Abstract
Free full text

Spotlight on childhood blindness
Abstract
Leber congenital amaurosis (LCA) is a rare disease that severely affects vision in early life. It is characterized by genetic and clinical heterogeneity due to complex and not fully understood pathogenetic mechanisms. It is also now widely known as a disease model for gene therapy. In this issue of the JCI, two independent research groups report valuable new data on LCA. Specifically, they provide important insights into the pathophysiological mechanisms of LCA and offer strong hope that the outcome of gene therapy for retinal degenerative diseases will be successful.
Highlights of LCA
Leber congenital amaurosis (LCA) is a rare retinal dystrophy with a prevalence at birth of approximately 1 in 80,000 (1). It is most often inherited in an autosomal-recessive manner. It typically becomes evident in the first year of life, with poor visual function often accompanied by nystagmus, sluggish or near-absent pupillary responses, photophobia, high hyperopia, and keratoconus. Profound visual impairment is usually present from birth.
LCA is caused by mutations in any one of at least 15 genes (2), and this accounts in part for its heterogeneous clinical presentation. To date, more than 400 mutations have been identified. Together, they account for approximately 70% of all LCA cases. The most frequently mutated genes are centrosomal protein 290 kDa (CEP290; also known as NPHP6), guanylate cyclase 2D (GUCY2D), and crumbs homolog 1 (CRB1) (2). Mutations in the retinal pigment epithelium–specific protein 65 kDa (RPE65) gene account for 5%–10% of all LCA cases (2, 3). RPE65 is highly expressed in the retinal pigment epithelium (RPE), where it encodes a retinoid isomerase enzyme essential for the production of chromophore, which forms the visual pigment in the rod and cone photoreceptors of the retina. Interestingly, the LCA-associated genes encode proteins with a wide variety of retinal functions, such as photoreceptor morphogenesis, phototransduction, vitamin A cycling, guanine synthesis, and outer segment (OS) phagocytosis (Figure (Figure1).1). Recently, several mutations were identified that are likely to affect intraphotoreceptor ciliary transport processes.
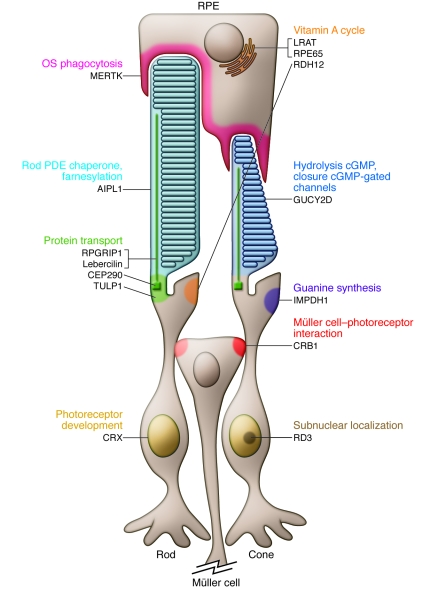
RPE65 and LRAT are located in the membranes of RPE cell endoplasmic reticulum. CEP290 is located in the basal bodies of rod and cone photoreceptors. Lebercilin, CEP290, and RPGRIP1 have prominent localization in the photoreceptor CC. GUCY2D localizes to the OS disc membranes of rod and cone photoreceptors. PDE, phosphodiesterase. Adapted with permission from Progress in Retinal and Eye Research (2).
Although nearly all identified LCA-associated genes are expressed preferentially or specifically in the retina, two genes recently identified as mutated in patients with LCA, CEP290 (4) and LCA5 (which encodes Lebercilin; ref. 5), are ubiquitously expressed. Furthermore, the identification of these genes as LCA associated highlights the important role of ciliary proteins in the pathogenesis of LCA, as has been observed for other retinal dystrophies (e.g., Bardet-Biedl and Usher syndromes). Mutation analysis in a French Canadian family identified an intronic mutation, c.2991+1655A>G (p.C998X), that results in the insertion of a cryptic exon in the CEP290 mRNA and introduces a premature stop codon in the protein. Strikingly, this mutation was found in 20% of LCA patients, rendering it one of the most important causes of LCA to date (4).
The diagnosis of LCA is based on clinical findings. Identification of the genetic cause by molecular genetic testing is important to both distinguish LCA from other retinal diseases and give a patient a more accurate visual prognosis based on genotype-phenotype correlations.
In this issue of the JCI, two independent research groups report significant new data on the molecular pathogenesis of LCA and on the potential of gene therapy in individuals with selected LCA-causing gene defects. Boldt et al. demonstrate a direct involvement of defective intraflagellar transport (IFT) in LCA and provide evidence that Lebercilin functions as an integral element of selective protein transport through photoreceptor cilia (6). Ashtari et al. demonstrate stable retinotopic functional improvement in humans after retinal gene therapy, as reflected by responses of the visual cortex (7).
Some mechanisms implicated in the pathogenesis of LCA
The mechanisms underlying LCA are multiple and not fully understood. Despite the striking genetic heterogeneity, the main clinical features of LCA can be categorized into a more limited number of phenotypes, a fact that points toward overlapping pathogenic disease mechanisms. Emerging data suggest that one of these possible mechanisms may affect transport across the photoreceptor connecting cilium (CC) — the sole link between the inner segment (IS) and OS of photoreceptors. As the photoreceptor IS (containing the metabolic machinery of the cell) and the OS (membranous disks containing phototransduction proteins) are linked solely by a CC, correct transport of proteins through the CC is crucial for the function and maintenance of the cell (Figure (Figure2).2). Boldt et al. analyze how mutations in the LCA5 gene affect the connectivity of the encoded protein, Lebercilin, and provide evidence that Lebercilin functions as an integral element of selective protein transport through the photoreceptor CC (6). This study represents the first molecular confirmation that disrupted IFT — an elaborate mechanism that mediates the polarized trafficking of proteins required for efficient phototransduction — is directly implicated in LCA. The primary cilium of photoreceptors mediates polarized trafficking of proteins for efficient phototransduction. During ciliogenesis, protein complexes are transported distally for growth of the axoneme using IFT (8). Since proteins and protein precursors are transported across the ciliary compartments via IFT, mutations in genes encoding proteins that participate in IFT can cause a spectrum of different ciliopathies.
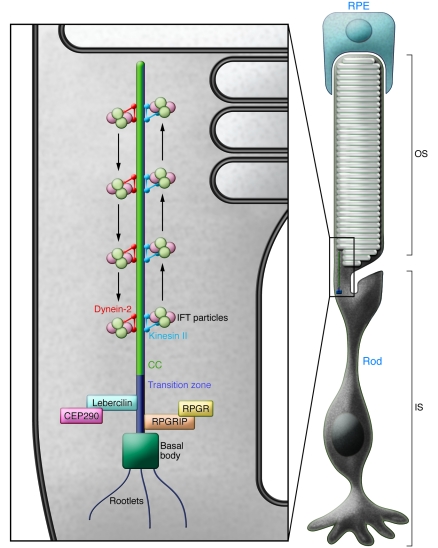
Cilia consist of a backbone (or axoneme), which contains microtubule doublets arranged in a circle. The cilium is anchored in the basal body, which regulates and organizes microtubule assembly. The photoreceptor CC is a specialized primary (nonmotile) cilium. It connects the photoreceptor IS, which contains the metabolic machinery of the cell, and the OS, which contains the photosensitive apparatus. As the OS is unable to synthesize proteins and lipids, the CC has an important role in transport from the IS to the OS. RPGR, retinitis pigmentosa GTPase regulator.
Connecting Lebercilin to LCA.
Lebercilin was first reported to localize to cilia of cultured cells and to the photoreceptor CC and was linked to ciliary and centrosomal function (9). It is noteworthy that dysfunction of primary cilia (an extension of the cell membrane formed by nucleation of microtubules) as a result of mutations in cilia-centrosomal proteins is associated with pleiotropic disorders. To understand the findings of Boldt et al. (6), it is important to remember that correct transport of proteins through the CC via the process of IFT is crucial for the function and maintenance of photoreceptors. Indeed, about 2,000 opsin molecules are transported per minute from the rod IS, where they are synthesized, via the CC, to the rod OS, where they maintain function and integrity. Therefore, any impairment of the IFT process leads to dysfunction of photoreceptors, ultimately leading to degeneration of the retina. For example, disruption of IFT in kinesin family member 3a (Kif3a) conditional knockout mice (10) results in opsin accumulation in ISs and, consequently, photoreceptor degeneration. In retinitis pigmentosa GTPase regulator interacting protein 1 (Rpgrip1) knockout mice, the degeneration of photoreceptors — and, subsequently, all retinal layers — is rapid (11). Interestingly, a common allele of RPGRIP1L is a modifier of retinal degeneration severity in ciliopathies (12).
Boldt et al. report their use of a powerful combination of a quantitative affinity proteomics approach using LCA5-encoded Lebercilin and two human LCA-causative mutations in LCA5, together with the development and detailed examination of a homozygous Lca5 gene trap mouse model (6). They convincingly demonstrated that Lebercilin physically interacts with the intracellular transport machinery. As LCA-associated LCA5 mutations abrogated this interaction, these results demonstrated a direct involvement of defective IFT in the LCA disease phenotype. Boldt et al. further demonstrated that physical association of Lebercilin with IFT proteins occurred in the retina. They assessed the effect of LCA-associated LCA5 mutations on the Lebercilin interactome and showed that mutations Lebercilin-p.P493TfsX1 and Lebercilin-p.Q279X caused the loss of all identified IFT and IFT-associated proteins from the protein complex. The observation that Lebercilin and IFT proteins partly colocalized in cilia of retinal cells indicates that Lebercilin and Lebercilin-associated proteins are likely to be essential for specialized transport by the IFT machinery. The authors also generated homozygous Lca5 gene trap mice, which exhibited phenotypes (restricted to the retina) characteristic of LCA in humans. The involvement of Lebercilin in the transport of phototransduction proteins along the CC was demonstrated by loss of transport of cone and rod opsins and reduced translocation of arrestin and transducin in response to light, thus pointing to a role for Lebercilin as a molecular scaffold, connecting the IFT core machinery to proteins involved in selecting and recruiting cargo.
Other CC-localized proteins connected to LCA.
CEP290, RPGRIP1, and Tubby-like protein 1 (TULP1) are other recently identified LCA-associated proteins that localize to CC of photoreceptor cells. TULP1 is a member of the Tubby-like family of proteins that play roles in neuronal development and function. An important role for TULP1 in retinal differentiation (in line with the early onset of the retinal degeneration in LCA) has also been suggested (13). Absence of Tulp1 in mice has been shown to induce mislocalization of rhodopsin to the plasma membrane of the ISs and budding into extracellular vesicles (14). CEP290 is a cilia-centrosomal protein that was initially identified as a tumor antigen. Mutations in CEP290, now known to be implicated in the pathogenesis of LCA, are also associated with syndromic ciliopathies (such as Joubert and Meckel-Gruber syndromes), as are mutations in the RPGRIP1-like (RPGRIP1L) gene. With the identification of mutations in LCA5, CEP290, RPGRIP1, and TULP1, the role of disrupted ciliary processes in the molecular pathogenesis of LCA is emphasized, pinpointing a growing group of LCA subtypes as ciliopathies.
Advances in therapy for LCA
Ocular gene therapy has been tried with success in mice, dogs, and, most recently, humans (15). It is most advanced for LCA caused by mutations in RPE65. Proof of principle for gene therapy came from studies in Briard dogs, a naturally occurring model of retinal degeneration resulting from mutations in RPE65 (16). Gene therapy using adeno-associated virus–mediated (AAV-mediated) delivery of RPE65 was shown to restore visual function in Briard dogs (16), with sustained success over many years in 95% of treated eyes (17). The results of three simultaneous phase I clinical treatment trials of AAV-mediated RPE65 gene therapy in humans (18, 19) demonstrated safety and showed slight improvement in vision in both bright and dim light. Clinical data on more than 30 patients are now available, showing stable clinical benefit in all patients, with no severe adverse effects (20, 21).
Ashtari et al. studied how the visual cortex responds to recovery of retinal function after prolonged sensory deprivation and provided the spectacular initial evidence that the visual cortex can be made responsive to visual input even after prolonged visual deprivation (up to 35 years; ref. 7). This study sheds light on the still-unanswered question concerning the Molyneux debate. More than 300 years ago, Molyneux formulated a problem as to “whether a man who has been born blind and who has learnt to distinguish and name a globe and a cube by touch, would be able to distinguish and name these objects simply by sight, once he had been enabled to see” (22). For modern neuroscientists, the answer to this question has an important bearing on contemporary issues concerning crossmodal identification and intermodal interactions — in other words, how the brain integrates information from the different senses. The results from the work of Ashtari et al. have a substantial effect on our understanding of the capacities of the visual cortex to be reactivated after visual deprivation. The study involved individuals with LCA2, a form of LCA caused by mutations in RPE65, who were injected unilaterally with AAV carrying normal RPE65 cDNA (AAV2-hRPE65v2) in their worst-seeing eye. Using psychophysical testing and functional MRI (fMRI) 1 year after the injection, Ashtari et al. provided an excellent demonstration that gene augmentation therapy in humans with LCA2 rendered both the retina and the visual cortex far more sensitive to dimmer light and lower-contrast stimuli (7). Cortical activation was found in regions corresponding to the area of the retina that had been exposed to AAV2-hRPE65v2, and the topography corresponded to visual field improvement. This is the first demonstration of stable, retinotopic improvement in humans for at least 2 years after retinal gene therapy, reflected by response of the visual cortex. The fMRI finding that the visual cortex can be reawakened after chronic visual deprivation in a rare eye disease may have much broader implications, providing promise for the outcome of gene therapy for both early- and late-onset retinal degenerative diseases.
Future directions.
Except for gene therapy for LCA2, no substantial treatment or cure for LCA exists; thus, care is currently supportive. One of the key issues in the near future will be the extension of results gained in individuals with RPE65 mutations to those with mutations in other genes. Emerging data suggest that CEP290 may account for a substantial percentage of LCA cases (5). Clinical and laboratory studies suggest that patients with CEP290-related LCA may be good candidates for gene therapy. Indeed, gene therapy of an RPE-expressed gene encoding a vitamin A cycle enzyme (RPE65), a photoreceptor gene encoding a phototransduction enzyme (GUCY2D; ref. 23), and a photoreceptor gene encoding a structural protein (RPGRIP1; ref. 11) have all been shown to be successful in different experimental models. These successes were reached in recessive disorders that were obviously more amenable to gene replacement strategies. However, large animal models are not available for GUCY2D and aryl hydrocarbon receptor interacting protein–like 1 (AIPL1) deficiency, and the canine model of RPGRIP1 deficiency has not yet been treated by gene therapy.
Translating genotypic and phenotypic insights into major therapeutic advances
Considering the clinical heterogeneity of LCA (24), a detailed genotype-phenotype correlation analysis is critical in order to understand the progression and pathogenesis of this devastating eye disease, as illustrated by the study by Boldt et al. on Lebercilin (6). Further elucidation of the molecular mechanisms of LCA in the retina and development of gene therapy approaches for different genetic subtypes of the disease are required. In particular, the status of remaining photoreceptors at the time of treatment will represent the most predictive parameter in terms of functional improvement. Moreover, the age of onset of profound visual impairment will determine the development of the visual cortex in the early years during the critical period. As LCA2 often spares significant visual function in the early years, sometimes into the second decade, this may represent a privileged situation for gene therapy: photoreceptors are affected secondary to RPE dysfunction, and cortical stimulation by formed images has occurred during the critical period, prior to the advent of legal blindness. The report by Ashtari et al. on cortical function restoration after therapy (7) brings great promise. Depending on the affected gene and severity of the phenotype, the extension of the landmark achievements in LCA2 to other types of LCA may prove more challenging depending upon the age of intervention.
Acknowledgments
The author thanks Katia Marazova (Institut de la Vision) for major help in preparing this commentary and Thierry Leveillard (Institut de la Vision) and Laurent Cohen (Institut du Cerveau et de la Moelle, Paris, France) for advice.
Footnotes
References
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci58300
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/58300/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Prime Editing for Human Gene Therapy: Where Are We Now?
Cells, 12(4):536, 07 Feb 2023
Cited by: 9 articles | PMID: 36831203 | PMCID: PMC9954691
Review Free full text in Europe PMC
Visual function restoration in a mouse model of Leber congenital amaurosis via therapeutic base editing.
Mol Ther Nucleic Acids, 31:16-27, 05 Dec 2022
Cited by: 18 articles | PMID: 36589710 | PMCID: PMC9792702
Application of prime editing to the correction of mutations and phenotypes in adult mice with liver and eye diseases.
Nat Biomed Eng, 6(2):181-194, 26 Aug 2021
Cited by: 78 articles | PMID: 34446856
Gene therapy for blindness.
Annu Rev Neurosci, 36:467-488, 31 May 2013
Cited by: 81 articles | PMID: 23724995
Review
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Disruption of intraflagellar protein transport in photoreceptor cilia causes Leber congenital amaurosis in humans and mice.
J Clin Invest, 121(6):2169-2180, 23 May 2011
Cited by: 71 articles | PMID: 21606596 | PMCID: PMC3104757
The human visual cortex responds to gene therapy-mediated recovery of retinal function.
J Clin Invest, 121(6):2160-2168, 23 May 2011
Cited by: 91 articles | PMID: 21606598 | PMCID: PMC3104779
Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement.
Proc Natl Acad Sci U S A, 110(6):E517-25, 22 Jan 2013
Cited by: 272 articles | PMID: 23341635 | PMCID: PMC3568385
Gene augmentation trials using the Rpe65-deficient dog: contributions towards development and refinement of human clinical trials.
Adv Exp Med Biol, 723:177-182, 01 Jan 2012
Cited by: 5 articles | PMID: 22183331
Review




