Abstract
Free full text

NOX ENZYMES IN ALLERGIC AIRWAY INFLAMMATION
Abstract
Chronic airway diseases such as asthma are linked to oxidative environmental factors and are commonly associated with increased production of reactive oxygen species (ROS). Therefore, it is commonly assumed that oxidative stress is an important contributing factor to asthma disease pathogenesis and that antioxidant strategies may be useful in treatment of asthma. A primary source of ROS production in biological systems is NADPH oxidase (NOX), originally associated primarily with inflammatory cells but currently widely appreciated as an important enzyme system in many cell types, which a wide array of functional properties ranging from antimicrobial host defense to immune regulation and cell proliferation, differentiation and apoptosis. Given the complex nature of asthma disease pathology, with the involvement of many lung cell types that all express NOX homologs, it is not surprising that the contributions of NOX-derived ROS to various aspects of asthma development and progression are highly diverse and multifactorial. It is the purpose of the present review to summarize the current knowledge with respect to the functional aspects of NOX enzymes in various pulmonary cell types, and to discuss their potential importance in asthma pathogenesis.
ASTHMA – A DISEASE ASSOCIATED WITH OXIDATIVE STRESS
Asthma is a chronic inflammatory disease of the airways, characterized by remodeling of the airways leading to enhanced airway hyperresponsiveness and increased mucus secretion. In addition to genetic factors, the occurrence and severity of asthma is determined by a variety of environmental factors, including allergens, bacterial or viral infection, physical stimuli such as exercise or cold air, or various airborne pollutants or occupational hazards, such as ozone, tobacco smoke, diesel particulates, isocyanates, etc. Because of the oxidative nature of most of these environmental pollutants, it is commonly thought that oxidative stress is an important contributing factor to asthma development and pathogenesis. Airway inflammation, a complex multi-cellular process that involves eosinophils, neutrophils, CD4+ T lymphocytes and mast cells, is also fundamental in asthma disease pathogenesis, and since many of these cell types are capable of production of reactive oxygen species (ROS), it is widely assumed that these endogenously produced ROS also contribute importantly to airway injury and disease pathogenesis. Analysis of several irreversible biomolecular oxidation markers that are diagnostic of oxidative chemistry by specific granulocytes (neutrophils, eosinophils) has indeed revealed the presence of increased levels of such stable oxidation markers within airway secretions or lung tissue sections from asthmatic subjects, often in correlation with the extent of ongoing inflammation and with the severity of clinical symptoms [1, 2]. While these findings clearly establish the presence of ongoing activation of neutrophils or eosinophils within the asthmatic airway, it has remained a matter of debate to what extent these ROS contribute to distinctive features of allergic airway inflammation. Indeed, direct evidence for an active contribution of specific oxidative events in asthma pathophysiology is still largely lacking, and attempts to curtail allergic inflammation or asthma symptoms by antioxidant supplementation strategies have so far been dissapointingly ineffective [3].
The concept of oxidative stress in asthma, and in inflammatory diseases in general, has been complicated by our growing appreciation of the diversity in biological sources of ROS production, with similarly diverse biological consequences. The NADPH oxidase system of activated granulocytes, which generates reactive oxygen species (ROS) as a key component of antimicrobial defense, is commonly considered the major source of oxidative stress during acute or chronic inflammation. However, with the discovery of other NADPH oxidase homologs over the past decade, and their presence in many diverse cell types and involvement in a broad range of physiological processes, ROS are increasingly appreciated as critical mediators in a broad range of cellular processes, such as cell proliferation, migration, differentiation, immunomodulation, and oxygen sensing, in virtually all aerobic organisms [4-7]. These actions are generally mediated by strictly controlled and localized production of ROS, which transmit signals through reversible oxidative modifications within specific target proteins, a process collectively known as redox signaling. Since different NOX isoforms are present within various different lung cell types that play key roles in asthma pathophysiology [8-11], it should not be surprising that NOX-derived ROS are not merely involved in pulmonary disease and injury, but may also have salutory roles in various aspects of lung biology and may even prevent injury associated with chronic inflammation. As a result, global antioxidant strategies or generic approaches to interfere with overall ROS production or NOX activation do not necessarily present effective therapeutic strategies to treat chronic diseases such as asthma, and development of more refined strategies based on selective targeting of NOX isoforms in specific cell types would be required. This review will discuss the general aspects of NOX biochemistry and biology, and summarize the current state of knowledge regarding their functional roles in within various cell types of the respiratory tract as well as their potential involvement in the development of allergic airway inflammation and remodeling.
GENERAL ASPECTS OF NADPH OXIDASES
The NOX family
Activated phagocytic cells produce ROS through assembly and activation of NADPH oxidase complex [12, 13], which comprises membrane-associated flavocytochrome b558 (gp91phox) and p22phox and various cytosolic cofactors (p47phox, p67phox, and p40phox, and the GTPase, Rac1), and mediates transmembrane electron transfer from the major cellular electron donor, NADPH, to reduce molecular O to superoxide anion (O2•−) and hydrogen peroxide (H2O2). A number of homologs of the main business end of NADPH oxidase, gp91phox, have been discovered, and mammalian systems are now known to contain seven NADPH oxidase (NOX) homologs, comprising NOX1-5 (NOX2 being the new name for gp91phox) and two larger Dual Oxidases, DUOX1 and DUOX2, which are widely expressed in many diverse cell types to regulate a variety of biological functions, including cell mitosis, differentiation, migration, and immune regulation. Similar to NOX2, activation of NOX1 and NOX3 also require association with p22phox, and assembly with Rac and cytosolic co-factors (p47phox and p67phox or their homologs, NOX organizer 1 (NOXO1) and NOX activator 1 (NOXA1)) [4, 5, 13]. NOX4 also requires p22phox, but appears to be constitutively active without the need for activation of other co-factors [5, 13]. NOX5 and DUOX1/2 differ from the other NOX homologs and contain additional intracellular Ca2+-binding EF-hand domain regions, and are primarily regulated by Ca2+ signaling, without the need for association with p22phox or other cytosolic co-factors [14-17]. The two DUOX proteins contain an additional extracellular peroxidase homology domain, with functional peroxidase activity in lower organisms but as yet unclear function in mammalian homologs [4, 5, 18]. The molecular biology and regulation of the various NOX/DUOX enzymes have been summarized in several excellent recent reviews [4, 5, 13, 19], and will not be further reviewed here.
General mechanisms of NOX-dependent signaling
The primary product of activated NOX/DUOX is superoxide anion (O2•−), which subsequently dismutates to hydrogen peroxide (H2O2), although DUOX1/2 and NOX4 appear to generate H2O2 without apparent intermediate production of O2•− [4, 15, 20]. Therefore, the biological actions of NOX/DUOX are thought to be mediated by either or O2•− or H2O2. In granulocytes, the primary target for NOX2-derived O2•−/H2O2 is a locally secreted heme peroxidase, such as neutrophil myeloperoxidase or eosinophil peroxidase, which catalyzes H2O2-mediated oxidation of various anionic substrates to generate antimicrobial oxidants [21]. Similar cooperative actions also exist between DUOX proteins and locally secreted heme peroxidases within the thyroid gland or at mucosal surfaces within the respiratory or gastrointestinal tract, with DUOX-derived H2O2 activating thyroperoxidase to promote the synthesis of thyroid hormone [22] or lactoperoxidase (LPO) to produce antimicrobial oxidants within the airway or intestinal lumen [4, 23, 24]. In most cases, however, the biological actions of NOX-derived O2•−/H2O2 are related to interactions with other target proteins at redox-sensitive metal centers or cysteine residues.
Collective studies towards the diverse biological actions of NOX-derived ROS indicate that these are largely related to modulation of a limited set of broadly used signaling mechanisms, primarily protein tyrosine phosphorylation and intracellular Ca2+ signaling. Following pioneering studies by Finkel and Rhee [25, 26], who first indicated a relationship between oxidant signaling and tyrosine phosphorylation through transient inactivation of a protein tyrosine phosphatase (PTP) by reversible oxidation of its invariant catalytic cysteine residue, a range of PTPs or other cysteine-containing phosphatases have since been identified as the oxidant target in NOX/DUOX-dependent cytokine and/or growth factor signaling [6, 27-30]. NOX/DUOX activation also appears to be intimately associated with Ca2+-mediated signaling, by oxidative cysteine modifications within Ca2+ channels [31, 32] or by increasing voltage-dependent Ca2+ channel opening [33, 34]. Since Ca2+-mobilizing stimuli are capable of activating various NOX enzymes, the ability of NOX/DUOX activation to regulate Ca2+ signaling is thought to represent a positive feedback mechanism allowing for rapid signal amplification in discreet cellular regions.
Specificity in oxidative signaling is ensured by strict spatial localization of NOX activation in association with the cytoskeleton [35, 36] or membrane rafts [37-39] and by direct interactions with locally present oxidant-sensitive proteins targets [6, 39, 40], which allows for effective oxidative signaling within discrete cellular regions in spite of the abundant presence of cytosolic antioxidant systems (Cu/Zn SOD, GSH peroxidase). In this regard, ROS can have opposing effects on certain biological processes depending on the location or extent of ROS production. For example, while a number of studies have demonstrated the ability of NOX enzymes to activate inflammatory signaling by promoting the activation of nuclear factor (NF)-κB [41, 42], several steps within the NF-κB pathway are also subject to inhibition by oxidative modification of critical protein cysteine residues [27]. Similarly, NOX-derived ROS production has also been intimately linked to regulation of matrix metalloproteinases MMPs), which often involve stimulatory effects on MMP expression or direct oxidative MMP activation (e.g. [28]), but inhibitory effects by elevated ROS production [43].
Although it is commonly assumed that the biological actions of NOX are related to ROS production, an aspect of NOX activation that is often overlooked is the fact that NOX activation is electrogenic and induces membrane depolarization resulting in compensatory activation of various ion channels. Oxidation of NADPH by NOX/DUOX activation also results in localized intracellular acidification, which promotes the activation of voltage-gated H+ channels, Na+/H+ exchangers (NHE) and/or alternative ion channels. Various lines of evidence indicate that these ROS-independent actions contribute importantly to the biological activity of NOX enzymes [44, 45]. For example, NOX2-mediated antimicrobial activity in phagocytes has been demonstrated to involve ROS-independent mechanisms that are related to activation of K+ or H+ channels within the phagosomal membrane to regulate the intraphagosomal activity of secreted proteases [46].
NOX ENZYMES IN LUNG CELL BIOLOGY AND ASTHMA PATHOLOGY
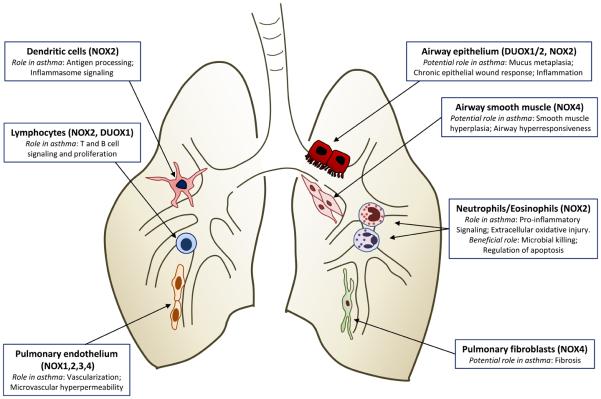
Sensitization and progression towards asthma is influenced by a delicate balance between the airway epithelium, innate immune cells and the induction of adaptive immunity [47, 48]. Interactions between airway epithelial cells and dendritic cells (DCs) are instrumental in recognition of allergens and the ability to mount appropriate responses to them, and alterations in epithelial barrier function or epithelial/DC interactions by exogenous stimuli, such as bacterial or viral infection or airborne pollutants or particulates, impact importantly on antigen presentation to DCs and on the overall innate and adaptive immune responses. Alveolar macrophages, the predominant immune effector cells within the alveolar space and conducting airways, can also impact on allergic inflammation by regulating DC function and adaptive immune responses [49]. The collective activation of innate and adaptive immune responses results in the variable recruitment and activation of effector cells, such as eosinophils and neutrophils, which are critical in host defense against pathogens but also contribute importantly to asthma pathology [50, 51]. Since each of these critical cell types express specific isoforms of NOX with specialized cell functions, activation of NOX and production of ROS in these different cell types is expected to have diverse implications for asthma pathophysiology (Table I). Moreover, NOX enzymes in other structural lung cell types, such as smooth muscle cells or fibroblasts, may play additional roles in cell proliferation and differentiation which are of importance in the context of airway remodeling and altered airway physiology. The following sections will summarize our current understanding with respect to the functional aspects of NOX enzymes in these various lung cell types and their potential relevance for asthma pathophysiology.
TABLE I
Biological and pathological aspects of NOX/DUOX enzymes within various lung cell types.
| Cell type | NOX | Functional role | Ref. |
|---|---|---|---|
| Airway/alveolar epithelium | DUOX1 | Inflammatory mediator production | [75, 76] |
| Cell migration and epithelial repair | [57, 87] | ||
| Acid secretion | [74] | ||
| DUOX2 | Antimicrobial host defense | [55, 58] | |
| NOX2 | Antiviral defense | [100] | |
| NOX4 | Inflammatory mediator production | [103] | |
| Neutrophils/eosinophils | NOX2 | Bacterial killing | [73] |
| Regulation of apoptotic clearance | [114] | ||
| Pro-inflammatory signaling | [107] | ||
| Macrophages/dendritic cells | NOX2 | Bacterial killing | [116] |
| Antigen processing | [120, 122] | ||
| Inflammasome activation | [124, 126] | ||
| T and B lymphocytes | NOX2 | T cell receptor signaling | [130] |
| DUOX1 | Cytokine regulation | [34, 132] | |
| Pulmonary endothelium | NOX1 | Hyperoxia-induced apoptosis | [104] |
| NOX2 | Barrier loss upon hyperoxia | [141] | |
| Proliferation after ischemia | [142] | ||
| NOX3 | Elastase activation and emphysema | [143] | |
| Smooth muscle cells | NOX4 | Proliferation, pulmonary hypertension | [11, 150] |
| Pulmonary fibroblasts | NOX4 | Myofibroblast differentiation, fibrosis | [151, 152] |
NOX in the Respiratory Epithelium
The respiratory epithelium represents an intimate cell layer connected by tight junctions and adherens junctions, that acts as a physical barrier against airborne pollutants and microorganisms and a molecular sieve that excludes inhaled antigens and pathogens. Disruptions in these barrier functions by environmental stressors, certain infections, or by allergens (e.g. Der p 1 allergen of house-dust mite) are important in enhancing antigen presentation to DCs and initiation of adaptive immune responses. The epithelium also impacts on inflammatory processes by mediating acute responses to luminal triggers, such as allergens and infections, and by pathogenic events occurring as a consequence of repeated epithelial damage-repair responses. The airway epithelium also coordinates transepithelial migration of leukocytes into the airway lumen, a critical process in regulation of inflammatory cell homeostasis. Based on these various important functional properties, the respiratory epithelium plays a central role in regulating inflammatory responses to commonly inhaled allergens and controlling airway responses to inhaled pathogens as well as allergic sensitization [47, 48]. Because airway and alveolar epithelial cells have long been known to be capable of ROS production in response to cell stimulation [52], epithelial ROS production may also be of importance in regulating asthma pathogenesis.
DUOX1/2 in Airway Host Defense
Analysis of NOX transcripts revealed the expression of several NOX isoforms and their co-factors in airway and alveolar epithelial cells [53, 54], but DUOX1 and DUOX2 were identified as the primary source of apical H2O2 production by airway or alveolar epithelia [9, 55-58]. In situ hybridization and immunohistochemical analysis revealed the presence of DUOX1 at the apical surface of tracheobronchial and alveolar epithelial cells, with DUOX2 largely localizing to salivary and submucosal glands [9, 53, 59-61]. DUOX1 and DUOX2 are co-expressed with their respective maturation factors, DUOXA1 and DUOXA2 [62-64], which determine DUOX1/2 subcellular localization and activation [65, 66]. Mature DUOX proteins are highly homologous N-glycosylated proteins (with 83% homology) localized to the plasma membrane, although substantial amounts of DUOX protein are also found intracellularly, presumably in association with the ER [22, 67]. Based on their anatomical location and involvement in apical H2O2 production, is was suggested that epithelial DUOX participates in innate host defense, which was first demonstrated in studies in Drosophila or zebrafish in which silencing of their single DUOX gene was found to increase gut infection [68, 69]. Localization of DUOX2 along the entire intestinal tract in mammalian systems suggests a similar mucosal host defense function [24, 70]. Analogously, DUOX proteins were also proposed to contribute to airway or alveolar mucosal host defense [4, 61], and studies with rat tracheal epithelial cells directly implicated a major role for DUOX2 in this regard [55]. This antimicrobial property relies on H2O2-dependent activation of secreted lactoperoxidase (LPO) to form a functional antimicrobial system [23, 55, 71, 72]. Consistent with such a host defense function of DUOX2 are observations that DUOX2 expression is strongly induced by various bacterial and viral stimuli [58, 59].
The importance of the major airway DUOX isoform, DUOX1, in airway innate host defense is not yet directly proven, but its ability to promote H+ secretion and acidification of the airway surface, due to its electrogenic properties, may represent an additional non-oxidative antimicrobial property similar to the electrogenic actions of phagocytic NOX [44, 73, 74]. In addition to these direct antimicrobial properties associated with DUOX activation, airway epithelial DUOX1 activation has also been shown to promote additional innate immune responses to airborne pathogens and environmental stimuli in response to activation of various TLR isoforms, and mediates the expression of various inflammatory mediators, such as the neutrophil chemokine interleukin (IL)-8 (CXCL8) [75-77], mucin genes (e.g. MUC1 and MUC5AC) [78, 79], matrix metalloproteinase-9 [57], and vascular endothelial growth factor (VEGF) [76]. These actions of DUOX1 are related to activation of intracellular signaling pathways involving extracellular signal-regulated kinases (ERK1/2) and/or nuclear factor (NF)-κB [57, 75-79], initiated by liberation of epidermal growth factor receptor (EGFR) ligands by activated cell surface sheddases such as TNFα-converting enzyme (TACE; ADAM17), a member of the a disintegrin and metalloproteinase (ADAM) family of metzincin metalloproteinases. The direct oxidant-sensitive target(s) involved in DUOX1-dependent EGFR signaling has (have) not yet been identified [76, 77]. A recent yeast two-hybrid screen identified an EF-hand binding protein (EFP1), which contains two thioredoxin domains, as a DUOX1-associated protein that may be involved in redox signaling [80], but its importance for airway epithelial biology is still unknown. Intriguingly, a recent report indicated activation of ADAM17 and TNFα-receptor shedding by double-stranded RNA through TLR3 activation, mediated by intermediate activation of DUOX2 rather than DUOX1 [81].
Activation of both DUOX enzymes typically involves Ca2+-mobilization [15, 22, 60, 82], but may also be mediated by phosphorylation by protein kinase C (PKC) and/or protein kinase A (PKA) [83]. One important mechanism of DUOX activation during cell activation or injury is the cellular release of ATP [57, 60, 77, 84], which can stimulate Ca2+ signaling and PKC activation by stimulation of purinergic P2Y receptors on the epithelial surface [28, 85]. However, other mechanisms may contribute to DUOX activation by bacterial infection as well, perhaps by yet unidentified TLR-mediated mechanisms [86]. Recently, intracellular NOD-like receptors have been related to DUOX2 activation in intestinal epithelial cells in response to certain infectious stimuli [70].
Role of DUOX in epithelial homeostasis
In addition to the various host defense properties discussed above, initial studies by us and others have demonstrated that airway epithelial DUOX1 also participates in wound responses to promote maintenance of epithelial integrity and barrier function. Studies using in vitro wound models in cultured human tracheobronchial epithelial cells demonstrated an important contribution of DUOX1 in wound responses, by promoting cell migration and wound closure through activation of EGFR/ERK signaling [57, 87]. By developing isoform-specific antibodies, Knaus and co-workers reported that both DUOX1 and DUOX2 localize to the leading edge of migrating cells, consistent with a role in cell motility and wound healing [65]. The in vivo importance of DUOX in wound repair responses was elegantly demonstrated in studies in zebrafish, in which DUOX was linked to ROS-dependent recruitment of leukocytes to the wounded tail-fin epithelial area [88]. Although this was attributed to direct leukocyte chemoattractant properties of H2O2, the involvement of human epithelial DUOX1 in production of the chemokine IL-8 illustrates an alternative mechanism of neutrophil recruitment as an additional feature of the overall epithelial wound response. Studies in Drosophila have shown that DUOX knockdown led to reduced and/or delayed JAK-STAT signaling in response to bacterial infection, an important signaling mechanism involved in stem cell signaling and epithelial renewal [89, 90]. Airway epithelial or alveolar DUOX1 expression is markedly associated with cell differentiation and are most prominent in fully differentiated epithelia [58, 59, 61, 91], further supporting its importance in epithelial homeostasis.
Collectively, these various observations indicate that DUOX plays an important role in mucosal immunity by mediating both direct antimicrobial activity as well as by regulating integrated epithelial responses that contribute to epithelial immunity and maintenance of epithelial integrity. While these functions appear to be associated to a single DUOX gene in Drosophila or zebrafish, the apparent gene duplication in mammalian systems suggests that these functions may be distributed between two DUOX homologs that are more independently regulated to support specialized functions in innate and adaptive immunity. This concept is supported by findings that DUOX1 and DUOX2 are subject to highly different transcriptional regulation [64], with relative constitutive airway epithelial expression of DUOX1, which is inducible by Th2 cytokines such as interleukin (IL)-4 and IL-13 [59], and strong induction of DUOX2 by viral or bacterial stimuli and by the Th1 cytokine IFN-γ [58, 59, 91, 92]. Moreover, in spite of the fact that DUOX1 may be the most abundant isoform within healthy airway epithelia, DUOX2 is thought to be the major isoform responsible for basal apical H2O2 production, while DUOX1 is primarily responsible for H2O2 production by exogenous stimuli [58]. In this regard, these various aspects of the different DUOX proteins are comparable to the nitric oxide synthases (NOS), of which the more constitutively expressed isoforms, NOS1 and NOS3, are primarily involved in homeostatic signaling whereas the high-output inducible NOS2 isoform is more important in innate host defense. By analogy, DUOX1 is most likely more important in epithelial homeostasis, whereas DUOX2 may be more important in specific conditions of infection.
Potential role of epithelial NOX/DUOX in asthma physiology
To date, very little is known to date regarding the potential contribution of DUOX1/2 to pulmonary disease [28], but various recent findings suggest a potential contribution of DUOX1 in asthma pathophysiology. As mentioned, epithelial DUOX1 is induced by the Th2 cytokines IL-4 and IL-13 [57, 59], which are commonly elevated within asthmatic airways. Our preliminary studies indeed indicate induction of DUOX1 in lung tissues from mice subjected to experimental allergic asthma (unpublished results). Moreover, several cardinal features of asthmatic airways can be associated with DUOX1 activation (Fig. 1). These include: increased production of EGFR ligands and increased EGFR activation [93], enhanced production of mucus and other inflammatory mediators (including IL-8 or MMP-9) [48, 94], and increased airway acidification [95]. Recent evidence also suggests an important role for extracellular ATP in asthma physiology [96]. Although this was primarily attributed to a role for ATP in dendritic cell maturation and activation, the potential involvement of ATP-mediated epithelial DUOX activation cannot be ruled out. Given the postulated role for DUOX1 in epithelial wound responses, increased expression and persistent activation of epithelial DUOX1 may also contribute to the proposed scenario of a chronic epithelial wound response as a central mediator of asthma development and airway remodeling [48]. However, no direct evidence is yet available with regard to altered DUOX1 expression or activation in asthmatic subjects. Some recent reports indicate reduced expression of DUOX1 in bronchial biopsies from smokers or patients with COPD [97, 98] and reduced DUOX2 expression in the lungs of patients with severe cystic fibrosis [99], which may contribute to reduced airway host defense and epithelial homeostasis in these subjects.
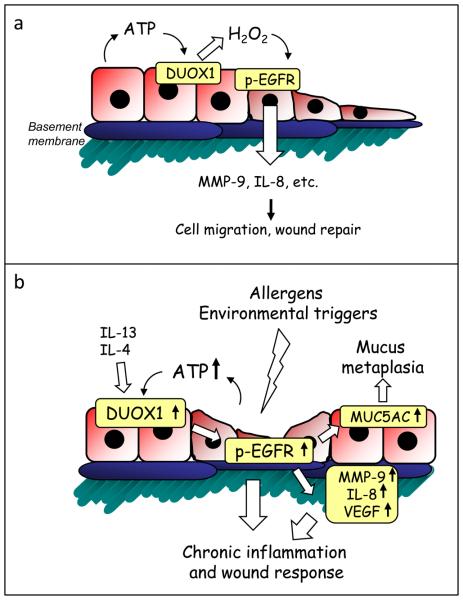
Illustration of suggested properties of epithelial DUOX in epithelial homeostasis and chronic inflammation and remodeling in asthma. (a) Release of ATP as a result of epithelial injury activates DUOX and subsequent EGFR to activate various genes involved in epithelial wound repair. (b) Induction of DUOX1 by Th2 cytokines (IL-13, IL-4) and augmented ATP release are expected to lead to amplified EGFR activation and dysregulated wound responses in the asthmatic airway, as a critical mediator of airway remodeling.
Other epithelial NOX's
In addition to DUOX, other NOX isoforms are expressed in airway and alveolar epithelia and appear to contribute to airway or alveolar epithelial responses to viral infection or environmental stress. For example, antiviral responses to respiratory syncytial virus (RSV) are associated with activation of RIG-1 helicase and NF-κB, which was found to involve the intermediate activation of NOX2 [100, 101]. Moreover, epithelial responses to oxidative stress or diesel exhaust particles have been linked to upregulation of NOX4, which contributes to regulating epithelial signaling pathways that promote the production of MUC5AC or MMP-1 [102, 103]. In light of the important associations between viral infections and environmental pollution and asthma, these findings might suggest a contribution of these epithelial NOX isoforms as well, but this has not been addressed to date. Recent studies using knockout mice have indicated a role for NOX1, but not NOX2, in alveolar injury during hyperoxia [104]. NOX1 expression was found to be induced within alveolar epithelial and endothelial cells in response to hyperoxia, and promotes epithelial and endothelial apoptosis. However, whether epithelial NOX1 also plays a role in asthma is not known.
NOX in Myeloid and Lymphoid Cells
Perhaps the major source of ROS during allergic inflammation is the activation of myeloid cell types, such as eosinophils and neutrophils, which generate ROS by their NADPH oxidase (NOX2) systems. As mentioned earlier, eosinophils play important roles in the pathophysiology of asthma and exacerbations [50, 105], but neutrophils are usually the first cells to be recruited to the site of the allergic reaction, and their presence may influence clinical presentation and has been linked to the development of severe chronic asthma and sudden severe attacks [105, 106]. Based on observations of stable oxidation markers within the airways of asthmatics, representative of neutrophil- and eosinophil-derived ROS, in association with the degree of allergic inflammation and other asthma symptoms [1, 2], it is popular belief that ROS production by these myeloid cells contributes to asthma development. However, as can be judged from the following sections, the molecular actions of hematopoietic cell NOX-derived ROS are multifactorial and their contribution to overall asthma pathology may be variable.
Oxidative killing and immunomodulation by myeloid cells
A well recognized function of NOX2 in granulocytes is its host defense function, by producing intraphagosomal ROS to kill ingested microorganisms by oxidative mechanisms. In addition, NOX2-dependent microbial killing is also mediated by its role in regulating phagosomal pH or K+ to control the activation of secreted proteolytic enzymes. Moreover, the activation of neutrophil NOX2 in response to e.g. TLR or Fcγ receptor activation has also been associated with intracellular signaling pathways that modulate and inflammatory gene regulation through the activation of transcription factors such as NF-κB and AP-1 [107]. The involvement of NOX2-derived ROS in these signaling events is thought to include the oxidative inhibition of tyrosine phosphatases, such as CD45 and the SH2 domain-containing PTP, SHP-1, resulting in enhanced activation of tyrosine kinases such as Syk, Hck, Lyn, Fgr, Yes, and Btk, and downstream signaling [107]. Thus, myeloid-derived ROS may contribute to disease by inducing direct oxidative injury or by promoting pro-inflammatory signaling pathways.
In addition to these pro-inflammatory actions, NOX2-derived ROS are also important in regulating of neutrophil/eosinophil apoptosis and thereby contribute to resolution of inflammation. Indeed, neutrophils from patients with CGD (which lack functional NADPH oxidase) can undergo normal constitutive apoptosis, but are defective in ROS-dependent oxidation of phospholipids that promotes externalization of phosphatidylserine, a critical event in the phagocytic clearance of apoptotic neutrophils [108, 109]. Accordingly, patients with CGD often suffer from hyperinflammatory lesions that are unrelated to infection, although no clear association has been found between CGD and atopic diseases such as asthma [110, 111]. Studies in NOX2-deficient mice are consistent with this notion, and show enhanced and prolonged inflammatory responses to Aspergillus fumigatus [112], branched fungal β-glucan [113], zymosan or LPS [114] or acid inspiration [115], often with persistent inflammatory foci containing abundant apoptotic and necrotic cells. Restoration of NADPH oxidase in bone marrow-derived cells was able to restore the normal inflammatory response, presumably related to oxidative activation of Nrf2 as a mechanism to terminate inflammation [114]. These observations suggest that inhibition of NOX2 within these cell types is not necessarily beneficial in chronic inflammatory diseases such as asthma, but may in fact contribute to chronic inflammation and worsen disease outcome.
Macrophages/dendritic cells
Macrophages and dendritic cells (DCs) also express NOX2, in which it may play similar direct antimicrobial properties, as well as phagosomal recruitment of the autophagy protein LC3, indicating an oxidative mechanism in antibacterial autophagy [116]. Production of ROS by NOX2 in macrophages or DCs is considerably less robust than in neutrophils or eosinophils, suggesting roles of NOX2 in other aspects of macrophage biology rather than direct microbial killing. Indeed, activation of macrophage NOX2 also coordinates production of pro-inflammatory cytokines in response to e.g. TLR activation, which has been linked to regulation of NF-κB and AP-1 signaling by oxidative inactivation of PTPs such as PTP-1B and PTEN [117-119]. Another important aspect of NOX2 activation in macrophages or DCs is its critical involvement in antigen processing and cross-presentation, which is mediated by oxidative inactivation of cysteine cathepsins [120] and by intraphagosomal pH regulation [121-123], key mechanisms involved in proteolytic control within the phagosome.
Macrophage/DC activation also leads to production of the important pro-inflammatory cytokines, IL-1β and IL-18, which is mediated by activation of the so-called inflammasome, a signaling complex that is activated in response to various damage-associated “danger-signaling” molecules, including uric acid, extracellular ATP, and environmental particulates [124]. Indeed, NOX2 activation by ATP-mediated P2X7 receptor stimulation was found to promote ROS production in LPS-primed macrophages [125]. Accordingly, several pharmacological NADPH oxidase inhibitors or shRNA knockdown of p22phox, an essential NOX2 cofactor, were found to inhibit inflammasome activation. However, the role of NOX2 in inflammasome activation is controversial, and recent studies with macrophages from CGD patients indicated no defect in inflammasome activation, and in fact indicated exaggerated processing and secretion of IL-1β, suggesting inhibitory actions of NOX2 on inflammasome activation [126, 127], presumably related to oxidative inactivation of caspase-1 [128].
NOX in lymphocyte signaling
Initial observations that H2O2 can mimic antigen receptor stimulation, by negatively regulating the SH2 domain-containing PTPs, SHP-1 and SHP-2, suggested the potential involvement of NADPH oxidase in antigen-dependent signaling in T and B lymphocytes [129]. Indeed, T cells were found to express essential NADPH oxidase (NOX2) components, which contribute to oxidant production and signaling upon T cell receptor (TCR) activation, thereby regulating inflammatory cytokine production and T cell adhesion [130, 131]. More recently, Ca2+-dependent activation of DUOX1 was found to contribute to TCR signaling in CD4+ T cells, which promotes oxidative inactivation of SHP-2 and activation of the tyrosine kinase Lck, as a potential positive feedback loop to sustain TCR activation [132]. Similarly, antigen receptor stimulation in B cells has been found to involve the activation of NADPH oxidase, apparently to serve similar amplification of BCR signaling, through inactivation of the tyrosine phosphatase SHP-1 and increased activation of the tyrosine kinase Syk [129], and more recent studies suggest that this may also involve DUOX1 [34].
Potential implications for asthma
In aggregate, it follows from the above that NOX homologs play diverse roles in phagocytic function, antigen processing and presentation, and T or B cell activation and expansion, which would suggest that selective inhibition of these NOX enzymes might minimize the development of allergic inflammatory responses and be potentially beneficial in allergic asthma. For example, the pro-inflammatory action of granulocyte NOX2 may contribute to exacerbations in asthma that are associated with increased neutrophilia. Likewise, the recently established role of eosinophils as early responders and regulators of innate and adaptive immune responses during allergic inflammation [50], might also involve a contribution of NOX2-dependent pro-inflammatory signaling, although this possibility has not yet been tested. Conversely, the involvement of NOX2 in phagocyte apoptosis and clearance and in termination of inflammation would imply that inhibition of NADPH oxidase activity might also have adverse consequences. Indeed, studies using gp91phox−/− or p47phox−/− mice demonstrated that genetic NADPH oxidase deficiency was in many cases associated with enhanced inflammation and injury [43, 112, 115, 133]. Surprisingly, no reports appear to exist that address the importance of gp91phox (NOX2) or p47phox deficiency in models of asthma. The only demonstrated direct involvement of NOX2 in allergic asthma comes from a study using chimera of NOX2-deficient mice, in which NOX2 was absent only in non-hematopoietic cell types, which showed that only non-hematopoietic NOX2 contributes to development of airways eosinophilia, indicating a role for NOX2 within structural cell types rather than inflammatory cells [134].
NOX in Other Lung Cell Types
NOX in pulmonary endothelium
Vascular endothelial cells express various NOX isoforms (NOX1, NOX2, and NOX4) that can control several vascular processes, such as vascular tone, vascular cell growth and angiogenesis, and inflammation [135, 136]. Accordingly, pulmonary endothelial cells have been demonstrated to generate NADPH-derived oxidants in response to pulmonary ischemia [137, 138] as well as hyperoxia [104, 139]. Endothelial NOX activation in response to hyperoxia was found to occur in association with the actin cytoskeleton via the activation of the non-receptor tyrosine kinase Src, and has been suggested to contribute to decreased alveolar-capillary barrier function as a mechanism of hyperoxia-induced lung injury [140, 141]. Pulmonary endothelial cell depolarization during ischemia/hypoxia results in activation of NOX2, which promotes ROS-mediated endothelial proliferation [142]. Induction of NOX3 in pulmonary endothelial cells was observed in relation to features of pulmonary emphysema due to genetic TLR4 deficiency, and NOX3 was found to actively contribute to ROS generation and elastolytic activity in this model [143]. Through these various actions, NOX enzymes might contribute to increased airway vascularization and increased microvascular hyperpermeability that are commonly observed in asthmatic subjects [144]. However, the significance of these various endothelial NOX isoforms for asthma pathology has not yet been addressed, although the contribution of non-hematopoietic NOX2 to allergic inflammation [134] might point to a role for endothelial NOX2.
Pulmonary smooth muscle cells and fibroblasts
Pulmonary fibroblasts and smooth muscle cells express NOX4 and several NOX cofactors (p47phox, p67phox, p22phox) and respond to inflammatory mediators, such as tumor necrosis factor α (TNF-α) or transforming growth factor β (TGF-β), with increased production of ROS which is primary associated with selective upregulation of NOX4 [145-148]. Expression of NOX4 expression in smooth muscle cells is closely associated with smooth muscle differentiation markers such as smooth muscle α-actin (SMA) or myosin heavy chain [146, 149], and studies with NOX4-targeted siRNA demonstrated that NOX4 is required for the expression of smooth muscle differentiation markers, and maintenance of SMA-based stress fibers [149]. Induction of NOX4-mediated ROS production in human pulmonary artery smooth muscle cells by TGF-β mediates smooth muscle cell proliferation and hypertrophy, which is related to activation of ERK1/2 signaling and phosphorylation of nuclear or ER proteins, such as retinoblastoma protein and eukaryotic translation initiation factor 4E binding protein-1 [146, 150]. Analysis of lung tissues from patients with pulmonary arterial hypertension revealed a ~2.5-fold upregulation of NOX4 [11], which may contribute to pulmonary hypertension by promoting ROS-mediated smooth muscle cell proliferation. Likewise, TGFβ –mediated NOX4 induction in pulmonary fibroblasts or smooth muscle cells also suggest its importance in pulmonary fibrosis or airway remodeling during chronic lung diseases such as asthma [147, 150]. Indeed, TGF- β is recognized as a major contributing factor to development of pulmonary fibrosis by promoting differentiation of fibroblasts to pathological myofibroblasts. Myofibroblast differentiation is associated with increased NOX4 expression, and pulmonary fibroblast from patients with idiopathic pulmonary fibrosis (IPF) have been found to express increased amounts of NOX4 [151, 152]. Using pharmacological or genetic approaches it was determined that NOX-4 actively contributes to fibrogenesis in murine models of lung fibrosis, and is a critical mediator of the myofibroblast phenotype in fibrosis [151, 152]. In addition to promoting extracellular H2O2 production in response to TGF-β1, which may induce apoptotic cell death in neighboring pulmonary epithelial cells [147], NOX-4-dependent generation of H2O2 is also required for TGF-β-induced myofibroblast differentiation, which increased expression of SMA and extracellular matrix production [151].
FINAL THOUGHTS
It is clear from this overview that diverse expression of various NOX isoforms in different cell types within the respiratory tract, and their highly diverse functions in lung cell growth and differentiation and other specialized functions in innate or adaptive immunity, indicates the great complexity with respect the biological actions of ROS and their contributions to multifactorial lung disorders such as asthma. Increased diversity of NOX isoforms during mammalian evolution appears to have coincided with increased biologic complexity [153], and with the appearance of larger organisms that depend on aerobic metabolism with co-development of respiratory and cardiovascular systems to enable effective transport of atmospheric O2 to internal organs. Moreover, the various NOX isoforms appear to have evolved to exploit the chemical properties of O2 and ROS in the regulation of a wide range of biological processes throughout the organism. As such, the various NOX enzymes have been found to serve important physiologic functions, often related to tissue development and innate immunity, although their inappropriate activation may also contribute to disease pathogenesis. These detrimental properties of NOX are most commonly found in chronic disorders that are typically associated with ageing, such as cardiovascular disorders [154], various cancers [155] or chronic pulmonary diseases [143, 156, 157]. These dichotomous roles of NOX are an example of antagonistic pleiotropy, which allows for the development and maintenance of potentially harmful ROS-producing NOX genes during evolution because they confer early survival advantages due to their importance in e.g. host defense [158]. Nevertheless, as discussed in this review, the contributions of various NOX isoforms in chronic lung diseases such as asthma are likely to be highly diverse, because of their variable roles in immune regulation and tissue repair and remodeling. The potential contributions of cell-specific NOX isozymes to various pathophysiologic hallmarks of asthma are schematically illustrated in Fig. 2. Because of the complex and multifaceted nature of asthma, with variable contributions of inflammation and remodeling determining the overall disease pathology, global approaches to inhibit NOX activation or ROS may not be equally effective in each case.
Over the past several years, our basic understanding of various NOX enzymes has increased dramatically and development of genetically engineered mice that lack specific NOX isoforms have helped clarify some of their biological roles or contributions to disease. Future studies on the physiological roles of specific NOX isoforms in the lung will provide important insights into their pathologic roles in pulmonary disease and opportunities for therapeutic targeting. With cell specific deletion of specific NOX enzymes, using conditionally inducible tissue-selective expression systems, the specific importance of specific cellular NOX enzymes in disease development can be more definitively assessed. However, since animal models do not always faithfully recapitulate complex human diseases, and further evaluation of potential alterations in expression, activation, or tissue localization of NOX enzymes in association with disease status in patients is critically needed, as our current understanding of alterations in NOX expression or activity in human disease is incomplete. Such efforts, and the continued development of selective pharmacological inhibitors targeted at specific NOX isozymes [159], may lead to useful additional therapeutic approaches to help treat and manage asthma in the future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.bbagen.2011.03.004
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3139819?pdf=render
Citations & impact
Impact metrics
Article citations
Redox Pathogenesis in Rheumatic Diseases.
ACR Open Rheumatol, 6(6):334-346, 25 Apr 2024
Cited by: 1 article | PMID: 38664977
Review
Diet-induced obesity worsens allergen-induced type 2/type 17 inflammation in airways by enhancing DUOX1 activation.
Am J Physiol Lung Cell Mol Physiol, 324(2):L228-L242, 10 Jan 2023
Cited by: 2 articles | PMID: 36625485 | PMCID: PMC9942905
Immune Metabolism-An Opportunity to Better Understand Allergic Pathology and Improve Treatment of Allergic Diseases?
Front Allergy, 3:825931, 09 Mar 2022
Cited by: 6 articles | PMID: 35386646 | PMCID: PMC8974690
A proposed population-health based metric for evaluating representativeness of air quality monitoring in cities: Using Hong Kong as a demonstration.
PLoS One, 16(5):e0252290, 28 May 2021
Cited by: 2 articles | PMID: 34048462 | PMCID: PMC8162681
Role of Mitochondria in the Redox Signaling Network and Its Outcomes in High Impact Inflammatory Syndromes.
Front Endocrinol (Lausanne), 11:568305, 23 Sep 2020
Cited by: 19 articles | PMID: 33071976 | PMCID: PMC7538663
Review Free full text in Europe PMC
Go to all (27) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Molecular mechanisms of oxidative stress in asthma.
Mol Aspects Med, 85:101026, 06 Oct 2021
Cited by: 76 articles | PMID: 34625291
Review
Molecular insights of NADPH oxidases and its pathological consequences.
Cell Biochem Funct, 39(2):218-234, 25 Sep 2020
Cited by: 20 articles | PMID: 32975319
Review
NADPH oxidases in lung biology and pathology: host defense enzymes, and more.
Free Radic Biol Med, 44(6):938-955, 05 Dec 2007
Cited by: 136 articles | PMID: 18164271 | PMCID: PMC2323509
Review Free full text in Europe PMC
The Antioxidant Rosmarinic Acid Ameliorates Oxidative Lung Damage in Experimental Allergic Asthma via Modulation of NADPH Oxidases and Antioxidant Enzymes.
Inflammation, 43(5):1902-1912, 01 Oct 2020
Cited by: 17 articles | PMID: 32519269
Funding
Funders who supported this work.
NHLBI NIH HHS (5)
Grant ID: HL085646
Grant ID: R01 HL074295
Grant ID: R01 HL085646
Grant ID: R01 HL085646-03
Grant ID: R01 HL068865