Abstract
Free full text
A Chemokine-Dependent Stromal Induction Mechanism for Aberrant Lymphocyte Accumulation and Compromised Lymphatic Return in Rheumatoid Arthritis
Abstract
According to the current model for tissue-specific homing, specificity is conferred by the selective recruitment of lymphocyte populations from peripheral blood, based on their expression of chemokine and adhesion receptors (endothelial selection). In this study, we provide evidence for an alternative stromal induction mechanism that operates in chronic inflammation. We show that the human rheumatoid synovial microenvironment directly induces functional inflammatory (CCR5 and CXCR3) and constitutive (CCR7 and CXCR4) chemokine receptors on infiltrating CD4+ T cells. Expression of the corresponding inflammatory chemokine ligands (CCL5 and CXCL11) was confined to stromal areas in the synovium. However, expression of the constitutive ligands (CCL19 and CXCL12) was inappropriately high on both vascular and lymphatic endothelium, suggesting that the vascular to lymphatic chemokine gradient involved in lymphatic recirculation becomes subverted in the rheumatoid synovium. These results challenge the view that leukocyte trafficking is regulated solely by selective recruitment of pre-existing chemokine receptor-positive cells from peripheral blood, by providing an alternative explanation based on aberrant lymphocyte retention and compromised lymphatic return.
The persistence of an inflammatory infiltrate within tissues requires an imbalance between those factors that enhance cellularity (recruitment and proliferation) and those that decrease cellularity (emigration and apoptosis) (1). Although current interest has focused predominantly on those mechanisms that increase cellularity, through identifying endothelial-specific homing mechanisms or Ags that induce proliferation, there has been less focus on mechanisms that might alter cell accumulation through changes in the retention of leukocytes once they enter tissue.
It is now well established that the differential expression of chemokines, chemokine receptors, and adhesion molecules plays an important role in determining tissue-specific trafficking and the positioning of leukocyte subsets within both normal and inflamed tissues (2-4). For example, whereas naive T cells home to secondary lymphoid tissue using the adhesion molecules L-selectin, LFA-1, and chemokine receptor CCR7, T cells destined to home to the skin and small intestine are of a memory phenotype and express the combination code cutaneous lymphocyte Ag (CLA)2/CCR4 and α4β7/CCR9, respectively (5-7). Therefore, naive T cells have a very restricted pattern of recirculation (only secondary lymphoid tissue), whereas memory T cell subsets can migrate into any tissue in the body depending on their expression of appropriate adhesion and chemokine receptors (3).
Chemokines and their receptors have been traditionally classified into two main functional groups: constitutive chemokines (involved principally in regulating basal trafficking of naive lymphocytes to lymphoid organs) and inflammatory chemokines (involved primarily in regulating the recruitment of effector memory leukocyte subsets to nonlymphoid inflamed tissue) (3). Although this classification system provides an explanation for the homing of naive and memory T cells to distinct tissue microenvironments in noninflammatory settings, it is not clear whether the same rules apply during chronic inflammation. For example, recent studies have surprisingly shown that in chronic inflammatory conditions ectopic expression of constitutive as well as inflammatory chemokines is observed (8).
Rheumatoid arthritis (RA) is a chronic persistent inflammatory disease characterized by the accumulation of T lymphocytes within defined microdomains in synovial tissue. For example, CD4+ T cells often accumulate within lymphoid-like structures in the synovial membrane (9, 10). However, naive CD4+ T cells are effectively excluded from the inflamed synovium, suggesting that the endothelial selection mechanism that discriminates between naive and memory CD4+ T cells remains intact, despite ongoing inflammation (11, 12). We and others have previously shown that CD4+ T cells within the rheumatoid synovium express levels of the constitutive chemokine receptor CXCR4 that are out of keeping with their highly differentiated memory phenotype (13-15). Moreover, we found that the high levels of expression of CXCR4 on CD4+ T cells were induced on these cells following their entry into the synovium by TGF-β produced by stromal cells within the synovial microenvironment (13). Taken together, these findings suggested to us that the induction of chemokine receptors on CD4+ T cells following their entry into inflamed tissue might play a more important role in lymphocyte accumulation, during chronic inflammation, than has previously been recognized.
We have found that the rheumatoid synovial microenvironment induces the expression of a distinct subset of inflammatory as well as constitutive chemokine receptors. Expression of these receptors is functionally relevant, and their ligands are differentially expressed on either synovial tissue or vascular and lymphatic endothelium. Our results challenge the current view that the enrichment of leukocytes expressing particular subsets of chemokine receptors is regulated solely by selection at their point of entry through the inflamed endothelium. Furthermore, these results provide a mechanism that accounts for lymphocyte accumulation within the ectopic lymphoid-like structures seen in the inflamed synovium, based on aberrant stromal retention and compromised lymphatic return.
Materials and Methods
Patient samples
Samples from peripheral venous blood and synovial fluid (SF) were collected into preservative-free heparin. Synovial tissue was taken at the time of joint replacement and snap frozen in liquid nitrogen. All 39 patients with RA fulfilled 1987 American College of Rheumatology criteria for RA (16). Tonsils were removed from patients undergoing routine tonsillectomy, and salivary gland tissue was taken from patients with Sjögren’s syndrome undergoing lip biopsy. All five patients with Sjögren’s fulfilled the revised classification for Sjögren’s syndrome (17). PBL and SF lymphocytes were isolated, as previously described (13). SF was immediately centrifuged to remove cells and debris before storage in aliquots at −70°C. Ethical approval for the use of tissue samples taken at the time of this study was obtained from the Birmingham Research Ethics Committee (REC 2002/088 and LREC 5735).
Medium, cytokines, and Abs
Cells were cultured in RPMI 1640 (Sigma-Aldrich) with 0.5% BSA (Sigma-Aldrich), supplemented with antibiotics, as previously described (13), sometimes in the presence of IFN-β (1000 U ml−1; BioSource International).
Abs used to detect chemokine receptors by flow cytometry were: CCR1 (53504.111, mouse IgG2b), CCR2 (48607 mouse IgG2b), CCR3 (61828, rat IgG), CCR4 (1G1, mouse IgG1), CCR5 (45531.111, mouse IgG2b), CCR6 (53103.111, mouse IgG2b), CCR7 (150503, mouse IgG2a), CCR9 (112509, mouse IgG2a), CXCR1 (42705.111, mouse IgG2a), CXCR2 (48311.211, mouse IgG2a), CXCR3 (49801 mouse IgG1), CXCR4 (44708 mouse IgG2a), CXCR5 (51505.111, mouse IgG2b), and CXCR6 (56811.111, mouse IgG2b) (R&D Systems); CLA (HECA-452, rat IgM), CD4-PE (SK3, mouse IgG1), and CD45RA-Cy5 (2H4, mouse IgG1) (all from BD Biosciences); and α4β7 (Act-1, mouse IgG1) (a kind gift from G. Nash, University of Birmingham, Birmingham, U.K.). Chemokines CCL5, CCL19, CXCL9, and CXCL12 were purchased from PeproTech. These chemokines were titrated to achieve maximal migration for both peripheral blood and SF samples, and were used at CXCL12 (1 μg ml−1), CCL5 (200 ng ml−1), CCL19 (1 μg ml−1), and CXCL9 (1 ng ml−1).
For immunohistology using fluorescence, as detected by confocal microscopy (laser-scanning confocal microscope LSM510 from Zeiss), primary Abs used were: CCR5 (mouse IgG2a 555991), CXCR4 (mouse IgG2a 555972), and CCR2-Alexa647 (mouse IgG2b 557913) (BD Pharmingen); CXCR3 FITC (mouse IgG1 MCA1834) (Serotec); CCR7 FITC (mouse IgG2a FAB197F), CCL5 (goat 278-NA), CXCL11 (mouse IgG2a MAB672), CCL19 (mouse IgG2b MAB361), and CCL2 (mouse IgG2b MAB2791) (R&D Systems); CXCL12 (mouse Ig2a clone K15C) (a gift from A. Amara, Pasteur Institute, Paris, France); CD31 (mouse IgG2a clone Hec7 MA3100) (Pierce); CD31 (mouse IgG1 clone BAG 85D10) (as supplied to the Seventh Human Leukocyte Differentiation Antigen Workshop by M. Hadam, Kinderklinik, Medizinische Hochschule, Hannover, Germany); von Willebrand factor (vWF) (polyclonal rabbit Ig A0082) (DakoCytomation); LYVE-1 (mouse IgG1 clone 8C) (18), CD3 (mouse IgG2b clone UCHT-1), and CD4 (mouse IgG1 clone QS412) (gifts from P. Beverley, Edward Jenner Institute for Vaccine Research, Newbury, U.K.); and CD3 (mouse IgG2a clone OKT3) and CD4 (mouse IgG2b clone OKT4) (American Type Culture Collection).
CCR5 and CXCR4 were detected using anti-mouse IgG2a Cy5. CXCR3 FITC and CCR7 FITC detection was amplified, and CXCL12 was detected using anti-mouse IgG2a FITC. CCL2 was detected and CCR2-Alexa 647 was amplified using anti-mouse IgG2b Cy5, CCL19 was detected using anti-mouse IgG2b FITC, and CXCL11 was labeled with biotin anti-mouse IgG2a (Southern Biotechnology Associates). CCL5 was detected using anti-goat Alexa 546. CXCL11 labeled with biotin was detected using streptavidin Alexa 633, and the signal from CCR7 was further amplified with anti-FITC Alexa 488 (Molecular Probes). To provide four colors when comparing vWF, LYVE-1, CCL19, and CXCL12, reagents used were donkey anti-rabbit IgG Cy5 (Jackson ImmunoResearch Laboratories), goat anti-mouse IgG1 tetramethylrhodamine (TRITC), goat anti-mouse IgG2b FITC, and goat anti-mouse IgG2a biotin (Southern Biotechnology Associates) with streptavidin-7-amino-4-methylcoumarin-3-acetic acid (Jackson ImmunoResearch Laboratories). Combinations of reagents used for triple-color experiments included CCR5 and CXCR4 with CD4 (QS412 with anti-mouse IgG1 FITC) and CD3 (UCHT-1 with anti-mouse IgG2b TRITC) (Southern Biotechnology Associates); CCR2 with CD4 (QS412 as used previously) and CD3 (OKT3 with anti-mouse IgG2a TRITC) (Southern Biotechnology Associates); CXCR3 with CD4 (OKT4 with anti-mouse IgG2b TRITC, as used previously) and CD3 (OKT3 with anti-mouse IgGa Cy5, as used previously); CCR7 with CD4 (QS412 with anti-mouse IgG1 TRITC, as used previously) and CD3 (UCHT-1 with anti-mouse IgG2b Cy5, as used previously); CCL2, CCL19, CXCL12, and CXCL11 with CD31 (BAG 85D10 with anti-mouse IgG1 TRITC, as used previously) and vWF (detected with anti-rabbit Cy5, as used previously); and CCL5 with CD31 (Hec7 with anti-mouse IgG2a FITC, as used previously) and vWF (detected as previously).
Flow cytometry
Analysis of cell surface molecules was performed using three-color immunofluorescence, as previously described (13). For analysis of chemokine receptor expression, PBL and SF lymphocytes were stained with anti-chemokine receptor Abs, followed by FITC-labeled secondary Abs (Southern Biotechnology Associates). The samples were analyzed on an EPICS XL flow cytometer (Beckman Coulter). Cytometer calibration was standardized using fluorospheres (Immunocheck and Standardbrite; Beckman Coulter). Data were analyzed using WinMDI version 2.8 (The Scripps Institute).
Cell culture
To study the effect of the synovial microenvironment on chemokine receptor expression, freshly isolated SF T cells were transferred to 24-well flat-bottom culture plates (1 × 106 cells/well) and maintained in culture in the presence of IFN-β. This did not affect expression of the chemokine receptors used in this study, but prevented T cell apoptosis (13). Autologous SF was added back at a 50% dilution. Cell surface expression of chemokine receptors was measured on matched peripheral blood T cells at the time of SF aspiration.
Transmigration assays for functional chemokine receptor expression
Viable cells (determined by trypan blue exclusion) were placed in 5-μm transwell inserts (Corning Glass). Chemokines used for the assay were prewarmed in 0.5% BSA/RPMI 1640 at 37°C before adding them to the top chamber of the transwell. After 4 h, chemokine receptor expression on T cell subsets was analyzed using three-color flow cytometry. Specific migration was calculated as: (amount of cells in lower chamber/number of cells added to top well at time zero) × 100 (% input).
Immunofluorescence staining of human tissue
The 5-μm cryostat sections of rheumatoid synovium, Sjögren’s syndrome salivary gland, and normal donor palatine tonsil were fixed in cold acetone for 15 min. Sections were stored at −70°C. Chemokine and chemokine receptor expression in rheumatoid synovium was compared with vessel and T lymphocyte staining, respectively. Vessels were identified using CD31 and vWF, while T cells were identified as being CD4+ and CD3+. The levels of expression of CCL19 and CXCL12 on vascular and lymphatic endothelium were compared using tonsil, rheumatoid synovium, and salivary glands from patients with Sjögren’s syndrome. vWF and LYVE-1 were used as markers of vascular and lymphatic endothelium, respectively. Primary Abs against CCL19 and CXCL12 were incubated on sections overnight at +4°C. All other incubations were conducted on the second day for 30 min at room temperature, and slides were washed for 20 min in PBS, pH 7.4, between incubations. The primary Abs used on the second day were vWF and LYVE-1. Stained slides were analyzed by confocal microscopy using a 510 laser-scanning microscope system (Zeiss). The sensitivity of the system was set to eliminate nonspecific background levels of staining given by the binding of secondary reagents either directly to the tissue samples or across species. For each comparison, a minimum of 10 different 320 × 320-μm adjacent sections was analyzed. Comparisons were made of the percentage expression of either CCL19 or CXCL12 on vWF- or LYVE-1-positive endothelial structures in tonsil, rheumatoid, and Sjögren’s tissue.
Results
Chemokine receptor expression on naive vs memory peripheral blood CD4+ T cells
We first determined the expression of a range of inflammatory as well as constitutive chemokine receptors on CD45RA+ naive, as well as CD45RA− memory CD4+ T cells in the peripheral blood of healthy volunteers (Fig. 1). As expected, there were distinct patterns of chemokine receptor expression on naive (CD45RA+) compared with memory (CD45RA−) CD4+ T cells. Naive (CD45RA+) CD4+ T cells showed high and restricted expression of the constitutive chemokine receptors CXCR4 and CCR7, which are implicated in homing to lymphoid tissue. In contrast, memory (CD45RA−) CD4+ T cells exhibited a more varied phenotype expressing CCR4, CCR6, CXCR3, CXCR4, and CXCR5 as well as slightly lower levels of CCR7 compared with peripheral blood CD45RA+ T cells. These findings are in keeping with current models of leukocyte homing, which predict that tissue-infiltrating CD4+ T cells are recruited from dedicated memory T cell subsets in peripheral blood that express specific combinations of chemokine and adhesion receptors (19).
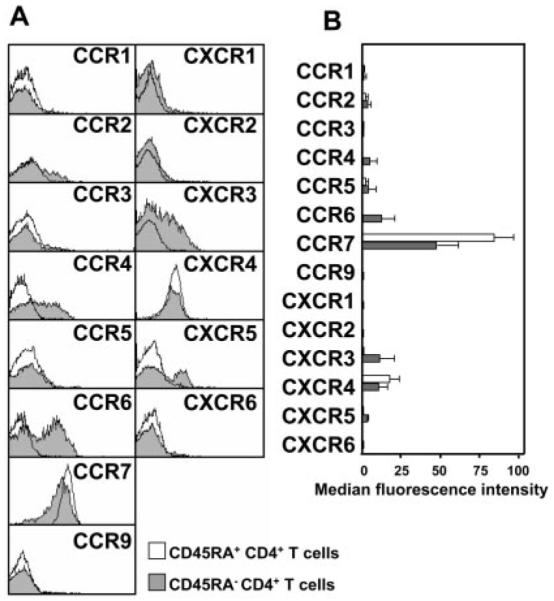
Comparison of chemokine receptor expression on peripheral blood CD45RA+ (naive) and CD45RA− (memory) CD4+ T cells. A, Overlaid histograms from a representative sample, of chemokine receptor expression on CD45RA+ naive and CD45RA− memory CD4+ T cells. CD45RA+ T cells predominantly express constitutive chemokine receptors (CCR7 and CXCR4), whereas CD45RA− T cells express a wider range of chemokine receptors. B, Chemokine receptor expression (median fluorescence intensity ± SD) on CD45RA+ and CD45RA− cells from a minimum of six different donors.
Chemokine receptor expression on matched peripheral blood and SF T cells in RA
CD4+ T cells within the rheumatoid synovium like all other extralymphoid tissue are exclusively of the memory phenotype (CD45RA−) and display markers of an activated phenotype (CD69+, MHC class II+) (Fig. 2A). This activation phenotype is at odds with the functional state of the cells (CD25−, quiescent non-cycling) and has been termed the frustrated phenotype (20). Therefore, any comparison of the expression of chemokine receptors on SF T cells (exclusively CD45RA−) vs peripheral blood T cells (a mixture of CD45RA+ and CD45RA−) must take these differences into account.
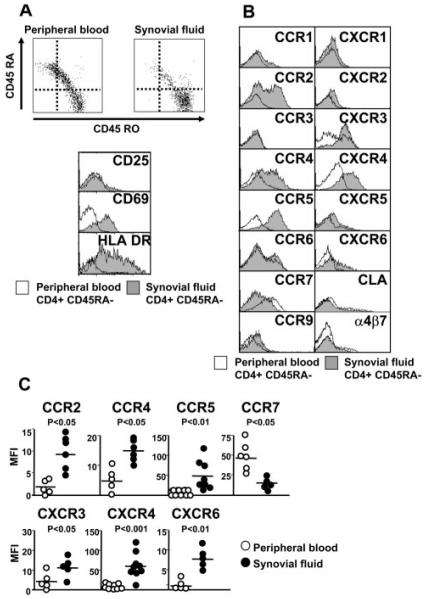
Expression of chemokine receptors on SF compared with peripheral blood T cells from patients with RA. A, SF CD4+ T cells are exclusively of the memory CD45RA− phenotype. These cells are of a frustrated phenotype (CD69+ HLA DR+ CD25−). B, Representative example of chemokine receptor, CLA, and α4β7 expression on CD4+CD45RA− cells in peripheral blood and SF from a patient with RA. C, Scatter-plot representation of chemokine receptor expression (median fluorescence intensity: bar is mean) from a range (minimum of five) of different patients.
We therefore compared the expression of chemokine receptors on CD4+CD45RA− T cells in SF to matched CD4+CD45RA− peripheral blood cells in patients with RA (Fig. 2, B and C). For comparison, we also studied the adhesion receptors, CLA and α4β7, which, according to current models of tissue-specific homing, should be excluded from the synovium as they are restricted to cells destined to home to the skin (CLA+ CCR4+) and intestine (α4β7+ CCR9+), respectively (5-7). We found that the expression of CCR2, CCR4, CCR5, CXCR3, CXCR4, and CXCR6 was significantly enriched on rheumatoid SF CD4+ T cells, compared with their circulating CD4+ memory T cell counterparts. CCR7 expression was significantly lower, but not absent on SF CD4+ T cells. As others have previously noted, SF contained CLA+ as well as α4β7+ memory T cells in proportions similar to those found in peripheral blood memory CD4+ T cells (12). Taken together, these results suggested to us that either there might be loss of the tissue-homing specificity within the rheumatoid synovium or that some of these receptors might be induced on T cells after their entry into the synovium.
The rheumatoid synovial microenvironment induces selective chemokine receptor expression on synovial CD4 T cells
To distinguish between these two different, but not mutually exclusive possibilities, we isolated SF CD4+CD45RA− T cells, cultured them ex vivo in the absence of SF, and then re-exposed them to autologous SF to mimic re-exposure to the synovial stromal microenvironment. Synovial T cells are highly differentiated cells that are prone to apoptosis. Therefore, to maintain their survival in culture, we added IFN-β to the culture medium. Importantly, IFN-β is cytostatic, maintains the cells in their normally nonproliferative state, and does not affect the expression of any of the chemokine receptors examined in this study (13) (data not shown). Of the seven chemokine receptors found to be expressed on synovial CD4+CD45RA− T cells, the expression of four, namely CCR5, CXCR3, CCR7, and CXCR4, was reduced when cultured in the absence of SF. When re-exposed to autologous SF for 24 h, levels of expression were restored (Fig. 3, A and B). The kinetics of loss and reinduction of CXCR4 was slower (3 days) compared with CCR5, CCR7, and CXCR3 (1 day). In contrast, expression of CCR2, CCR4, and CXCR6 was not affected by re-exposure to autologous SF (Fig. 3C). This strongly suggests that the expression of CCR5, CCR7, CXCR3, and CXCR4 on CD4+CD45RA− T cells infiltrating the synovium is directly regulated by soluble factors present within the synovium, as mimicked by autologous SF. It is more likely that enrichment of CCR2, CCR4, and CXCR6 reflects recruitment of pre-existing receptor-positive populations from the peripheral blood memory T cell compartment.
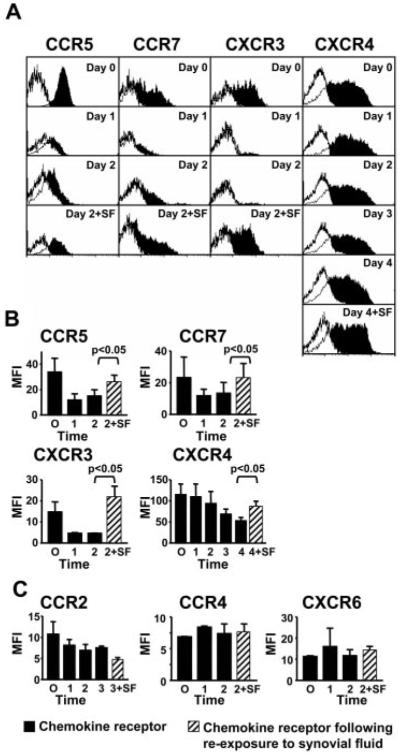
The synovial microenvironment regulates chemokine receptor expression. CD4+CD45RA− T cells were isolated and cultured in the absence of SF. At the time points indicated, cells were recultured with autologous SF. A, Histograms of a representative example of chemokine receptor expression (filled histograms) compared with negative isotype-matched control. B, Repeated experiments plotting MFI (±SD) are shown for CCR5, CCR7, CXCR3, and CXCR4, in which addition of SF ( ) leads to an increase in expression. C, SF reintroduction does not affect expression of CCR2, CCR4, and CXCR6. Data are representative of three to six independent experiments.
) leads to an increase in expression. C, SF reintroduction does not affect expression of CCR2, CCR4, and CXCR6. Data are representative of three to six independent experiments.
SF induces CXCR3 and CXCR4 expression on peripheral blood CD4+ T cells
To determine whether factors present within SF were both necessary and sufficient to induce CCR5, CCR7, CXCR3, and CXCR4 expression on CD4+ T cells upon their entry into the inflamed synovium, we exposed peripheral blood CD4+CD45RA− T cells to allogeneic SF derived from patients with RA. Importantly, exposure of CD4+CD45RA− T cells to SF did not induce either selective proliferation or apoptosis (data not shown). After 24 h, expression of CCR2, CCR4, CCR5, CCR7, CXCR3, CXCR4, and CXCR6 was determined (Fig. 4). SF did not induce expression of CCR2, CCR4, CCR5, CCR7, or CXCR6. However, expression of CXCR3 and CXCR4 was markedly increased upon exposure to SF. Taken together with previous data (Fig. 3), this suggests that SF contains all of the factors that are both necessary and sufficient to induce CXCR3 and CXCR4 expression on T cells, whereas the induction of CCR5 and CCR7 on synovial CD4+ T cells requires additional factors that are intrinsic to synovial T cells.
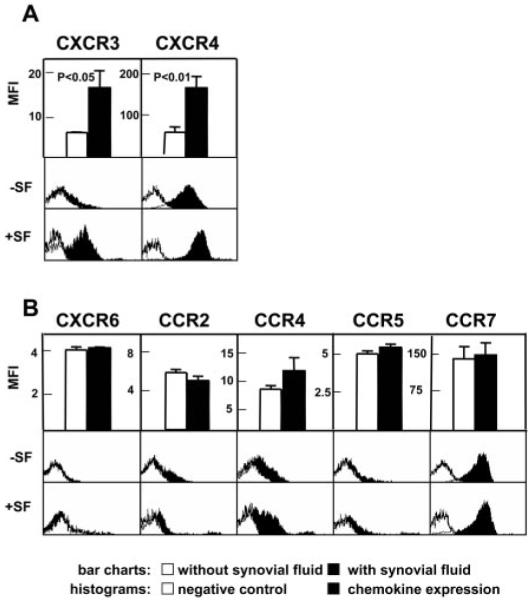
SF induces CXCR3 and CXCR4 expression on peripheral blood CD4+CD45RA− T cells. Comparison of chemokine receptor expression of T cells cultured with (+SF) and without (−SF) allogeneic SF. Data for each chemokine receptor are represented as bar charts with information from minimum of six experiments (±SD) per chemokine receptor and as histograms of a representative sample. A, Up-regulation of CXCR3 and CXCR4 by SF. B, No significant effect of SF on levels of CCR2, CCR4, CCR5, CCR7, and CXCR6 expression.
Functional relevance of chemokine receptor expression on synovial CD4+ T cells
To determine whether the increased expression of CCR5, CCR7, CXCR3, and CXCR4 on synovial CD4+ T cells was functionally relevant, we tested the ability of SF and matched peripheral blood CD4+CD45RA− T cells to migrate to some of their appropriate chemokine ligands using transwell filter assays (Fig. 5). The proportion of CD4+CD45RA− T cells that migrated in response to CCL5 (RANTES, ligand for CCR5), CXCL9 (monokine induced by IFN-γ, ligand for CXCR3), or CXCL12 (stromal cell-derived factor-1, ligand for CXCR4) correlated well with levels of expression of their associated chemokine receptors. Migration was consistently higher for SF T cells compared with their blood counterparts for all of the chemokines tested over a broad range of concentrations. Despite the lower levels of expression of CCR7 on SF T cells, CCL19 (EBV-induced gene 1 ligand chemokine, ligand for CCR7) still induced higher levels of chemotaxis on SF T cells compared with matched peripheral blood T cells over all concentrations tested (Fig. 5).
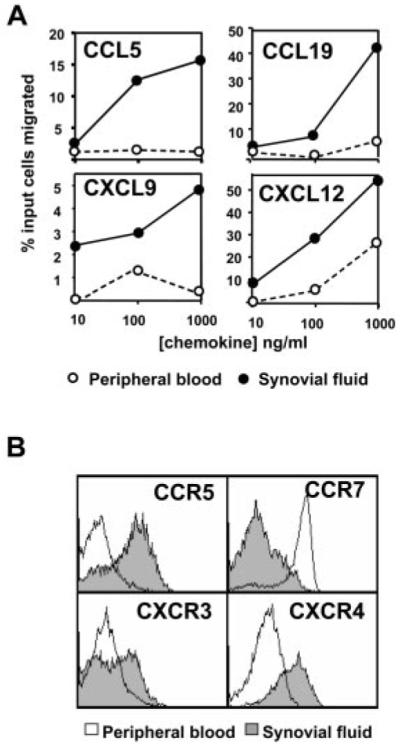
CCR5, CCR7, CXCR3, and CXCR4 receptors on CD4+CD45RA− T cells are functional. A, SF or peripheral blood CD4+ T cell migration in response to the chemokines CCL5, CCL19, CXCL9, and CXCL12 was measured (at different concentrations of chemokines ranging from 10 to 1000 ng/ml) after 4 h. Data are from one representative experiment of four. B, Chemokine receptor expression was analyzed to verify expression of receptors on peripheral blood and SF CD4+CD45RA− cells that had migrated. Data are from one representative experiment of four.
Expression of chemokines and their receptors in synovial tissue
To confirm that the high levels of expression of CCR5, CCR7, CXCR3, and CXCR4 on SF CD4+ T cells reflected those in synovial tissue, we performed immunohistochemistry studies on rheumatoid synovial tissue (Fig. 6). We included CCR2 as an example of one of the chemokine receptors whose expression was higher in SF, but was not modified following entry into the synovium. Synovial tissue CD4+ T cells expressed high levels of CCR5, CXCR3, CXCR4, and CCR2, but lower levels of CCR7, supporting our findings in SF CD4+ T cells. We also examined whether the ligands for these receptors were expressed in synovial tissue in vivo (Fig. 7). Of particular interest was the finding that there was a striking demarcation in the location where the chemokines were expressed. Expression of CCL5 and CXCL11 (ligands for CCR5 and CXCR3, respectively) was confined to nonendothelial stromal sites (Fig. 7A). In complete contrast, the expression of CXCL12, CCL19, and CCL2 (ligands for CXCR12, CCR7, and CCR2, respectively) was broader, with expression on stromal as well as endothelial cells, as evidenced by colocalization with the pan-endothelial marker CD31 (Fig. 7B). Although these findings support earlier observations that CXCL12 and CCL19 are produced by stromal cells, but displayed on the endothelium (13, 21), it presents a paradox: why, if chemokines involved in the recruitment of naive CD4+ T cells are posted on synovial endothelial cells, are naive cells so efficiently excluded from the inflamed synovium?
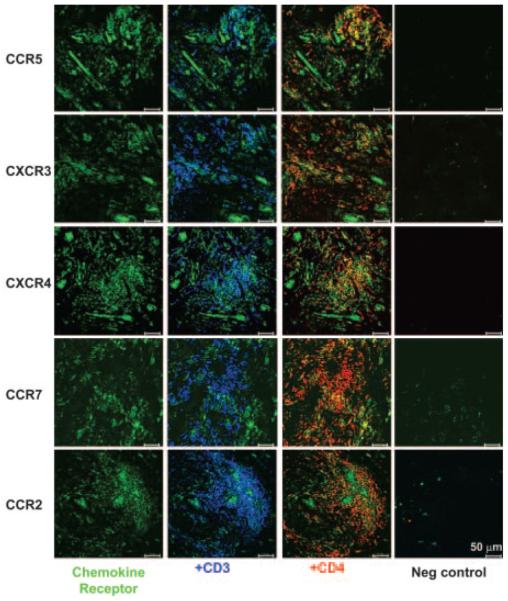
Expression of chemokine receptors in synovial tissue. Expression of the chemokine receptors CCR5, CXCR3 and CXCR4, and CCR7 and CCR2 (all green). Localization of chemokine receptors in areas in which CD3+ cells (blue) that are present are cyan, and in which CD4+ T cells (red) that are present are yellow. Isotype-matched negative controls for each chemokine receptor are shown.
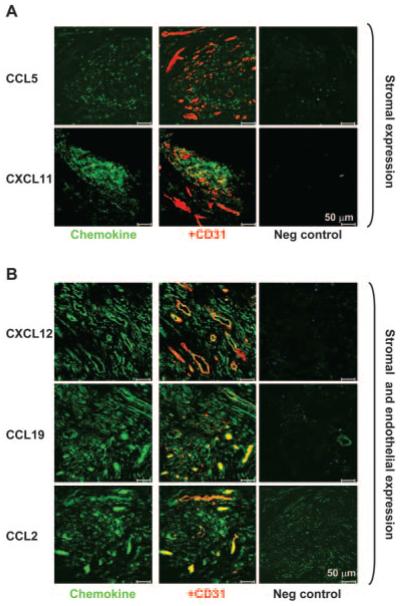
Differential expression of chemokine ligands on stromal and endothelial cells in synovial tissue. A, Chemokines CCL5 and CXCL11 (green) are expressed within the tissue stroma in areas of T cell infiltration, but not on endothelial cells (as marked by CD31, a pan endothelium marker in red). B, In contrast, the chemokines CXCL12, CCL19, and CCL2 (green) are expressed on both stromal cells as well as endothelial cells (yellow colocalization with CD31 red). Isotype-matched negative controls for each chemokine are shown.
Expression of CCL19 and CXCL12 on vascular and lymphatic endothelium in the rheumatoid synovium
We wondered whether the lack of naive CD4+ T cells in the rheumatoid synovium, despite high levels of expression of CCL19 and CXCL12 on endothelial cells, might reflect differential expression of these chemokines on lymphatic as opposed to vascular endothelium. Endothelial cells can be divided into two main functional types: vascular and lymphatic. Much work has gone into characterizing the former, but comparatively little is known about the latter, due in part to the lack of markers of sufficient quality to identify such cells. Using vWF and LYVE-1, a recently identified hyaluronan receptor specific for lymphatic vessels, we compared the distribution of CXCL12 and CCL19 on vascular (vWF+) and lymphatic (LYVE-1+) endothelium in the rheumatoid synovium. For comparison, we examined inflamed tonsils as well as salivary glands from patients with Sjögren’s syndrome (Fig. 8).
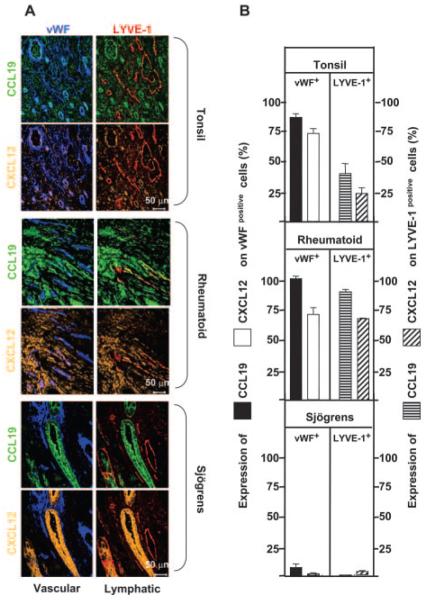
CCL19 and CXCL12 expression on vWF+ and LYVE-1+ cells in tonsil, rheumatoid synovium, and Sjögren’s salivary glands. A, Four-color confocal micrographs show CCL19 (green), CXCL12 (gold), vWF (blue), and LYVE-1 (red). Colocalization is indicated as: CCL19 and vWF (cyan), CCL19 and LYVE-1 (yellow), CXCL12 and vWF (pink), and CXCL12 and LYVE-1 (russet). The group of four images for each tissue is the same field of view. B, Percentage of expression of either CCL19 or CXCL12 on vWF-positive vascular endothelium (left panels) or LYVE-1-positive lymphatic structures (right panels) in tonsil, rheumatoid, or Sjögren’s tissue. At least 10 different fields of view were analyzed. Comparing tonsil with rheumatoid synovium, the following differences were statistically significant: CCL19 expression on vWF, p < 0.002; CCL19 on LYVE-1, p < 0.0001; CXCL12 on LYVE-1, p < 0.0001. CXCL12 on vWF was not significantly different (p = 0.94).
Three distinct patterns of CCL19 and CXCL12 expression were observed. In inflamed lymphoid tissue (tonsil), the vascular endothelium (vWF+) displayed high levels of CXCL12 and CCL19, while the lymphatic endothelium (LYVE-1+) expressed lower levels of both chemokines. This was quite unlike the pattern seen in rheumatoid synovium, where expression of CXCL12 and CCL19 was high on both vascular endothelium (vWF+) and lymphatic endothelium (LYVE-1+). This pattern appears to be specific to the rheumatoid synovium and not a generic property of inflamed tissue, because there was very little expression of CXCL12 and CCL19 on vascular or lymphatic vessels in salivary glands in Sjögren’s syndrome. In Sjögren’s tissue, CXCL12 and CCL19 were abundantly expressed on ductal epithelial tissue rather than endothelium, a pattern that we have observed previously for CXCL12 (22). This suggests that in lymphoid tissue (tonsil), high levels of CCL19 and CXCL12 on vascular, compared with lymphatic endothelium might provide a chemokine gradient for the entry and retention of naive CD4+ T cells. However, a similar gradient does not exist in the rheumatoid synovium, and may thereby account for the lack of naive T cells.
Discussion
Tissue-specific accumulation of lymphocyte subsets provides for functional compartmentalization within the immune system (19). For example, naive T cells are programmed to recirculate through secondary lymphoid tissue, where they meet and are activated by their cognate Ag, whereas effector/memory cells recirculate through extralymphoid tissue to limit tissue damage and facilitate organ repair (3, 23). There is now overwhelming evidence to support the concept that tissue specificity is conferred by the restricted expression of adhesion molecules and chemokines on endothelial cells within target tissues (5, 19). In addition, small populations of lymphocytes expressing the appropriate adhesion and chemokine receptors pre-exist in peripheral blood and are enriched within tissues that express their corresponding ligands (12). More recently, studies in mice have shown that lymphocytes acquire the ability to home to a broader range of sites by regulating their profile of tissue-specific adhesion and chemokine receptors at the time of activation within lymph nodes (24).
Although this endothelial area code model (2) undoubtedly explains why certain T cell subsets accumulate within tissues during physiological levels of lymphocyte homing (e.g., immune trafficking to lymphoid tissue), we wondered whether this model also applied in pathological conditions, especially when neovascularization occurs and lymphocyte trafficking is increased. We therefore examined the expression of a range of chemokine receptors on CD4+ T cells infiltrating the rheumatoid synovium and compared their expression with circulating counterparts in blood.
SF CD4+ T cells, like those isolated from all other inflamed tissues to date examined, are highly differentiated T cells that are exclusively of the CD4+CD45RA−CD69+ phenotype, and therefore are best defined as effector memory cells (20). This dramatic enrichment of CD4+ memory cells and exclusion of naive cells in extralymphoid tissue have been consistently observed by many other groups (5-7, 11-13, 20, 23). Consistent with this phenotype, SF CD4+ T cells expressed disproportionately higher levels of the chemokine receptors CCR2, CCR4, CCR5, CXCR3, and CXCR6 compared with matched peripheral blood cells. Unexpectedly, synovial CD4+ T cells also expressed CXCR4 and CCR7, markers more characteristic of primary and secondary lymphoid-homing T cells rather than CD4+ T cells with a tropism for extralymphoid tissue. Recent studies by Sallusto et al. (25) have suggested that the T cell effector memory phenotype is best defined in peripheral blood as CD45RA− CCR7−, and therefore we were surprised to find low, but significant and functionally relevant levels of CCR7 on synovial CD4+ T cells. In fact, our findings are more consistent with those of Campbell et al. (26), who also found that synovial CD4+ T cells express CCR7 and have argued against the hypothesis proposed by Sallusto et al. (25), that CCR7-expressing cells can only enter lymphoid tissue. Finally, we noted that SF CD4+ T cells also expressed markers more characteristic of skin (CLA+ CCR4+) and intestinal (α4β7+) homing T cells at comparable levels to their memory CD4+ T cell blood counterparts, in agreement with the findings of Kunkel et al. (12).
Although these findings could potentially reflect a reduction in homing specificity at sites of persistent inflammation, an alternative possibility, which we have explored in this study, is that expression of chemokine receptors on CD4+ T cells might be modulated once lymphocytes enter the inflamed synovium. Using a series of withdrawal and replacement studies, we found that the expression of CCR5, CCR7, CXCR3, and CXCR4 on SF CD4+ T cells, taken directly ex vivo, was maintained by soluble factors present within the synovial microenvironment. This was not a general feature for all chemokine receptors whose expression was enriched in the inflamed synovium, as expression of CCR2, CCR4, and CXCR6 was not affected. We also observed that rheumatoid SF was able to up-regulate the expression of CXCR3 and CXCR4 on peripheral blood CD4+CD45RA− T cells. In contrast, exposure to SF was not sufficient to up-regulate the expression of CCR5 and CCR7 on peripheral blood CD4+ cells, suggesting that factors intrinsic to synovial T cells, such as transendothelial migration or prolonged residence within the synovium, might be required for up-regulation of these two receptors once the cells enter the synovium. These observations provide an alternative explanation as to why CCR7-expressing effector memory CD4+ T cells are found in some nonlymphoid tissues (26) because modulation of chemokine receptor expression is likely to be important in regulating the migration of lymphocytes within tissues and from tissue into the lymphatic system.
We have previously shown that TGF-β, produced in the synovium, regulates the expression of CXCR4 on CD4+ T cells in vivo (13). Up-regulation by TGF-β appears to be unique to CXCR4 because the expression of the other chemokine receptors examined in this study was not modified by TGF-β (data not shown). Despite a large screen of cytokines and other potential regulatory factors, we have been unable to identify the factor(s) present in SF that is responsible for up-regulating CXCR3, CCR5, and CCR7 expression by SF. We are currently examining whether other physico-chemical factors such as redox potential (27) and oxygen tension (28) can affect expression of CXCR3, CCR5, and CCR7 on CD4+ T cells in the rheumatoid synovium.
The expression of CCR5 and CXCR3 on T cells has been proposed to mark subsets of circulating memory T cells with a pre-dilection for homing to inflammatory sites dominated by Th1-type reactions (29, 30). Our findings suggest that CCR5 and CXCR3 may not play a key role in determining tissue specificity, but may play a more general role in regulating T cell retention and compartmentalization within inflamed tissue. In support of this notion, Kunkel et al. (12) have reported that unlike inflamed tonsil, all other inflammatory tissues examined (skin, intestine, liver, lung, and synovium) contained CD4+ memory T cells, which expressed high levels of CXCR3 and CCR5. Our findings that CCL5 and CXCL11, ligands for CCR5 and CXCR3, respectively, are expressed on stromal tissue, but not endothelial cells within the inflamed synovium (Fig. 7A) lend further support to this idea. Moreover, studies in humans (31), confirmed in a mouse model of granulomatous liver disease (32), have shown that CXCL10 (IFN-γ-inducible protein-10), another ligand for CXCR3, plays an important role in the compartmentalization of CD4+ T cells within the liver and in their retention within hepatic lymph nodes. Although we cannot formally exclude a role for CXCR3 and CCR5 in T cell recruitment into inflamed tissue (such studies will require adoptive transfer of T cells from CXCR3 and CCR5 knockout mice), our results support the idea that CXCR3 and CCR5 play a role in the retention rather than recruitment of T cells within inflamed nonlymphoid tissue (12). However, it is important to note that CXCR3 and CCR5 bind a total of seven chemokines, of which only two (CCL5 and CXCL11) have been investigated in this study. It is possible that these other chemokines could compensate for the lack of CCL5 and CXCL11 expression on RA blood vessels. Finally, the presence of CCL2 (the ligand for CCR2) on RA blood vessels and the lack of any effect of SF on CCR2 expression support the concept that this chemokine/chemokine receptor pair directly contributes to the recruitment of T cells from the blood into the rheumatoid synovium.
We also found that synovial CD4+ T cells expressed unexpectedly high levels of the constitutive chemokine receptors CCR7 and CXCR4. In addition, there was elevated expression of their ligands CCL19 and CXCL12 on both vascular and lymphatic vessels in rheumatoid, but not other inflamed tissues such as tonsil and Sjögren’s salivary glands. A number of reports, including overexpression studies in transgenic mice, have suggested that expression of CCL19, CCL21, and CXCL12 on endothelial cells contributes to the formation of ectopic lymphoid structures similar to those seen in a range of autoimmune diseases (33-35). We and others have also shown that the constitutive expression of the B cell-attracting chemokine CXCL13 on endothelial cells is also associated with the formation of lymphoid structures in Sjögren’s syndrome, an autoimmune disease with classical ectopic germinal center-like structures in salivary glands (22). Weninger et al. (36) have suggested that ectopic expression of CCL21 by vascular cells within the inflamed synovium contributes to the perivascular accumulation of naive CD3+ T cells and the development of lymphoid neogenesis in RA. However, in this study, the role of CD45RA+ memory CD8+ T cells (37) as a potential explanation for the accumulation of naive T cells and the exact type of endothelial cell expressing CCL21 was not rigorously examined. Using a novel maker for lymphatic endothelium (LYVE-1), we have found differential expression of CCL19 and CXCL12 on vascular and lymphatic vessels in the rheumatoid synovium compared with Sjögren’s salivary glands and inflamed tonsil. Importantly, in Sjögren’s salivary glands, a site of abundant ectopic lymphoid neogenesis, there was no vascular or lymphatic expression of CCL19 or CXCL12. Instead, expression was confined to epithelial ductal cells. It is tempting to speculate that aberrant expression of CCL19 and CXCL12 on vascular and lymphatic vessels in the rheumatoid synovium, in conjunction with the overexpression of the chemokine receptors CCR7 and CXCR4, drives the development of lymphoid-like structures with perivascular cuffing of CD4+ T cells in RA. Interestingly, expression of the B cell chemoattractant CXCL13 has a reciprocal distribution to CCL19 and CXCL12 in Sjögren’s with expression on vascular, but not epithelial structures (22).
Several observations have shown that the microenvironment in which T cells are activated dictates the resulting homing phenotype of the cell (24, 38). For example, the imprinting of gut tropism on naive T cells is mediated by an as yet unidentified mechanism involving dendritic cells from Peyer’s patches. Dendritic cells from spleen or peripheral lymph nodes are unable to do this (24). Our findings suggest that a similar stromal instruction mechanism involving the chemokine receptors CXCR4 and CCR7 and CCR5 and CXCR3 also occurs for memory CD4+ T cells in inflamed tissues, such as the rheumatoid synovium. In normal circumstances, reciprocal expression of CXCR4 and CCR7 might facilitate the exit of CD4+ T cells from tissues via draining lymphatics during the resolution phases of inflammation. In contrast, CCR5 and CXCR3 might be required to support stromal retention of infiltrating lymphocytes within tissues during ongoing inflammation.
Our studies therefore suggest that one of the consequences of ectopic expression of constitutive chemokines such as CCL19 and CXCLl2 on vascular and lymphatic endothelium is subversion of the normal physiological retention (CXCR3- and CCR5-dependent) and lymphatic re-entry (CCR7- and CXCR4-dependent) mechanisms. This would lead to the wrong cell (effector memory CD4+ T cells) accumulating in the wrong place (lymphoid aggregates) at the wrong time (during the resolution phase of inflammation) (39). Therefore, strategies aimed at preventing inflammatory T cell recruitment (targeting CCR2) may need to be combined with complementary strategies to augment lymphatic return (inhibiting CXCR4) to facilitate the resolution of inflammation and aid tissue repair in the rheumatoid synovium.
Footnotes
2Abbreviations used in this paper:
- CLA
- cutaneous lymphocyte Ag
- RA
- rheumatoid arthritis
- SF
- synovial fluid
- TRITC
- tetramethylrhodamine
- vWF
- von Willebrand factor
References
Full text links
Read article at publisher's site: https://doi.org/10.4049/jimmunol.174.3.1693
Read article for free, from open access legal sources, via Unpaywall:
http://www.jimmunol.org/content/174/3/1693.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4049/jimmunol.174.3.1693
Article citations
Immune-Related Genes in the Pathogenesis of Atherosclerosis: Based on Sex Differences.
J Inflamm Res, 16:4713-4724, 18 Oct 2023
Cited by: 0 articles | PMID: 37872959 | PMCID: PMC10590557
Fibroblast heterogeneity: Keystone of tissue homeostasis and pathology in inflammation and ageing.
Front Immunol, 14:1137659, 28 Feb 2023
Cited by: 8 articles | PMID: 36926329 | PMCID: PMC10011104
Review Free full text in Europe PMC
Crosstalk in the diseased plasma cell niche - the force of inflammation.
Front Immunol, 14:1120398, 21 Feb 2023
Cited by: 0 articles | PMID: 36895566 | PMCID: PMC9989665
A Review of Acquired Autoimmune Blistering Diseases in Inherited Epidermolysis Bullosa: Implications for the Future of Gene Therapy.
Antibodies (Basel), 10(2):19, 17 May 2021
Cited by: 3 articles | PMID: 34067512 | PMCID: PMC8161452
Review Free full text in Europe PMC
Predicting the Key Genes Involved in Aortic Valve Calcification Through Integrated Bioinformatics Analysis.
Front Genet, 12:650213, 11 May 2021
Cited by: 10 articles | PMID: 34046056 | PMCID: PMC8144713
Go to all (79) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium.
J Immunol, 165(6):3423-3429, 01 Sep 2000
Cited by: 202 articles | PMID: 10975862
Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium.
J Immunol, 165(11):6590-6598, 01 Dec 2000
Cited by: 262 articles | PMID: 11086103
Phenotypic and functional characterisation of CCR7+ and CCR7- CD4+ memory T cells homing to the joints in juvenile idiopathic arthritis.
Arthritis Res Ther, 7(2):R256-67, 12 Jan 2005
Cited by: 37 articles | PMID: 15743472 | PMCID: PMC1065323
Chemokines in rheumatoid arthritis.
Springer Semin Immunopathol, 20(1-2):115-132, 01 Jan 1998
Cited by: 64 articles | PMID: 9836372
Review
Funding
Funders who supported this work.
Versus Arthritis (1)
Fate determination in the rheumatoid synovium
Prof Mike Salmon
Grant ID: 16390




