Abstract
Objective
To assess patients' self-estimates of the burden of disease in vasculitis and to compare data across patient populations from the US, Germany, and the UK. Outcome assessment in vasculitis primarily focuses on physician evaluations of disease activity and damage. Little is known about the patients' perspectives regarding the burden of disease.Methods
Patients ranked (scale 0-5) a list of vasculitis-related items to estimate a combination of pain, interference with daily function, discomfort and/or annoyance, anxiety/psychological impact, and medical importance. Patients were also asked to list the 5 most troublesome aspects of the disease.Results
Data from 264 patients with vasculitis from 3 countries were collected. Wegener's granulomatosis was the predominant disease, comprising 63% of the cohort. Diagnoses were confirmed by physicians in 98% of cases. The most common item from the free-text sections was fatigue (75%), followed by pain (31%), musculoskeletal symptoms (24%), difficulty breathing (19%), financial aspects (13%), nasal discharge/crusting (14%), and weight gain (10%). Rankings of individual items varied substantially: fatigue (3.5), loss of energy (3.4), weight gain (3.1), joint pain (3.0), and sinusitis (3.0) were the highest-ranked symptoms among those manifestations experienced by at least 50% of patients. Several severe manifestations (e.g., dialysis, seizures, oxygen dependency) were ranked lower (<3.0).Conclusion
Fatigue and reduced energy level are considered the most important disease burdens by patients with vasculitis; manifestations associated with organ damage were rated lower. The patients' perspectives of the impact of vasculitis differ from the physicians' ratings. Future outcome assessment in vasculitis should include the patients' perspectives.Free full text

Patient-Reported Outcome Assessment in Vasculitis May Provide Important Data and a Unique Perspective
Abstract
Objective
To assess patients’ self-estimates of the burden of disease in vasculitis and to compare data across patient populations from the US, Germany, and the UK. Outcome assessment in vasculitis primarily focuses on physician evaluations of disease activity and damage. Little is known about the patients’ perspectives regarding the burden of disease.
Methods
Patients ranked (scale 0–5) a list of vasculitis-related items to estimate a combination of pain, interference with daily function, discomfort and/or annoyance, anxiety/psychological impact, and medical importance. Patients were also asked to list the 5 most troublesome aspects of the disease.
Results
Data from 264 patients with vasculitis from 3 countries were collected. Wegener’s granulomatosis was the predominant disease, comprising 63% of the cohort. Diagnoses were confirmed by physicians in 98% of cases. The most common item from the free-text sections was fatigue (75%), followed by pain (31%), musculoskeletal symptoms (24%), difficulty breathing (19%), financial aspects (13%), nasal discharge/crusting (14%), and weight gain (10%). Rankings of individual items varied substantially: fatigue (3.5), loss of energy (3.4), weight gain (3.1), joint pain (3.0), and sinusitis (3.0) were the highest-ranked symptoms among those manifestations experienced by at least 50% of patients. Several severe manifestations (e.g., dialysis, seizures, oxygen dependency) were ranked lower (<3.0).
Conclusion
Fatigue and reduced energy level are considered the most important disease burdens by patients with vasculitis; manifestations associated with organ damage were rated lower. The patients’ perspectives of the impact of vasculitis differ from the physicians’ ratings. Future outcome assessment in vasculitis should include the patients’ perspectives.
INTRODUCTION
Outcome assessment in vasculitis is based on items that physicians consider relevant in terms of disease activity, disease extent, and damage. The primary systemic vasculitides are a group of heterogeneous multisystem diseases with manifestations that range from mild to severe (1). Existing assessment tools, such as the Birmingham Vasculitis Score (BVAS) (2), the BVAS for Wegener’s granulomatosis (WG) (3), the Disease Extent Index (4), and the Vasculitis Disease Index (5), were created by the consensus of expert physicians (6). Similarly, weighting schema employed by these instruments were developed using expert opinion (7,8). Little is known about patients’ perspectives in systemic vasculitis. Despite the development of instruments measuring disease extent, activity, and damage, there are no vasculitis-specific instruments measuring patient-estimated burdens of disease. Prior work on patient-reported outcomes (PROs) in vasculitis included a few small studies of patients with WG (9–13) or giant cell arteritis (14,15).
Patients’ subjective experiences may represent key domains of illness that differ from clinicians’ views (16). While physician- and patient-derived measures should complement each other, they need not be identical; however, the aspects of disease believed by patients to be the most important must be identified and included in a set of outcome assessments in vasculitis.
Since patients are the true experts of their disease, their subjective experience should be part of the outcome measurement process. The vasculitis clinical research community and international experts in medical outcome assessment (the Outcome Measures in Rheumatology Clinical Trials initiative) agreed that patient-reported ratings of the burden of vasculitis and multidimensional health-related quality of life (HRQOL) are important domains of illness. There is a strong consensus that the development of instruments to measure these domains is an important addition to the overall research agenda in vasculitis, and such instruments should be included in a “core set” of measures applied to clinical research in vasculitis (8). The burden of disease in vasculitis, defined as the impact of permanent damage on the patient and its assessment, has not been investigated to date and was the focus of this study.
MATERIALS AND METHODS
Development of the questionnaire
After a comprehensive literature review of publications regarding PRO studies in vasculitis, a thorough investigation of the small number of existing studies, and input from a panel of expert physicians, a questionnaire was developed to allow patients to assess different aspects of vasculitis.
The 3-page questionnaire included items on patient demographics, vasculitis type and duration, current disease activity, changes in daily life due to vasculitis (numerical rating scale from 0–10), and whether the diagnosis was confirmed by a physician.
Additionally, using a 5-point Likert scale, patients ranked a list of 40 vasculitis-related items according to the following instructions: “We are interested in knowing how ‘bad’ you personally think different aspects of having vasculitis are. We want to know your opinion based on your experience and feelings. We ask below that you rate how bad each item is in terms of a combination of pain, interference with your daily function, discomfort and/or annoyance, anxiety or other psychological impact (such as how you look or feel), medical importance (per your opinion). We only want you to rate those items you have experienced since the date of your first symptoms of vasculitis and ask you to average the impact you have felt. For example, if you had nerve damage to your right leg, tell us how bad it has been on average, not just at the beginning. If you never were affected by the listed symptom during the course of your vasculitis, please circle ‘not applicable = 0.’”
The response scale was defined as 1 = not too bad, 2 = a little bit bad, 3 = moderately bad, 4 = quite bad, and 5 = extremely bad. Patients who did not experience a burden were not included in the analysis of this item. The items included were manifestations of disease activity and permanent damage. To maximize acceptance and feasibility, the instrument was limited to 3 pages. Respondents were also asked to list the 5 most important aspects of the disease in their daily life in open-text spaces.
Data collection
The questionnaire was distributed to patients at the Vasculitis Foundation Symposium in Baltimore, Maryland, in 2006, at a national German vasculitis support group meeting in Bad Bramstedt, Germany, in 2007 (the questionnaire was translated into German by a bilingual translator and back-translated into English), and at the Vasculitis Foundation Symposium in Cambridge, UK, in 2007. No incentive was offered to the patients. Inclusion criteria comprised the diagnosis of vasculitis (self-reported) and the ability to understand and complete the questionnaire.
Statistical analysis
The data were analyzed with the SPSS statistics software program, version 16.0 (SPSS). Means were calculated for the 40 vasculitis-related manifestations for those patients who experienced the particular symptom (range 1–5). Comparisons between groups were calculated by performing Mann-Whitney U tests and t-tests, as appropriate.
RESULTS
A total of 264 patients in 3 countries completed the questionnaires. The demographic and disease summary information from the 3 patient groups and the combined cohort is outlined in Table 1. The mean age of all patients was 55.5 years. Sixty-eight percent of the subjects were women. The antineutrophil cytoplasmic antibody–associated vasculitides (WG, microscopic polyangiitis, and Churg-Strauss syndrome) were the most common diagnoses (81%); the German cohort was more heterogeneous than the US and UK groups regarding diagnoses. Seventeen of the 18 patients with giant cell arteritis were German. The diagnosis was confirmed by a physician for 98% of the patients, as reported by the patients.
Table 1
Baseline characteristics*
| US | Germany | UK | Combined cohort | |
|---|---|---|---|---|
| Patients, no. | 135 | 79 | 50 | 264 |
| Men/women, % | 30/70 | 41/51 | 24/76 | 32/68 |
| Age, mean ± SD years | 53 ± 13 | 59 ± 12 | 55 ± 17 | 55.5 ± 14 |
| Time since first symptoms, median (range) months | 60 (3–471) | 67 (3–289) | 79 (15–480) | 67 (3–480) |
| Time since date of diagnosis, median (range) months | 41 (1–462) | 56 (2–275) | 62 (3–257) | 51 (1–462) |
| Confirmed diagnosis, % | 99 | 96 | 96 | 98 |
| Type of vasculitis | ||||
| Wegener’s granulomatosis | 108 (80) | 27 (34) | 31 (62) | 166 (63) |
| Churg-Strauss syndrome | 11 (8.1) | 15 (19) | 12 (24) | 38 (14.4) |
| Microscopic polyangiitis | 5 (3.7) | 2 (3) | 2 (4) | 9 (3.4) |
| Polyarteritis nodosa | 2 (1.5) | 3 (4) | 1 (2) | 6 (2.3) |
| Giant cell arteritis | 1 (0.8) | 16 (20) | 1 (2) | 18 (7) |
| Other | 8 (6) | 16 (20) | 3 (6) | 27 (10) |
| Disease activity | ||||
| Remission | 70 (52) | 19 (24) | 18 (36) | 107 (41) |
| Mildly active | 46 (34) | 28 (35) | 20 (40) | 94 (36.6) |
| Moderate | 12 (9) | 25 (32) | 10 (20) | 47 (18) |
| Very active | 4 (3) | 2 (4) | 2 (4) | 8 (3.5) |
| Overall health | ||||
| Poor | 1 (0.7) | 3 (4) | 6 (12) | 10 (4) |
| Fair | 45 (33) | 37 (47) | 21 (42) | 103 (39) |
| Good | 60 (44) | 32 (41) | 15 (30) | 107 (42) |
| Very good | 22 (16) | 4 (5) | 7 (14) | 33 (13) |
| Excellent | 4 (3) | 1 (1) | 1 (2) | 5 (2) |
| Change of daily life, mean ± SD† | 4.4 ± 2.4 | 4.4 ± 2.3 | 5.1 ± 2.4 | 4.6 ± 2.4 |
Forty-one percent of the patients rated their disease activity as in remission, 36.6% as mildly active, 18% as moderately active, and 3.5% as very active. Patients were asked to rate, “How much does the vasculitis affect your daily life?” on a scale of 0–10; the mean ± SD score was 4.6 ± 2.4. No differences between female and male patients were observed for disease activity (P = 0.777), overall health (P = 0.883), or disease duration (P = 0.742).
The questionnaire took ~10–15 minutes to complete. Subjects noted it was clear and easy to complete. Almost no missing data occurred (6.1%). Informal feedback from many participating patients indicated that patients were excited that investigators were taking patients’ views of their own disease seriously, and the patients were highly enthusiastic about the research program.
The frequencies and ratings of disease burden by people with vasculitis are shown in Table 2. Means were calculated for those patients who experienced the particular symptom. Fatigue, loss of energy, weight gain, joint pain, and sinusitis were the highest-ranked symptoms among those manifestations experienced by at least 50% of the patients. Ninety-five percent of all patients experienced both fatigue and energy loss and rated these manifestations as severe. Severe organ manifestations (seizures, kidney failure, and oxygen dependency) were ranked lower. Stroke, heart attack, blindness, dialysis, cancer, seizures, loss of extremity, and stomach ulcers occurred in ≤10% of the patients. Saddle nose deformity and thrombosis were relatively rare, but were rated as severe.
Table 2
Frequencies and ratings of manifestations of vasculitis in 3 different populations
| US (n = 135)* | Germany (n = 79)* | UK (n = 50)* | Combined cohort (n = 264) | |||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Mean ± SD | No. (%) | Mean ± SD | No. (%) | Mean ± SD | No. (%) | Mean ± SD | |
| Nasal septum perforation | 23 (17) | 4.1 ± 1.2 | 10 (13) | 2.5 ± 1.4 | 10 (20) | 3.4 ± 1.8 | 43 (16) | 3.6 ± 1.5 |
| Tiredness/fatigue | 129 (96) | 3.5 ± 1.1 | 73 (92) | 3.3 ± 0.9 | 50 (100) | 3.8 ± 1.1 | 252 (95) | 3.5 ± 1.0 |
| Loss of energy | 128 (95) | 3.4 ± 1.1 | 68 (86) | 3.3 ± 1.0 | 49 (98) | 3.7 ± 1.1 | 245 (95) | 3.4 ± 1.1 |
| Saddle nose deformity | 16 (12) | 3.3 ± 1.7 | 7 (9) | 1.9 ± 1.5 | 11 (22) | 3.9 ± 1.6 | 34 (13) | 3.2 ± 1.7 |
| Thrombosis (blood clot) | 16 (12) | 3.3 ± 1.4 | 11 (14) | 2.6 ± 1.4 | 8 (16) | 3.8 ± 1.8 | 35 (13) | 3.2 ± 1.5 |
| Weight gain | 109 (81) | 3.1 ± 1.3 | 48 (61) | 2.9 ± 1.3 | 48 (96) | 2.8 ± 1.7 | 200 (76) | 3.1 ± 1.3 |
| Joint pain | 119 (89) | 3.0 ± 1.2 | 57 (72) | 2.7 ± 1.1 | 42 (84) | 2.8 ± 1.2 | 218 (83) | 2.9 ± 1.2 |
| Sinusitis | 102 (76) | 3.0 ± 1.4 | 42 (53) | 2.6 ± 1.3 | 41 (82) | 3.2 ± 1.4 | 185 (70) | 3.0 ± 1.4 |
| Kidney failure | 41 (30) | 3.0 ± 1.3 | 13 (16) | 3.2 ± 1.5 | 12 (24) | 3.6 ± 1.1 | 66 (25) | 3.1 ± 1.3 |
| Stroke | 6 (4) | 3.0 ± 1.9 | 5 (6) | 3.0 ± 1.2 | 3 (6) | 4.0 ± 1.0 | 14 (5) | 3.2 ± 1.5 |
| Heart attack | 3 (2) | 3.0 ± 2.0 | 3 (4) | 4.0 ± 1.0 | 1 (2) | 1.0 | 7 (3) | 3.1 ± 1.7 |
| Nasal discharge/crusting | 111 (82) | 2.9 ± 1.4 | 45 (57) | 2.6 ± 1.2 | 41 (82) | 3.5 ± 1.4 | 198 (75) | 3.0 ± 1.4 |
| Difficulty breathing | 104 (77) | 2.9 ± 1.3 | 46 (58) | 2.6 ± 1.1 | 35 (70) | 3.1 ± 1.2 | 185 (70) | 2.8 ± 1.3 |
| Muscle weakness | 113 (84) | 2.8 ± 1.3 | 44 (56) | 2.8 ± 1.0 | 39 (78) | 2.6 ± 1.1 | 196 (74) | 2.7 ± 1.2 |
| Foot or wrist drop | 13 (10) | 2.8 ± 1.6 | 14 (18) | 2.9 ± 1.6 | 10 (20) | 3.2 ± 1.1 | 37 (14) | 2.9 ± 1.5 |
| Cancer | 10 (7) | 2.8 ± 1.2 | 7 (9) | 4.1 ± 1.2 | 2 (4) | 3.0 | 19 (7) | 3.3 ± 1.3 |
| Pain (overall) | 128 (95) | 2.7 ± 1.2 | 55 (70) | 2.5 ± 1.2 | 46 (92) | 2.9 ± 1.3 | 229 (87) | 2.7 ± 1.2 |
| Easy bruising | 94 (70) | 2.7 ± 1.2 | 53 (67) | 2.6 ± 1.3 | 40 (80) | 2.8 ± 1.3 | 187 (71) | 2.7 ± 1.2 |
| Dialysis | 14 (10) | 2.7 ± 1.6 | 3 (4) | 3.3 ± 1.5 | 3 (6) | 2.0 ± 1.0 | 20 (8) | 2.7 ± 1.5 |
| Blindness | 4 (3) | 2.8 ± 2.1 | 5 (6) | 3.0 ± 1.2 | 2 (4) | 5.0 ± 0.0 | 11 (4) | 3.3 ± 1.6 |
| Anxiety | 105 (78) | 2.6 ± 1.2 | 41 (52) | 2.3 ± 1.2 | 39 (78) | 2.4 ± 1.3 | 185 (70) | 2.5 ± 1.2 |
| Skin ulcers | 43 (32) | 2.5 ± 1.3 | 16 (20) | 2.4 ± 1.5 | 10 (20) | 2.7 ± 1.4 | 69 (26) | 2.5 ± 1.4 |
| Seizures | 6 (4) | 2.5 ± 1.0 | 21 (27) | 2.9 ± 1.7 | 0 | 0 | 27 (10) | 2.8 ± 1.5 |
| Numbness or tingling | 105 (78) | 2.4 ± 1.3 | 54 (68) | 2.8 ± 1.2 | 40 (80) | 2.8 ± 1.5 | 199 (75) | 2.6 ± 1.3 |
| Depression | 99 (73) | 2.4 ± 1.1 | 39 (49) | 2.1 ± 1.1 | 32 (64) | 2.4 ± 1.3 | 170 (64) | 2.3 ± 1.1 |
| Hearing loss | 78 (58) | 2.4 ± 1.2 | 35 (44) | 2.6 ± 1.1 | 33 (66) | 2.6 ± 1.4 | 146 (55) | 2.5 ± 1.2 |
| High blood pressure | 68 (50) | 2.4 ± 1.1 | 45 (57) | 2.5 ± 1.0 | 26 (52) | 2.7 ± 1.4 | 139 (53) | 2.5 ± 1.1 |
| Require oxygen therapy | 38 (28) | 2.4 ± 1.4 | 8 (10) | 2.3 ± 1.4 | 8 (16) | 2.1 ± 1.6 | 54 (20) | 2.4 ± 1.4 |
| Bone fractures | 16 (12) | 2.4 ± 1.3 | 11 (14) | 2.1 ± 1.0 | 12 (24) | 2.5 ± 1.4 | 39 (15) | 2.4 ± 1.3 |
| Ear ringing | 73 (54) | 2.3 ± 1.4 | 39 (49) | 2.7 ± 1.3 | 26 (52) | 2.9 ± 1.4 | 138 (52) | 2.5 ± 1.4 |
| Asthma | 33 (24) | 2.3 ± 1.2 | 19 (24) | 3.2 ± 1.0 | 18 (36) | 2.7 ± 1.4 | 70 (27) | 2.6 ± 1.2 |
| Diabetes mellitus | 20 (15) | 2.3 ± 1.2 | 10 (13) | 2.8 ± 1.0 | 4 (8) | 3.5 ± 1.3 | 34 (13) | 2.6 ± 1.2 |
| Abdominal pain | 56 (41) | 2.2 ± 1.3 | 27 (34) | 2.0 ± 1.1 | 18 (36) | 2.2 ± 1.1 | 101 (38) | 2.2 ± 1.2 |
| Muscle pain | 110 (81) | 2.1 ± 1.4 | 59 (75) | 3.0 ± 1.2 | 42 (84) | 2.6 ± 1.3 | 211 (80) | 2.7 ± 1.2 |
| Weight loss | 58 (43) | 2.1 ± 1.2 | 27 (34) | 1.7 ± 1.1 | 21 (42) | 2.8 ± 1.2 | 106 (40) | 2.2 ± 1.2 |
| Cataract | 41 (30) | 2.1 ± 1.2 | 14 (18) | 2.6 ± 1.1 | 11 (22) | 2.7 ± 1.3 | 66 (25) | 2.3 ± 1.2 |
| Loss of extremity | 3 (2) | 2.0 ± 1.0 | 0 | 0 | 2 (4) | 4.0 ± 1.4 | 5 (2) | 2.8 ± 1.5 |
| Angina, chest pain | 17 (13) | 1.9 ± 0.7 | 29 (37) | 2.1 ± 1.2 | 6 (12) | 2.0 ± 0.9 | 52 (20) | 2.0 ± 1.0 |
| Limited vision | 63 (47) | 1.8 ± 0.9 | 52 (66) | 2.3 ± 1.0 | 29 (58) | 2.1 ± 1.3 | 144 (55) | 2.1 ± 1.0 |
| Stomach ulcer | 8 (6) | 1.8 ± 0.9 | 9 (11) | 1.7 ± 0.7 | 3 (6) | 2.7 ± 1.2 | 20 (8) | 1.9 ± 0.9 |
Fatigue, energy loss, and nasal symptoms were rated as more severe in younger patients. Patients age <55 years (mean age) rated septum perforation with a mean of 3.8 and saddle nose deformity with a mean of 3.4, while patients age >55 years rated these manifestations 3.2 (P < 0.05) and 2.7 (P < 0.01), respectively. No significant differences between the vasculitis entities were observed besides the nasal and sinus symptoms (almost exclusively present in patients with WG).
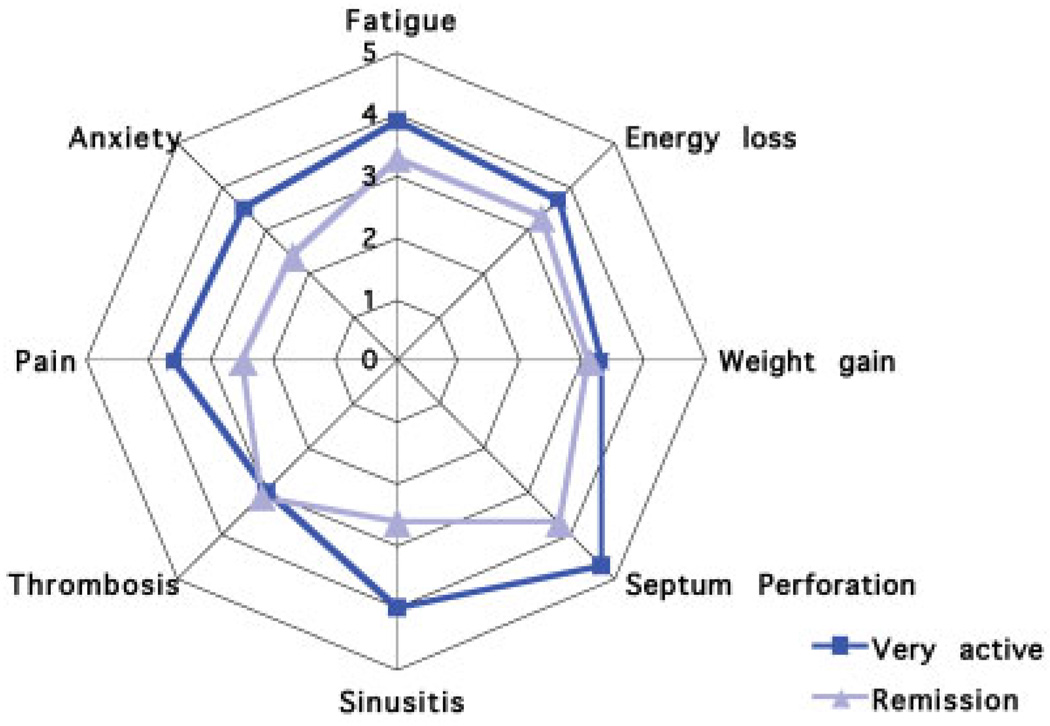
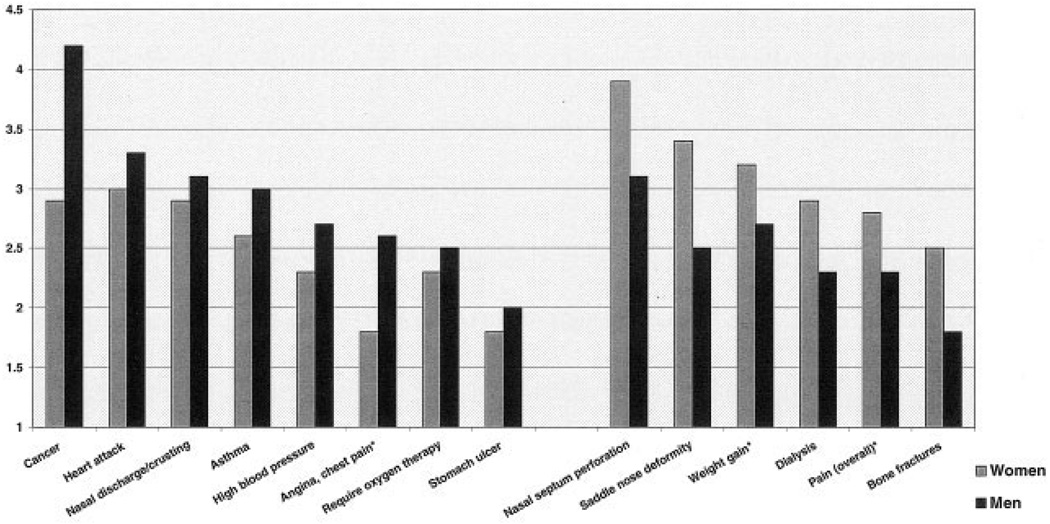
Patients from different nationalities rated their burdens similarly (Table 3). In general, patients in (self-declared) remission estimated their disease manifestations as less severe, with lower mean scores than patients who rated their disease as active or very active (Figure 1). Rating of many manifestations differed between women and men (Figure 2).
Table 3
Most frequent burdens of disease listed in the free-text section of the questionnaire
| Reported disease burden | US, no. (%) | Germany, no. (%) | UK, no. (%) | Combined cohort, no. (%) |
|---|---|---|---|---|
| Fatigue/energy loss | 83 (62) | 73 (91) | 42 (82) | 198 (75) |
| Pain | 52 (39) | 11 (14) | 20 (40) | 83 (31) |
| Musculoskeletal symptoms | 30 (23) | 21 (26) | 12 (24) | 63 (24) |
| Financial (work, insurance, costs) | 25 (19) | 5 (6) | 5 (10) | 35 (13) |
| Difficulty breathing | 23 (17) | 15 (19) | 13 (26) | 51 (19) |
| Anxiety | 13 (10) | 14 (18) | 23 (46) | 50 (19) |
| Treatment-related aspects | 18 (13) | 9 (11) | 17 (34) | 44 (17) |
| Nasal discharge | 17 (13) | 6 (9) | 14 (28) | 37 (14) |
| Weight gain | 15 (12) | 6 (9) | 5 (10) | 26 (10) |
The main results of the free-text data are shown in Table 3. In the free-text section, fatigue/energy loss, pain, and musculoskeletal symptoms were cited most frequently. Seventy-five percent of patients stated that fatigue and energy loss were both severe burdens of their vasculitis. Patients also commonly reported pain (31%) and musculoskeletal symptoms (24%) as burdens of disease. Financial aspects are more often mentioned by the American patients than the European patients (19% versus 6%). Additional frequently mentioned burdens are difficulty breathing (19%), anxiety (19%), treatment-related aspects (17%), nasal discharge (14%), and weight gain (10%).
DISCUSSION
This study of PROs among patients with vasculitis indicates that there are many manifestations of disease that are quite important to patients but not measured with the outcome instruments currently included in clinical trials of vasculitis. This exploration of the ratings that patients assign to various burdens of disease highlights the need for the full development and validation of a PRO instrument in vasculitis.
Improved treatment strategies have led to increased long-term survival among patients with systemic vasculitis and a shift in the frequencies of some forms of disease- and treatment-related damage and morbidity. Consequently, it becomes more important to detect subtle differences, differentiate disease states, and detect persisting functional limitations in the patients’ daily lives beyond chronic disease manifestations.
Fatigue, reduced energy level, and musculoskeletal symptoms were considered the most important burdens of disease by patients with vasculitis from 3 different nationalities. These problems are newly identified key domains in vasculitis. These symptoms are extremely common with systemic rheumatic diseases and other chronic illnesses (17,18), but are often not directly measured in clinical trials or are considered manifestations of the underlying disease. Additionally, several aspects of disease not covered by the list of physical and mental symptoms were commonly cited by patients, such as financial concerns, fear of work loss, and social limitations. The finding that vasculitis has a substantial negative impact on patients’ quality of life and economic situation is consistent with prior studies (9 –13). Patients also frequently mentioned and rated highly treatment-related complications that cause limitations such as infections and osteoporosis.
These data also indicate that both symptoms and items of damage rated highly by patients with vasculitis do not correlate with physician-estimated disease assessments (19,20). Patients in the current study ranked the more “subjective” manifestations (e.g., fatigue or pain) higher than the “objective” physician-defined measures that focus more on severe organ manifestations (e.g., renal failure or visual impairment). Physicians appear to focus on the physical findings, whereas patients’ judgments emphasize the psychological and social aspects of the disease. The measurement of burden of disease by patients and the assessment by physicians of disease activity and damage should complement each other. The observation that patient- and physician-reported burdens of disease in vasculitis differ considerably provides additional justification for the development of a validated PRO instrument. The inclusion of PROs is now standard research practice for multisystemic inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis (21–23).
HRQOL has been a focus in patient-related outcome research in several chronic diseases in the last 2 decades and has been identified as a key domain for the core set of outcome measures in vasculitis. Standardized, self-administered, and generic instruments provide data on physical, psychological, and social aspects of chronic illness, but do not address disease-specific issues, or allow the patient to contribute his or her observations. Many of the patient-reported burdens assessed in this study would have been missed by using available questionnaires. It therefore seems essential to implement a disease-specific questionnaire for patients with vasculitis to address this gap.
Adding standardized, validated PRO assessments to clinical studies in vasculitis would provide greater insight into the impact of the disease, and enable investigators to discriminate between side effects of treatment regimens and the natural course of the disease; this may aid the development of treatment strategies that improve both organ function and functional outcome simultaneously. In rheumatoid arthritis, PROs such as global assessments have better discriminant validity than most physician-assessed outcomes (24–26), suggesting that adding patient outcome assessment to the current measurements in vasculitis would improve outcome assessment and may optimize clinical decisions and the detection of treatment effects. Further research is needed to differentiate the impact of disease- versus treatment-related damage on the individual patient.
This study has several important strengths. The combined cohort sample size is large, especially for a study of a rare disease. Data were collected from cohorts in 3 countries from patients with a variety of vasculitides. Furthermore, the disease-related items rated by patients were identical to items routinely rated by physicians. Additionally, patients were given the option of listing items of importance that were not listed in the questionnaire; data from these free-text submissions were particularly valuable to investigators. The high level of completeness of forms by the participants is notable and indicates a high acceptability and practicability of the questionnaire.
The completion of the free-text section showed that patients listed a variety of non-physical dimensions that were not listed in the 40 items, including treatment-related issues and concerns about finances, employment, and insurance. Financial concerns were listed more often by the US patients than the European patients. Variations in the rating of symptoms by sex, disease status, and patient age should be considered in the development of PRO assessment instruments and can be assessed in more detail in focus groups and followup testing in different demographic study groups. Our finding that the results do not differ by nationality implies that an instrument assessing burdens of vasculitis would be applicable to patients in different countries.
The study also has some limitations. Selection bias may have occurred if only more motivated patients completed the questionnaire. It is also unclear to what extent participating patients were fully representative of all patients with vasculitis. Although it is likely that the diagnoses listed as having been confirmed by physicians were mostly accurate, diagnostic misclassification is possible. However, the lack of substantial differences in patient perspectives assessed in the cohorts from 3 countries provides reassurance regarding the generalizability of this study’s results. Conducting focus groups with patients with vasculitis could expand the understanding of patients’ burdens of disease, particularly of fatigue and energy loss.
These data provide strong evidence for the need for a PRO assessment tool in clinical trials in vasculitis. Further development of a fully-validated PRO instrument in vasculitis should take into consideration the need for a comprehensive item list, weighting of items, differences according to sex and age, and socioeconomic variances. Such an instrument would be an essential addition to the core set of domains in vasculitis assessment.
ACKNOWLEDGMENTS
The authors thank the many patients who participated in this study and who shared their experiences with vasculitis. We also thank the staff of the Vasculitis Foundation (online at: http://www.vasculitisfoundation.org) for their tremendous assistance in conducting this study.
The Vasculitis Clinical Research Consortium was supported by the NIH (grants U01-AR-1874, U54-RR-019497, and U54-AR-057319).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Herlyn had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Herlyn, Hellmich, Seo, Merkel.
Acquisition of data. Herlyn, Merkel.
Analysis and interpretation of data. Herlyn, Seo, Merkel.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1002/acr.20276
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3123033?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/169905969
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/acr.20276
Article citations
A systematic review of patient-reported outcome measures in patients with anti-neutrophil cytoplasmic antibody associated vasculitis.
Rheumatology (Oxford), 63(10):2624-2637, 01 Oct 2024
Cited by: 2 articles | PMID: 38310326 | PMCID: PMC11443012
Review Free full text in Europe PMC
Treatment goals in ANCA-associated vasculitis: defining success in a new era.
Front Immunol, 15:1409129, 13 Jun 2024
Cited by: 1 article | PMID: 38938575
Review
Enteric-coated Mycophenolate Sodium therApy versus cyclophosphamide for induction of Remission in Microscopic PolyAngiitis (EMSAR-MPA trial): study protocol for a randomised controlled trial.
BMJ Open, 14(3):e074662, 11 Mar 2024
Cited by: 0 articles | PMID: 38471694
Developing a disease-specific patient reported outcome measure to enhance understanding of the lived experiences of ANCA associated vasculitis: A protocol paper.
PLoS One, 19(3):e0298796, 07 Mar 2024
Cited by: 0 articles | PMID: 38451929 | PMCID: PMC10919579
Prospective study of complications and sequelae of glucocorticoid therapy in ANCA-associated vasculitis.
RMD Open, 10(1):e003956, 29 Feb 2024
Cited by: 0 articles | PMID: 38428978 | PMCID: PMC10910690
Go to all (55) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Renal vasculitis in Japan and the UK--are there differences in epidemiology and clinical phenotype?
Nephrol Dial Transplant, 23(12):3928-3931, 19 Jun 2008
Cited by: 59 articles | PMID: 18565978
Discordance of patient and physician health status concerns in systemic lupus erythematosus.
Lupus, 27(3):501-506, 01 Aug 2017
Cited by: 36 articles | PMID: 28764617
Survival and vasculitis activity in patients with end-stage renal disease due to Wegener's granulomatosis.
Nephrol Dial Transplant, 13(7):1713-1718, 01 Jul 1998
Cited by: 27 articles | PMID: 9681717
[Wegener's granulomatosis and microscopic polyangiitis].
Rev Prat, 58(5):522-532, 01 Mar 2008
Cited by: 4 articles | PMID: 18524109
Review
Funding
Funders who supported this work.
NCRR NIH HHS (2)
Grant ID: U54-RR-019497
Grant ID: U54 RR019497
NIAMS NIH HHS (6)
Grant ID: U01 AR051874
Grant ID: K24 AR002224
Grant ID: U01 AR055057
Grant ID: U54-AR-057319
Grant ID: U54 AR057319
Grant ID: U01-AR-1874