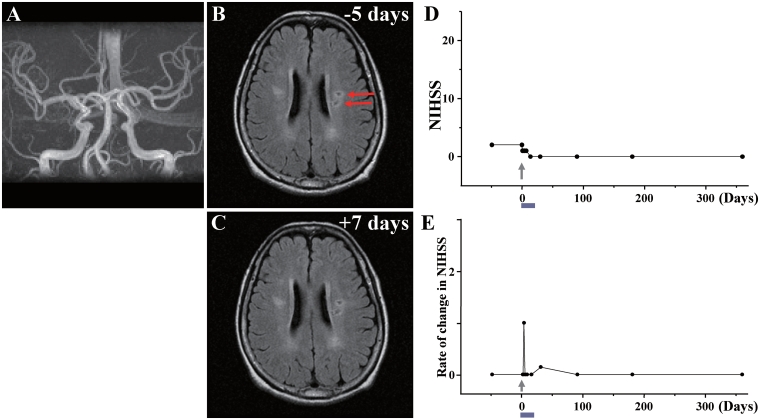
Case 9
This 63-year-old female, with a history of diabetes mellitus/Kienbock’s disease, sustained a lacunar stroke. MRA showed that major cerebral arteries were intact (Fig. 9A). MRI demonstrated a lacunar lesion in the left corona radiata (Fig. 9B). The patient displayed moderate right hemiparesis. NIHSS/Rankin scores were 2(3) on Day −49 and 2(3) on Day 0, immediately prior to autologous human MSC infusion. Eighty-eight days after the stroke, the patient received an intravenous infusion of 0.6 ×
× 108 autologous human MSCs. NIHSS/Rankin scores were 1(3) on Days 1, 2 and 4, 1(2) on Day 7 following autologous human MSC infusion, 0(2) on Day 14, and 0(1) thereafter (Fig. 9D and E). There were no major cell injection-related adverse events except slight appetite loss. MRI lesion volume was 2.68
108 autologous human MSCs. NIHSS/Rankin scores were 1(3) on Days 1, 2 and 4, 1(2) on Day 7 following autologous human MSC infusion, 0(2) on Day 14, and 0(1) thereafter (Fig. 9D and E). There were no major cell injection-related adverse events except slight appetite loss. MRI lesion volume was 2.68 cm3 on Day −5 prior to cell infusion, and 1.85
cm3 on Day −5 prior to cell infusion, and 1.85 cm3 on Day 7 post-infusion, without new lesions (Fig. 9C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 9C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
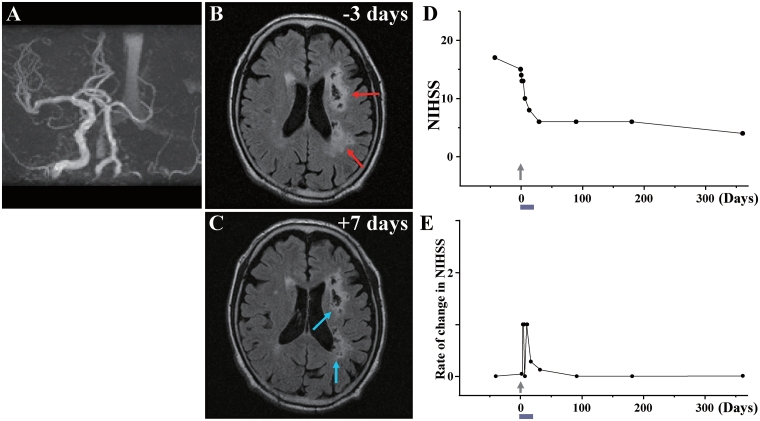
Case 10
This 60-year-old male, with a history of hypertension/diabetes mellitus/hyperlipidaemia/asymptomatic cerebral infarction/inguinal hernia, sustained an atherosclerotic stroke due to left internal carotid artery occlusion (Fig. 10A). MRI demonstrated infarction of left frontal and parietal grey matter and white matter (Fig. 10B). The patient displayed right hemiparesis and aphasia. NIHSS/Rankin scores were 17(5) on Day −42 and 15(5) on Day 0, immediately prior to cell injection. The patient received an intravenous infusion of 1.2 ×
× 108 autologous human MSCs 74 days after the stroke. NIHSS/Rankin scores were 14(5) on Day 1 after autologous human MSC infusion and 13(5) on Days 2 and 4, and 10(4) on Day 7. NIHSS/Rankin scores were 8(3) on Day 14, 6(3) on Days 30, 90 and 180 and 4(3) at 1 year (Fig. 10D and E). There were no major cell injection-related adverse events. MRI lesion volume was 43.03
108 autologous human MSCs 74 days after the stroke. NIHSS/Rankin scores were 14(5) on Day 1 after autologous human MSC infusion and 13(5) on Days 2 and 4, and 10(4) on Day 7. NIHSS/Rankin scores were 8(3) on Day 14, 6(3) on Days 30, 90 and 180 and 4(3) at 1 year (Fig. 10D and E). There were no major cell injection-related adverse events. MRI lesion volume was 43.03 cm3 on Day −3 prior to cell infusion, and 28.15
cm3 on Day −3 prior to cell infusion, and 28.15 cm3 on Day 7 post-infusion, without new lesions (Fig. 10C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 10C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
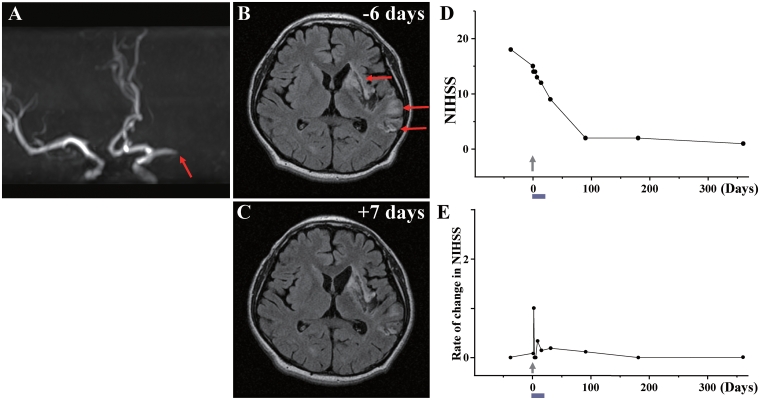
Case 11
A 41-year-old male, with a history of urinary calculus, sustained an embolic stroke due to left middle cerebral artery occlusion (Fig. 11A). MRI demonstrated infarction of grey and white matter in the left frontal, temporal and parietal lobes (Fig. 11B). The patient displayed right hemiparesis and aphasia. NIHSS/Rankin scores were 18(5) on Day −38 and 15(3) on Day 0, immediately prior to cell infusion. Sixty-six days after the stroke, the patient received an intravenous infusion of 1.5 ×
× 108 autologous human MSCs. NIHSS/Rankin scores were 14(3) on Days 1, 2 and 4 after autologous human MSC infusion, 13(3) on Days 7 and 12(3) on Day 14. NIHSS/Rankin scores were 9(3) on Day 30 and 2(2) on Day 90 and 1(1) at 1 year (Fig. 11D and E). There was local pain at the site of bone marrow aspiration just after bone marrow collection, but no other major cell injection-related adverse events. Lesion volume on MRI was 55.50
108 autologous human MSCs. NIHSS/Rankin scores were 14(3) on Days 1, 2 and 4 after autologous human MSC infusion, 13(3) on Days 7 and 12(3) on Day 14. NIHSS/Rankin scores were 9(3) on Day 30 and 2(2) on Day 90 and 1(1) at 1 year (Fig. 11D and E). There was local pain at the site of bone marrow aspiration just after bone marrow collection, but no other major cell injection-related adverse events. Lesion volume on MRI was 55.50 cm3 on Day −6 prior to cell infusion, and was 52.22
cm3 on Day −6 prior to cell infusion, and was 52.22 cm3 on Day 7 post-infusion, without new lesions (Fig. 11C). Diffusion tensor axonography showed recovery of anisotropy in the left pyramidal tract 6 months after cell injection (Fig. 13G and H). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 11C). Diffusion tensor axonography showed recovery of anisotropy in the left pyramidal tract 6 months after cell injection (Fig. 13G and H). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
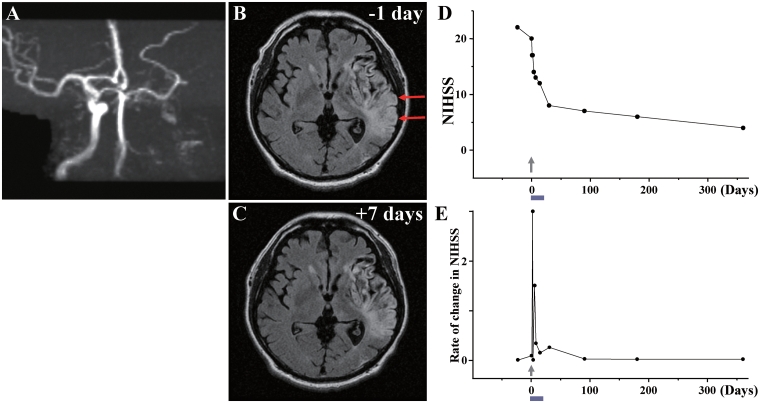
Case 12
A 61-year-old male, with a history of gall-stone/hyperlipidaemia/prostate hypertrophy, sustained an atherosclerotic stroke due to left internal carotid artery occlusion (Fig. 12A). MRI demonstrated infarction of grey and white matter in the left frontal, temporal and parietal lobes (Fig. 12B). The patient displayed right hemiparesis and aphasia. NIHSS/Rankin scores were 22(5) on Day −24 and 20(5) on Day 0, immediately prior to autologous human MSC infusion. The patient received an intravenous infusion of 1.2 ×
× 108 autologous human MSCs 56 days after the stroke. NIHSS/Rankin scores were 17(4) at Days 1 and 2 after autologous human MSC infusion, 14(4) on Day 4, 13(4) on Day 7 and 12(4) on Day 14. NIHSS/Rankin scores were 8(3) at 30 days and 4(3) at 1 year (Fig. 12D and E). There were no major cell injection-related adverse events. MRI lesion volume was 181.92
108 autologous human MSCs 56 days after the stroke. NIHSS/Rankin scores were 17(4) at Days 1 and 2 after autologous human MSC infusion, 14(4) on Day 4, 13(4) on Day 7 and 12(4) on Day 14. NIHSS/Rankin scores were 8(3) at 30 days and 4(3) at 1 year (Fig. 12D and E). There were no major cell injection-related adverse events. MRI lesion volume was 181.92 cm3 on Day −1 prior to cell infusion and 168.46
cm3 on Day −1 prior to cell infusion and 168.46 cm3 on Day 7 post-infusion, without new lesions (Fig. 12C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
cm3 on Day 7 post-infusion, without new lesions (Fig. 12C). MRIs following cell injection showed no tumour or abnormal cell growth over 1 year.
Selective studies
Case 3 presented with predominantly white matter lesion (internal capsule) with disruption in the adjacent basal ganglia (Fig. 13A). A series of fluid attenuated inversion recovery images are presented in Fig. 13 at 4 h before cell infusion (Fig. 13A) and at 4
h before cell infusion (Fig. 13A) and at 4 h (Fig. 13B), 1 (Fig. 13C) and 2 weeks (Fig. 13D) post-autologous human MSC infusion. The high intensity area (21.67
h (Fig. 13B), 1 (Fig. 13C) and 2 weeks (Fig. 13D) post-autologous human MSC infusion. The high intensity area (21.67 cm3 on Day 0 immediately prior to cell infusion) was reduced at the 1 week (14.61
cm3 on Day 0 immediately prior to cell infusion) was reduced at the 1 week (14.61 cm3) and 2 week (11.13
cm3) and 2 week (11.13 cm3) post-infusion time points. Perfusion imaging was carried out in Case 3 (Fig. 13E). Cerebral blood flow in the regions of interest (red ovals) was increased at 1 week post-cell infusion (Fig. 13F).
cm3) post-infusion time points. Perfusion imaging was carried out in Case 3 (Fig. 13E). Cerebral blood flow in the regions of interest (red ovals) was increased at 1 week post-cell infusion (Fig. 13F).
3D diffusion tensor axonography was carried out on Case 11 and is shown before and 6 months after autologous human MSC infusion, in Fig. 13G and H, respectively. The black area (Fig. 13G; arrow) indicates loss of anisotropy (limited movement of water) in internal capsule axons suggesting axonal swelling (but not disruption) prior to autologous human MSC infusion. At 6 months after cell infusion, there was return of anisotropy (Fig. 13H; arrow).
Summary of NIHSS scores and MRI observations for all cases
Figure 14 shows NIHSS scores plotted for 1 year for the 12 patients in this study. Open circles represent the onset of stroke for each of the patients. Testing did not begin until the patients were medically stabilized. The solid colour-coded symbols represent serial scoring on the NIHSS at 1, 2, 4, 7 days and at 2 weeks, 1, 3, 6 and 12 months. From the first day of testing to immediately before autologous human MSC infusion, the patients displayed a median daily rate of change in NIHSS score (dividing by the number of days between assessments to give an estimate of daily rate of change prior to cell infusion) of 0.04 (interquartile range 0.016–0.08). The median daily rate of change in NIHSS scores during the first week, following autologous human MSC infusion, was 0.36 (interquartile range 0.14–0.64). Subsequent median rates of change were: 2–1 week post-infusion: 0.142; 4–2 weeks: 0.01; 3–1 month: 0.016; 12–3 months: 0.002. As indicated in the case descriptions, the interval between initial NIHSS scoring and autologous human MSC infusion varied between patients, precluding a strict comparison of the daily rate of change in NIHSS scores over the entire pre-infusion period with the rate of change over an identical period precluding infusion. However, if those patients with intervals from initial pre-infusion NIHSS scoring until infusion of 24–38 days (Patients 3, 4, 5, 7, 8, 11 and 12) are considered as a group, their median daily rate of change in NIHSS score during the 24–38 day pre-infusion period was 0.079 (mean 0.068), compared with a median daily rate of change of 0.133 (mean 0.167) for the same patients over the first 30 days post-infusion. For the four patients with intervals of 42–54 days between initial pre-infusion NIHSS scoring and autologous human MSC infusion (Patients 1, 6, 9 and 10), the median daily rate of change in NIHSS score prior to cell infusion was 0 (mean 0.012), compared with 0.15 (mean 0.167) for the first 30 days following cell infusion. For the patient with a 92
day pre-infusion period was 0.079 (mean 0.068), compared with a median daily rate of change of 0.133 (mean 0.167) for the same patients over the first 30 days post-infusion. For the four patients with intervals of 42–54 days between initial pre-infusion NIHSS scoring and autologous human MSC infusion (Patients 1, 6, 9 and 10), the median daily rate of change in NIHSS score prior to cell infusion was 0 (mean 0.012), compared with 0.15 (mean 0.167) for the first 30 days following cell infusion. For the patient with a 92 day interval between initial pre-infusion NIHSS scoring and autologous human MSC infusion, the daily rate of change in NIHSS score was 0.033 for the 92
day interval between initial pre-infusion NIHSS scoring and autologous human MSC infusion, the daily rate of change in NIHSS score was 0.033 for the 92 day period prior to infusion and was 0.056 for the 90 days following cell infusion. Figure 15 shows the median, interquartile range and range of the NIHSS change per day during the period prior to cell infusion, over the week following cell infusion, and for the 2/1 week, 4/2 week, 3/1 month and 12/3 month time periods.
day period prior to infusion and was 0.056 for the 90 days following cell infusion. Figure 15 shows the median, interquartile range and range of the NIHSS change per day during the period prior to cell infusion, over the week following cell infusion, and for the 2/1 week, 4/2 week, 3/1 month and 12/3 month time periods.
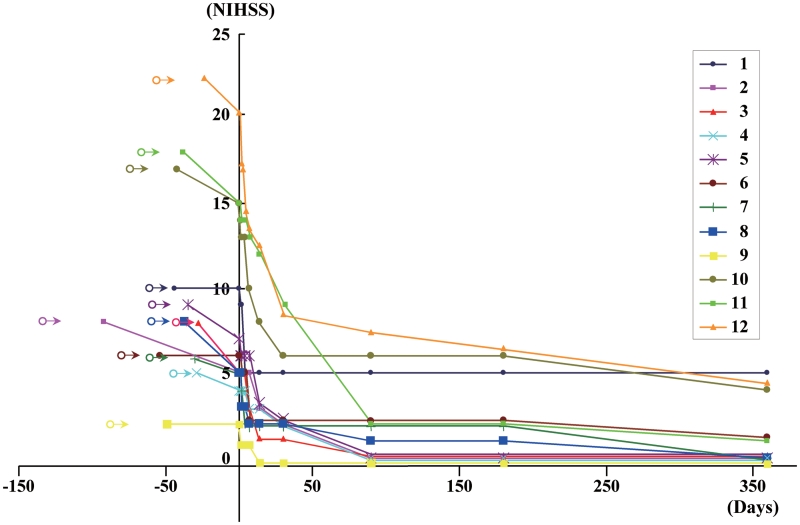
NIHSS scores at the time of autologous human MSC infusion and for 1 year following autologous human MSC infusion for the 12 patients.
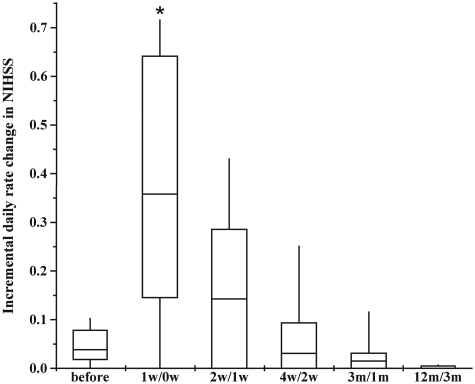
Median incremental daily rate of change in NIHSS (horizontal bars within boxes), interquartile ranges (boxes) and ranges (vertical lines) at several times before and after cell injection. *P <
< 0.001 compared with pre-infusion (before) rate.
0.001 compared with pre-infusion (before) rate.
Lesion volumes are shown for each case in Fig 16A and B, with mean lesion volume reduction of >20% at 1 week post-infusion (Fig. 16C). As shown in Fig. 16D, the mean reduction in lesion volume tended to correlate with the mean change in NIHSS score at 1 and 2 days, 1 and 2 weeks post-infusion (r =
= 0.98; P
0.98; P =
= 0.0025).
0.0025).
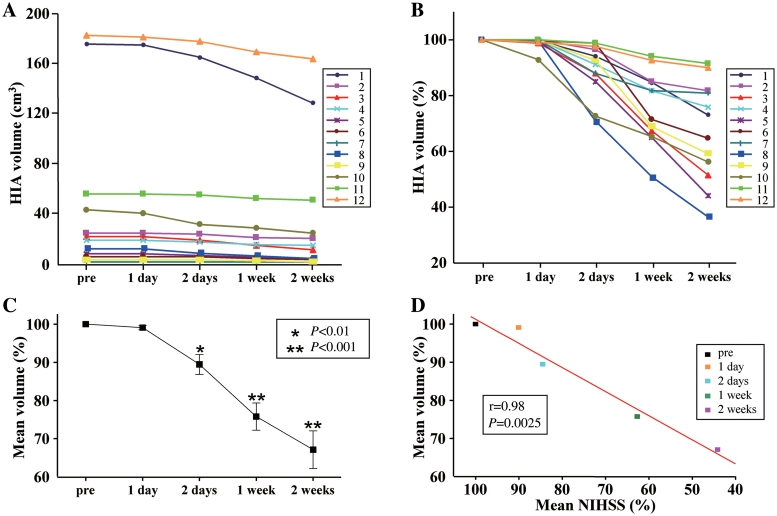
(A) Summary of high intensity magnetic resonance (fluid attenuation inversion recovery) lesion volumes (HIA) for all cases, pre-infusion and at 1 day, 2 days, 1 week and 2 weeks post-infusion. (B) Data re-plotted to show % change in lesion volume. (C) Mean % change in lesion volume compared with pre-infusion for 1 and 2 days, 1 and 2 weeks post-infusion. *P
day, 2 days, 1 week and 2 weeks post-infusion. (B) Data re-plotted to show % change in lesion volume. (C) Mean % change in lesion volume compared with pre-infusion for 1 and 2 days, 1 and 2 weeks post-infusion. *P <
< 0.01; **P
0.01; **P <
< 0.001 compared with pre-infusion value. (D) Mean % change in lesion volume plotted against mean change in NIHSS, compared with pre-infusion values. The results are correlated with r
0.001 compared with pre-infusion value. (D) Mean % change in lesion volume plotted against mean change in NIHSS, compared with pre-infusion values. The results are correlated with r =
= 0.98; P
0.98; P =
= 0.0025.
0.0025.
Discussion
In the present study, we found that intravenous administration of a well-defined population of autologous bone marrow-derived human MSCs, expanded in autologous human serum, into 12 subjects with stroke did not result in any significant adverse events. We observed slight itching at the injection site in one patient, a mild fever and nausea that recovered within 6 h in one patient, and slight appetite loss in one patient. One patient complained of local pain at the site of bone marrow aspiration just after marrow collection. We did not observe any CNS tumours, abnormal cell growths or neurological deterioration, and there was no evidence for venous thromboembolism, systemic malignancy or systemic infection in any of the patients following autologous human MSC infusion.
h in one patient, and slight appetite loss in one patient. One patient complained of local pain at the site of bone marrow aspiration just after marrow collection. We did not observe any CNS tumours, abnormal cell growths or neurological deterioration, and there was no evidence for venous thromboembolism, systemic malignancy or systemic infection in any of the patients following autologous human MSC infusion.
We would stress a number of caveats about our observations and their interpretation. In this phase I study, there was no control group or blinding, and we would emphasize that we cannot exclude placebo effects or observer bias. There are limitations on extracting meaningful conclusions from the rating scales that we used. These scales do not address the different functional consequences of different types of deficits, e.g. a motor deficit of the arm versus a facial palsy, and the degree to which changes in NIHSS score of the magnitude that we observed translate into clinically meaningful functional improvement in any given patient remains to be studied. Moreover, we used broad inclusion criteria, and studied patients at a range of ages (41–73 years old), with strokes in both cortical and subcortical locations, with a spectrum of aetiologies, and with different pre-infusion observation times in different patients. Some degree of clinical recovery occurred in most of our patients pre-autologous human MSC infusion, and we cannot exclude a contribution of spontaneous recovery to the changes we observed post-autologous human MSC infusion.
We observed a daily rate of change of the median NIHSS score of 0.36 in the week following infusion, compared with a median daily rate of change of 0.04 in the variable period before infusion, and there was a reduction in NIHSS score of four or more points in 4 of the 12 patients studied, and a reduction of five or more points in 3 of the 12 patients studied, within the first 7 days after autologous human MSC infusion. The improvement in NIHSS score was maintained for 1 year in all patients. In seven patients, there was a reduction of at least 15% in lesion volume at 7 days post-human MSC infusion, compared with pre-infusion. As shown in Fig. 16D, there tended to be a correlation between the change in lesion volume and the change in NIHSS scores. Gaudinski et al. (2008), in a study on stroke lesion volume measured by MRI, concluded that lesions continue to evolve between 5 and 90 days post-infarction, but approach final infarct volume by 30 days, and suggested that lesion volumes measured at 30 days post-infarction may provide a sufficient approximation of stroke infarct volumes for use in clinical trials. We cannot exclude a contribution of spontaneous recovery to post-infusion changes in these patients and would emphasize that these unblinded observations do not prove a therapeutic effect with the current safety study design. Nonetheless, the changes in NIHSS score and lesion volume within the first weeks after cell infusion, which tended to be correlated, in patients who received autologous human MSC infusions 36–33 days after stroke, suggest that the short- and long-term effects of intravenous delivery of autologous human MSC expanded in human serum, and their implications in terms of clinically meaningful functional improvement, after stroke, warrant further investigation.
An important aspect of the present study is that the cells were delivered several weeks or months after stroke onset. Most interventional approaches for stroke are limited to the acute phase after stroke onset. For example, the therapeutic time window of fibrinolytic agents, i.e. tissue-type plasminogen activator, is within hours after stroke. Although dysfunction of the blood–brain barrier attenuates over 1 month after ischaemic onset, animal studies indicate that accumulation of injected cells into ischaemic lesions does not, and MSCs continue to migrate selectively into damaged brain lesions (Komatsu et al., 2010). The observations described here suggest that, in future studies, therapeutic time windows from the acute to the subacute and possibly the chronic phase following stroke should be explored.
The autologous human MSCs introduced into stroke patients in the present study are different from those reported in a previous clinical study (Bang et al., 2005). The phenotypes of stem cells such as human MSCs are significantly affected by culture conditions (Kobayashi et al., 2005; Shahdadfar et al., 2005; Gordon and Scolding, 2009). The protocols for cell culture and choice of culture medium and serum were different in the Bang et al. (2005) study and the present study, making a one-to-one comparison in results difficult. In contrast to the Bang et al. (2005) study that used foetal calf serum, cells were cultured with autologous human serum in the present study. This allowed more rapid expansion of human MSCs and resulted in stable gene expression of less highly differentiated and transcriptionally stable cells (Kobayashi et al., 2005; Shahdadfar et al., 2005). Culturing of human MSCs in human serum also results in a different gene expression pattern compared with those in foetal calf serum (Honmou et al., unpublished data). For example, angiopoietin-like 4, which inhibits apoptosis is upregulated in human MSCs expanded in human serum, suggesting the increased cumulative cell numbers in human MSC cultures. Ectonucleotide pyrophosphatase/phosphodiesterase 1, which acts as an antagonist of bone mineralization, is also increased in human MSC with human serum. In contrast, growth arrest-specific 1 and anti-proliferative protein 1 are overexpressed in human MSCs cultured with foetal calf serum, consistent with their slower rate of proliferation. In addition, foetal calf serum appears to induce differentiation into mesenchymal lineages, with high levels of expression of genes associated with differentiation into osteoblasts [cytokine receptor-like factor 1, glycoprotein (transmembrane) nmb], adipocytes (leptin receptor, inhibitor of DNA binding 4, members of the complement system) and chondrocytes (extracellular matrix genes, cytokine receptor-like factor 1, leptin receptor, ectonucleotide pyrophosphatase/phosphodiesterase 2, transforming growth factor-β, SMAD6, OLF-1/early B-cell factor-associated zinc finger gene), which is consistent with the observations in a previous study (Shahdadfar et al., 2005). Another important difference between the two studies is that our study included subjects with both grey and white matter lesions, whereas Bang et al. (2005) focused mainly on grey matter lesions.
If a functionally significant reduction of neurological deficit and lesion volumes following autologous human MSC infusion is confirmed in future studies, there will be a need to explore the underlying mechanisms. Several mechanisms have been suggested to contribute to reduction in lesion size and improved functional outcome following early intravenous administration of MSCs in the rat middle cerebral artery occlusion model (Nomura et al., 2005; Horita et al., 2006; Liu et al., 2006), including neuroprotection (Chen et al., 2001; Bang et al., 2005; Honma et al., 2006; Liu et al., 2006), angiogenesis (Onda et al., 2007; Ukai et al., 2007; Omori et al., 2008; Toyama et al., 2009), replacement of damaged cells (Chopp and Li, 2002) and/or anti-inflammatory mechanisms (Ohtaki et al., 2008). Whether a subset of neural tissue in long-term stroke lesions can be rescued, or entry of cells from the periphery of the lesion can promote axonal regeneration and reorganization within the lesion, is not yet clear. Evidence from animal models suggests that an increase in angiogenesis may contribute to the effects of MSCs, even if MSCs were injected in the chronic phase of stroke (Komatsu et al., 2010). Increased angiogenesis was observed within 3 days after cell injection in a rat stroke model, and recovery of cerebral blood flow became evident within 7 days after cell injection (Ukai et al., 2007). Human MSCs secrete neurotrophins such as brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor, which can contribute to anatomical and functional recovery of the ischaemic brain (Nomura, et al., 2005; Horita, et al., 2006; Liu, et al., 2006). Brain-derived neurotrophic factor can affect neuronal excitability and synaptic transmission (Levine et al., 1998; Rogalski et al., 2000; Mizoguchi et al., 2002; Tucker and Fadool, 2002), and upregulates a potassium chloride co-transporter KCC2 that maintains activation of inhibitory receptors, thereby reducing spasticity (Boulenguez et al., 2010). It is possible that reduced lesion size may be a result of anti-oedematous effect of neurotrophins produced by MSCs (Nomura et al., 2005; Horita et al., 2006), including brain-derived neurotrophic factor that can attenuate microvascular permeability disturbances (Sharma, 2003), blood cerebrospinal fluid barrier breakdown, blood–brain barrier breakdown and brain oedema (Sharma and Johanson, 2007) and glial cell line-derived neurotrophic factor, which can ameliorate brain oedema (Abe et al., 1997).
Future directions
Systemically delivered cell-based approaches are being examined in clinical studies for a number of neurological diseases including Krabbe’s disease (Escolar et al., 2005), Hurler’s syndrome (Koc et al., 2002; Staba et al., 2004), metachromatic leucodystrophy (Koc et al., 2002), multiple sclerosis (Freedman et al., 2010) and stroke (Bang et al., 2005). Intravenous administration of human MSCs may offer advantages in clinical settings, because intra-arterial injection may cause embolism and requires specialized interventional facilities, while local, direct injection of human MSCs into brain may require stereotactic targeting and may cause haemorrhage. Previous studies have suggested that intravenous injection of human MSC may be relatively safe in a number of clinical settings (Koc et al., 2000, 2002; Bang et al., 2005; Lazarus et al., 2005) but have not assessed autoserum expanded cells. The present study provides evidence supporting the safety and feasibility of expansion of autologous human MSCs in autologous human serum and cryopreservation before injection into patients. Cryopreservation provides time for pathogen screening and cell-characterization analysis, and may facilitate transport of the human MSCs from the cell processing centre to the site of patient treatment. Autologous human MSCs, if shown in future studies to be efficacious, may also present advantages compared with allogenic human MSC, which express HLA Classes I and II (Honmou et al., unpublished data) (Shahdadfar et al., 2005) that can trigger immunorejection, and introduce a requirement of immunosuppression. The latter consideration is especially important because a single preparation of 108 human MSCs grown under standard conditions in foetal calf serum would carry with it ~7–30 mg of foetal calf serum protein, which could elicit humeral immune responses against foetal calf serum proteins in the recipient (Spees et al., 2004).
mg of foetal calf serum protein, which could elicit humeral immune responses against foetal calf serum proteins in the recipient (Spees et al., 2004).
Blinded, controlled studies that include assessments of overall function, and of the relative functional importance of different types of deficits, will be needed to determine whether intravenous administration of autologous human MSCs is efficacious in stroke, and the optimal therapeutic protocol in terms of cell preparation, number of cells and times of delivery remain uncertain. The present results provide evidence supporting the safety of delivery of a relatively large dose of autologous human MSCs, cultured in autologous human serum, into human subjects with stroke. Although we would emphasize that the current study was unblinded and does not exclude placebo effects or recovery as a result of the natural history of stroke, our observations demonstrate the feasibility of studies on administration of human serum-treated autologous human MSCs, suggest that these studies can be carried out safely and underscore the need to assess functional outcome at early, as well as late, times after cell administration.
Funding
The Japanese Ministry of Education, Culture, Sports, Science and Technology (Coordination, Support and Training Program for Translational Research, 20390388); the National Multiple Sclerosis Society (USA) (RG2135;; CA1009A10); the National Institutes of Health (NS43432); the Medical and Rehabilitation Research and Development Research Services of the Department of Veterans Affairs; the Bumpus Foundation.
Glossary
Abbreviations
| MRA | magnetic resonance angiography |
| MSC | mesenchymal stem cell |
| NIHSS | National Institutes of Health Stroke Scale |
References
- Abe K, Hayashi T, Itoyama Y. Amelioration of brain edema by topical application of glial cell line-derived neurotrophic factor in reperfused rat brain. Neurosci Lett. 1997;231:37–40. [Abstract] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. [Abstract] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–7. [Abstract] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. [Abstract] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–9. [Abstract] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. [Abstract] [Google Scholar]
- Drach G, Maret A, Richard MF, Barbu E. [Transfer and induction of delayed hypersensitivity to methylated bovine serum albumin in the absence of adjuvant] C R Acad Sci Hebd Seances Acad Sci D. 1977;284:2435–7. [Abstract] [Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005;352:2069–81. [Abstract] [Google Scholar]
- Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H, et al. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. 2010;16:503–10. [Abstract] [Google Scholar]
- Gaudinski MR, Henning EC, Miracle A, Luby M, Warach S, Latour L. Establishing final infarct volume: stroke lesion evolution past 30 days is significant. Stroke. 2008;39:2765–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Gordon D, Scolding NJ. Human mesenchymal stem cell culture for neural transplantation. Methods Mol Biol. 2009;549:103–18. [Abstract] [Google Scholar]
- Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, et al. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. [Europe PMC free article] [Abstract] [Google Scholar]
- Honmou O, Houkin K, Matsunaka T, Niitsu Y, Ishiai S, Waxman SG, et al. Intravenous transplantation of autologous mesenchymal stem cells derived from bone marrow into stroke patients. Stroke. 2008;39:543. [Google Scholar]
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495–504. [Europe PMC free article] [Abstract] [Google Scholar]
- Kim S, Honmou O, Kato K, Nonaka T, Houkin K, Hamada H, et al. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res. 2006;1123:27–33. [Europe PMC free article] [Abstract] [Google Scholar]
- Kobayashi T, Watanabe H, Yanagawa T, Tsutsumi S, Kayakabe M, Shinozaki T, et al. Motility and growth of human bone-marrow mesenchymal stem cells during ex vivo expansion in autologous serum. J Bone Joint Surg Br. 2005;87:1426–33. [Abstract] [Google Scholar]
- Kobune M, Kawano Y, Ito Y, Chiba H, Nakamura K, Tsuda H, et al. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp Hematol. 2003;31:715–22. [Abstract] [Google Scholar]
- Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–22. [Abstract] [Google Scholar]
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–16. [Abstract] [Google Scholar]
- Komatsu K, Honmou O, Suzuki J, Houkin K, Hamada H, Kocsis JD. Therapeutic time window of mesenchymal stem cells derived from bone marrow after cerebral ischemia. Brain Res. 2010;1334:84–92. [Abstract] [Google Scholar]
- Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–98. [Abstract] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci USA. 1998;95:10235–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–23. [Abstract] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, et al. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–45. [Europe PMC free article] [Abstract] [Google Scholar]
- Mizoguchi Y, Monji A, Nabekura J. Brain-derived neurotrophic factor induces long-lasting Ca2+-activated K+ currents in rat visual cortex neurons. Eur J Neurosci. 2002;16:1417–24. [Abstract] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, et al. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000;31:1965–72. [Abstract] [Google Scholar]
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136:161–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Ohta T, Kikuchi H, Hashi K, Kudo Y. Nizofenone administration in the acute stage following subarachnoid hemorrhage. Results of a multi-center controlled double-blind clinical study. J Neurosurg. 1986;64:420–6. [Abstract] [Google Scholar]
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638–43. [Europe PMC free article] [Abstract] [Google Scholar]
- Omori Y, Honmou O, Harada K, Suzuki J, Houkin K, Kocsis JD. Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats. Brain Res. 2008;1236:30–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:329–40. [Europe PMC free article] [Abstract] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. [Abstract] [Google Scholar]
- Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241–3. [Abstract] [Google Scholar]
- Rogalski SL, Appleyard SM, Pattillo A, Terman GW, Chavkin C. TrkB activation by brain-derived neurotrophic factor inhibits the G protein-gated inward rectifier Kir3 by tyrosine phosphorylation of the channel. J Biol Chem. 2000;275:25082–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Scolding N, Marks D, Rice C. Autologous mesenchymal bone marrow stem cells: practical considerations. J Neurol Sci. 2008;265:111–5. [Abstract] [Google Scholar]
- Shahdadfar A, Fronsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–66. [Abstract] [Google Scholar]
- Sharma HS. Neurotrophic factors attenuate microvascular permeability disturbances and axonal injury following trauma to the rat spinal cord. Acta Neurochir Suppl. 2003;86:383–8. [Abstract] [Google Scholar]
- Sharma HS, Johanson CE. Intracerebroventricularly administered neurotrophins attenuate blood cerebrospinal fluid barrier breakdown and brain pathology following whole-body hyperthermia: an experimental study in the rat using biochemical and morphological approaches. Ann NY Acad Sci. 2007;1122:112–29. [Abstract] [Google Scholar]
- Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–56. [Abstract] [Google Scholar]
- Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med. 2004;350:1960–9. [Abstract] [Google Scholar]
- Toyama K, Honmou O, Harada K, Suzuki J, Houkin K, Hamada H, et al. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol. 2009;216:47–55. [Abstract] [Google Scholar]
- Tucker K, Fadool DA. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. J Physiol. 2002;542:413–29. [Abstract] [Google Scholar]
- Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007;24:508–20. [Europe PMC free article] [Abstract] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. [Abstract] [Google Scholar]
Articles from Brain are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/brain/awr063
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/brain/article-pdf/134/6/1790/1056062/awr063.pdf
Citations & impact
Impact metrics
Article citations
Efficacy and safety of mesenchymal stem cell therapies for ischemic stroke: a systematic review and meta-analysis.
Stem Cells Transl Med, 13(9):886-897, 01 Sep 2024
Cited by: 0 articles | PMID: 39159204 | PMCID: PMC11386217
Review Free full text in Europe PMC
The role of mesenchymal stem cells in attenuating inflammatory bowel disease through ubiquitination.
Front Immunol, 15:1423069, 09 Aug 2024
Cited by: 1 article | PMID: 39185411 | PMCID: PMC11341407
Review Free full text in Europe PMC
Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities.
Stem Cell Res Ther, 15(1):266, 26 Aug 2024
Cited by: 2 articles | PMID: 39183341 | PMCID: PMC11346273
Review Free full text in Europe PMC
Activation of cellular antioxidative stress and migration activities by purified components from immortalized stem cells from human exfoliated deciduous teeth.
Sci Rep, 14(1):15340, 03 Jul 2024
Cited by: 0 articles | PMID: 38961142 | PMCID: PMC11222459
Priming and Combined Strategies for the Application of Mesenchymal Stem Cells in Ischemic Stroke: A Promising Approach.
Mol Neurobiol, 61(9):7127-7150, 17 Feb 2024
Cited by: 1 article | PMID: 38366307
Review
Go to all (265) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series.
Clin Neurol Neurosurg, 203:106565, 18 Feb 2021
Cited by: 35 articles | PMID: 33667953
Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: a pilot study.
Indian J Med Res, 136(2):221-228, 01 Aug 2012
Cited by: 52 articles | PMID: 22960888 | PMCID: PMC3461733
The effects of intravenous infusion of autologous mesenchymal stromal cells in patients with subacute middle cerebral artery infarct: a phase 2 randomized controlled trial on safety, tolerability and efficacy.
Cytotherapy, 23(9):833-840, 12 May 2021
Cited by: 23 articles | PMID: 33992536
[Phase III clinical trial using autologous mesenchymal stem cells for stroke patients].
Nihon Rinsho, 74(4):649-654, 01 Apr 2016
Cited by: 5 articles | PMID: 27333754
Review
 1,2,3,4
1,2,3,4 




