Abstract
Free full text

Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells
Associated Data
Abstract
Notch signaling is often and aberrantly activated by hypoxia during tumor progression; however, the exact pathological role of hypoxia-induced Notch signaling in tumor metastasis is as yet poorly understood. In this study, we aimed to define the mechanism of Notch ligand activation by hypoxia in both primary tumor and bone stromal cells in the metastatic niche and to clarify their roles in tumor progression. We have analyzed the expression profiles of various Notch liagnds in 779 breast cancer patients in GEO database and found that the expression of Jagged2 among all five ligands is most significantly correlated with the overall- and metastasis-free survival of breast cancer patients. The results of our immunohistochemical (IHC) analysis for Jagged2 in 61 clinical samples also revealed that both Jagged2 and Notch signaling were strongly up-regulated at the hypoxic invasive front. Activation of Jagged2 by hypoxia in tumor cells induced EMT and also promoted cell survival in vitro. Notably, a γ-secretase inhibitor significantly blocked Notch-mediated invasion and survival under hypoxia by promoting expression of E-cadherin and inhibiting Akt phosphorylation. Importantly, Jagged2 was also found to be up-regulated in bone marrow stroma under hypoxia and promoted the growth of cancer stem-like cells by activating their Notch signaling. Therefore, hypoxia-induced Jagged2 activation in both tumor invasive front and normal bone stroma plays a critical role in tumor progression and metastasis, and Jagged2 is considered to be a valuable prognostic marker and may serve as a novel therapeutic target for metastatic breast cancer.
Introduction
Oxygen homeostasis plays a critical role in carcinogenesis and tumor progression (Schindl et al., 2002; Zhong et al., 1999; Bertout et al., 2009). Hypoxia is known to promote aggressive phenotypes of tumor and induces their invasiveness and metastasis; however, the exact molecular mechanism of hypoxia-mediated metastasis is not well defined yet. Under hypoxic condition, tumor cells up-regulate the expression of hypoxia-inducible factors (HIFs) which function as master regulators to control a series of down-stream events such as activating angiogenic and cell survival pathways as well as promoting epithelial to mesenchymal transition (EMT) (Pouyssegur et al., 2006; Haase et al., 2009). Hypoxic areas are often found in the central necrotic regions of a tumor mass (Lambin et al., 1998; Tomes et al., 2003). Interestingly, however, the expression levels of several hypoxia-related proteins, including HIF-1α, Glut-1(Glucose transporter 1) and CA9 (Carbonic anhydrase 9) were also found to be frequently elevated at the margins of solid tumors which is known as invasive front (Horree et al., 2007). In this context, it is noteworthy that cancer stem-like cells are often seen at the invasive front in several types of cancers (Das et al., 2008). Bone is one of the most frequently metastasized organs in breast cancer due to its unique microenvironment (Roodman, 2004). Many regions of the bone are known to be hypoxic and this hypoxic niche in bone microenvironment is believed to promote self renewal of hematopoietic stem cells (Parmar et al., 2007). Therefore, cancer stem-like cells may also take advantage of such an environment and establish metastatic colony in the bone. In fact, Das et al used “injured conditioned medium” which is derived from bone marrow stromal cells exposed to hypoxia and oxidative stress to isolate post-hypoxic side population of cancer cells with stem cell property, indicating a potential role of hypoxic bone microenvironment in promoting cancer stem cell growth (Das et al., 2008).
Notch signaling is an evolutionarily conserved pathway that plays a critical role in self-renewal of stem cells, and aberrant expression of this pathway is often observed in various types of cancers (Leong & Karsan, 2006; Koch & Radtke, 2007; Kiaris et al., 2004; Klinakis et al., 2006; Lee et al., 2007; Stylianou et al., 2006). Notch signaling is also known to be activated under hypoxia in aggressive tumors (Sahlgren et al., 2008). Recently, it has been shown that hypoxia-induced HIF1α forms a complex with Notch1 to enhance the stability of the protein and its activity (Sahlgren et al., 2008; Gustafsson et al., 2005; Bertout et al., 2009; Main et al., 2010).However, since the activation of Notch signaling relies on the receptor–ligand interaction, the exact role of hypoxic microenvironment in Notch activation is not well clarified. Previous studies showed that not only Notch signaling but also Notch ligands were up-regulated in high grade cancers, indicating the prognostic value of Notch ligands in clinical settings (Mullendore et al., 2009; Nam et al., 2008). There are five Notch ligands (Jagged1-2 and Delta like 1, 3 and 4) that have been identified in mammals (Leong & Karsan, 2006; D'Souza et al.). They are functionally similar and appear to be redundant; however, expressions of these genes are differentially regulated during embryogenesis as well as in tumor progression. Choi et al showed that despite their high sequence similarity, Jagged1 and Jagged2 are differentially regulated according to their distinct biological roles in non-small lung cancer cells (Choi et al., 2009). Notably, over-expression of Jagged2 has been found in over 90% of pancreatic cancer cell lines (Mullendore et al., 2009). In breast cancer, increased Jagged2 mRNA was found in the MDA-MB-435 derived Br4 variant cell line which specifically metastasizes to the brain, and knocking down of Jagged2 significantly decreased migration and invasion abilities of the Br4 cells (Nam et al., 2008), suggesting that Jagged2 plays an important role in tumor metastasis in breast cancer although the underlying mechanisms of the aberrant Jagged2 expression is yet to be defined.
By analyzing microarray data from multiple patient cohorts, we recently found that the expression of Jagged2 among all five ligands is most significantly correlated with the overall- and metastasis-free survival of breast cancer patients, indicating the critical role of Jagged2 in breast cancer metastasis. In this report, we provide evidence to show that Jagged2 is significantly elevated by hypoxia in both cancer and bone stromal cells, which concomitantly activate Notch signal in cancer cells. We also show that this hypoxia-induced Notch activation is essential to promote EMT and cell survival at the hypoxic invasive front and that the hypoxia-induced Jagged2 expression in bone marrow stromal cells promotes self-renewal of cancer stem-like cells by activating Notch signaling.
Results
Jagged2 expression is correlated with poor overall- and metastasis-free survival of breast cancer patients
To understand the role of Notch signaling in the progression of breast cancer, we first examined the expression profile of all known Notch ligands (Jagged1, Jagged2, DLL1, DLL3, DLL4) and their prognostic values in breast cancer patients, using three existing microarray cohort data bases that contain the information of overall- and metastasis-free survival for a total of 779 breast cancer patients. As shown in Fig. 1A–B, we found that only Jagged2 among all five Notch ligands is significantly correlated with overall- and metastasis-free survival. On the other hand, the expression of other ligands failed to predict overall or metastasis-free survival in patients. Importantly, this trend was observed in all three independent cohorts, suggesting that Jagged2 plays a critical role in breast tumor progression (supplemental Fig. S1A). We also examined the relationship between expression of Notch receptors (Notch 1,2,3,and 4) and overall- or metastasis- free survival of patients in the GSE7390 cohort, and found that none of the notch receptors had significant co-relation to patient survival (Supplemental Fig. S1B). Furthermore, we conducted a multivariate Cox regression analysis for Jagged2, tumor size and ER status using another independent cohort database. The results of this analysis revealed that Jagged2 is a statistically significant independent prognostic factor, which is consistent with our result of survival analysis (Fig. 1C). The odds ratio for Jagged2 is 2.874 (95% CI 1.446–5.712, P<0.003), indicating that the risk of patients with increased Jagged2 expression within a specific time is 2.9 times higher than the risk of patients with Jagged2 negativity. Thus, over-expression of Jagged2 can be a strong predicator of overall- and metastasis-free survival. Because Jagged2 has potential value as a prognostic marker, it is interesting to know whether the expression of this gene varies in different subsets of breast cancers. Therefore we performed immunohistochemical analysis for Jagged2 expression using a tissue microarray which included 71 patient samples. However, we found that the Jagged2 expression had no correlation to the status of ER, PR or Her2 (Supplemental Fig. S1C). This result was also confirmed by analyzing GSE7390 cohort data for which ER status of the patients was available (Supplemental Fig. S1D)
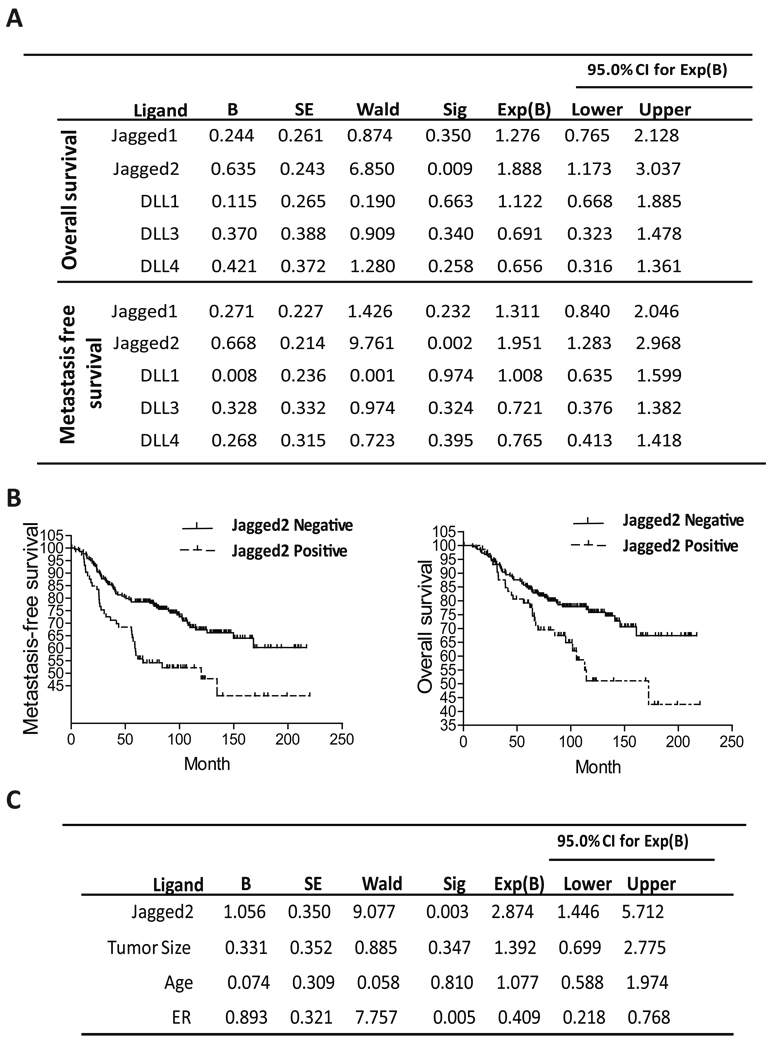
(A) Univariate survival analyses by log-rank were conducted to assess the contribution of the Notch ligands to disease prognosis in 779 patients using GEO database (GSE7390, GSE2034, and NKI-295). (B) Metastasis free- and overall- survival rates over a period of 5 years were analyzed in 295 patients (NKI-295) with breast cancer, in relation to Jagged2 expression. Solid line, Jagged2 negative patients; dotted line, patients with increased expression of Jagged2. (C) Multivariate Cox regression analysis of GSE7390 was conducted to assess the contribution of the indicated variables to disease prognosis. b, the estimation for the regression coefficient β; CI, confidence interval.
Notch signaling and Notch ligand are activated at hypoxic invasive front
To further evaluate the role of Notch signaling in breast tumor progression, we examined the expression of cleaved Notch1 in 61 human breast tumor samples by immunohistochemistry (IHC) using NICD (Notch intracellular domain)-specific antibody. As shown in Fig. 2A and B, we observed that NICD is strongly expressed in both the nucleus and cytosol in normal gland of breast tissue; however, it was gradually decreased during tumor progression. In ductal carcinoma in situ (DCIS), an early stage of breast cancer, the expression of NICD was slightly decreased although strong nuclear expression was still observed. On the other hand, overall NICD expression was significantly reduced inside poorly differentiated tumor. Our tissue array results indicate that the expression of NICD had no significant correlation with the status of ER, PR or Her2, although it was significantly correlated with the expression of Jagged 2 and metastasis status of the patients (Supplemental Fig. S2A–B). Interestingly, the expression of NICD was significantly up-regulated at the invasive front of the same tumors (Fig. 2C). This striking difference suggests the critical role of Notch signaling in invasion and metastatic tumor progression of breast cancer. Because activation of Notch signaling requires ligand interaction, we sought a possibility that Jagged2 expression, which is significantly correlated to patient survival as shown in Fig. 1, is also expressed at the invasive front. Indeed, we found that both NICD and Jagged2 are strongly expressed at the invasive front while they are drastically reduced inside the tumors (Fig. 2C), suggesting that Jagged2 is the major ligand to activate Notch signaling at the invasive front. To further verify this notion, we examined the expression of other Notch ligands (Jagged1, DLL1, DLL 3 and DLL4) using the consecutively sectioned slide for the same area of the tumor. We found that Jagged2 among all other Notch ligands was indeed most strongly expressed in the invasive front (Supplemental Fig. S2C). Because Notch signaling is often activated by hypoxia (Sahlgren et al., 2008) and the invasive front of tumor mass is known to be hypoxic (Horree et al., 2007), we tested our hypothesis that Jagged2 is up-regulated at the invasive front through hypoxic signaling by performing immunohistochemistry using antibodies for well established hypoxia markers, CA9 and Glut1, as well as NICD. As shown in Fig. 2D, we found that CA9 and Glu1 were strongly expressed at the invasive front and these markers coincided with the elevated expression of NICD. These results strongly suggest that the activated Notch signaling at the invasive front is caused by increased expression of Jagged2 which is mediated by local hypoxia.
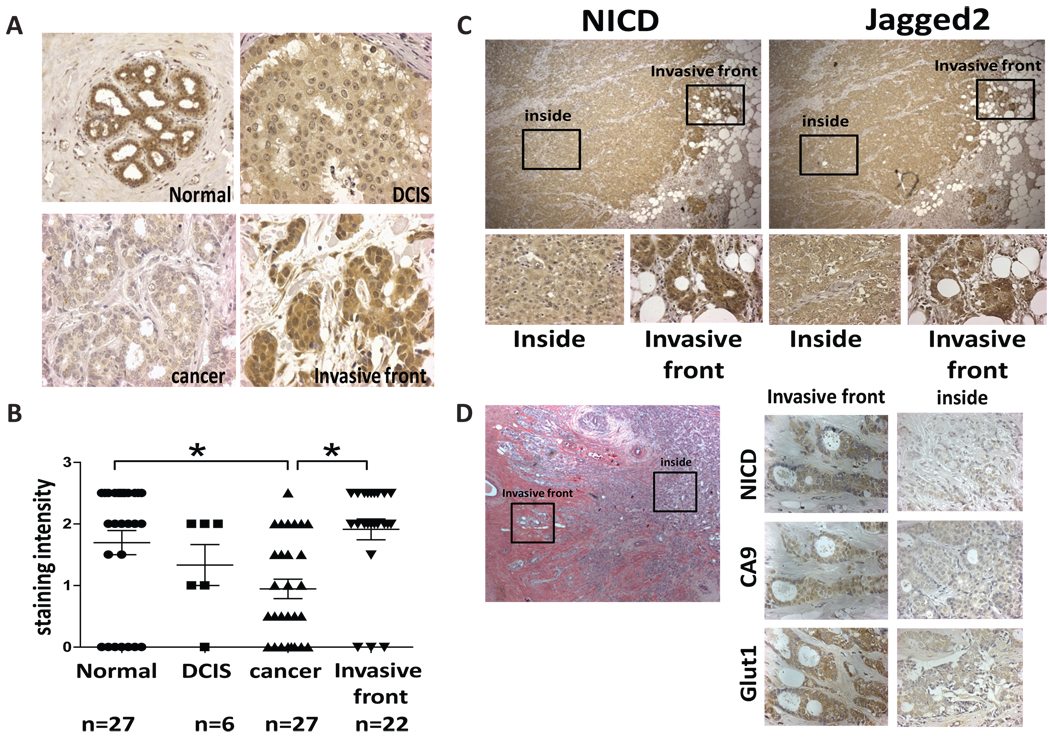
(A) Representative photos of breast tumor specimens at various stages after IHC with anti-NICD antibody. (B) Staining intensity of NICD at different stages was quantified. (C) Representative photos of two consecutive sections stained with anti-NICD and anti-Jagged2 antibodies at invasive front and inside of tumor. (D) Representative photos of IHC using antibodies to NICD, CA9 and Glut1 at invasive front and inside of tumor.
Hypoxia up-regulates Jagged2 in breast cancer cells
To provide further evidence that Jagged2 is activated by hypoxia in vitro, we examined the effect of hypoxia on Jagged2 expression in two different breast cancer cell lines, MCF7 and MDA-MB 231(MDA231), under normoxia (21%O2) and hypoxia (1%O2). As shown in Fig. 3A, we found that mRNA and protein levels of Jagged2 were significantly elevated in both MCF7 and MDA231 cells under hypoxia. However, we found no significant induction of other Notch ligands (Jagged1, DLL1, 2, 3 and 4) in either MDA231 or MCF7 cells under hypoxic condition (Supplemental Fig. S3A–B). In addition, the results of Jagged2 reporter assay indicate that hypoxia can significantly up-regulate the promoter activity of Jagged2 (Fig. 3A). However, we found no significant HIF1α binding sequence on Jagged2 promoter region. Therefore, to further verify that Notch signaling is indeed activated under hypoxia upon up-regulation of Jagged2, we examined the Notch reporter activity, protein level of NICD as well as HIF1α expression in MCF7 cells under normoxia and hypoxia. We found that Notch signal is indeed significantly augmented, which was accompanied by up-regulation of HIF1α level (Fig. 3B). We also examined the expression of NICD in the MDA-MB231 cells by immunocytochemistry and found that NICD was strongly expressed in the clustered cells but much less so in a separated single cell under hypoxic condition (Fig. 3C). These results suggest that hypoxia-induced NICD activation requires Jagged2-Notch interaction through cell-cell contact rather than stabilization of preexisting Notch signaling.
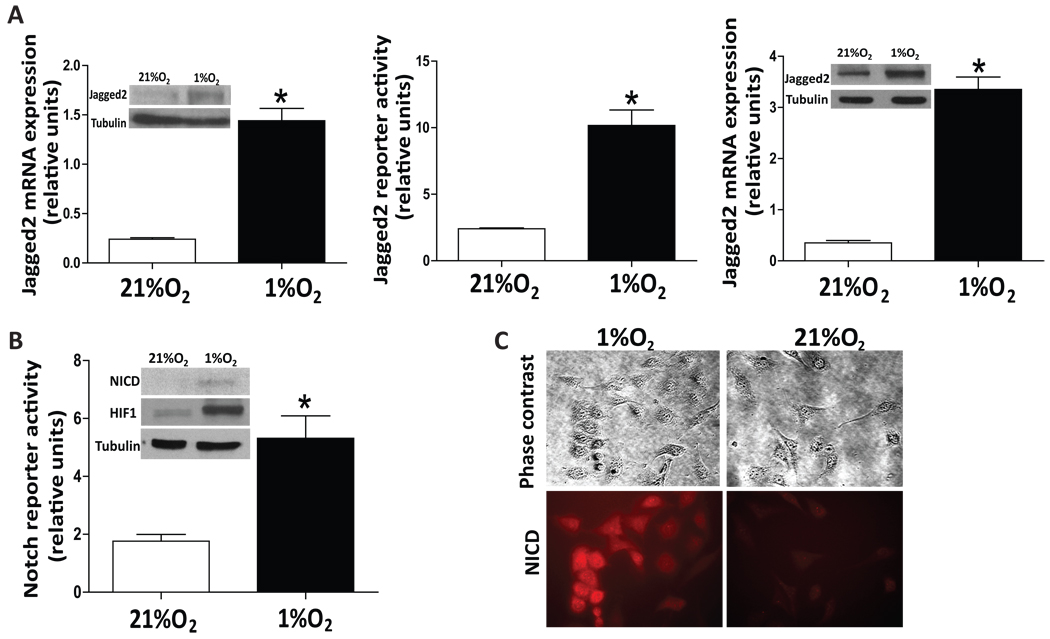
(A) Breast cancer cell lines, MCF7 and MDA-MB231, were cultured in two sets of 24-well plates under normoxic (21%O2) or hypoxic (1%O2) conditions for 24 hrs. One set of cells (in triplicate) was collected, and RNA was prepared. The samples were then subjected to qRT-PCR using primers for the Jagged2 and β-actin genes. Another set of cells was collected, and the cell lysates were subjected to Western blot analysis using anti-Jagged2 and anti-tubulin antibodies (inserted fig). For Jagged2 reporter assay, MCF7 cells were transfected with the Jagged2 reporter plasmid and they were incubated under normoxia or hypoxia. Luciferase activity was measured after 24hrs incubation. (B) MCF7 cells were transfected with Notch reporter plasmid (4XCBF-Luc) and they were incubated under normoxia or hypoxia for 24 hrs followed by luciferase activity assay. Western blot was performed to measure the protein level of NICD and HIF1α in MCF7 cells under normoxia or hypoxia (inserted figure). (C) MDA-MB231 were cultured under 1%O2 and NICD immunocytochemistry was performed. Values are means ± SD of triplicate measurements. *, P<0.01.
Hypoxia-induced cell survival and EMT require Notch signaling
How the up-regulation of Jagged2 by hypoxia and concomitant activation of Notch signaling affect tumor progression is the next important question. Rapidly growing tumor cells at the invasive front must survive under hypoxic condition and need to be invasive; therefore, we speculated that hypoxia-induced Jagged2 plays a role in this process. To test this possibility, Jagged2 expression in four different breast cancer cell lines were examined by RT-PCR and Western blot (Fig. 4A), and we found that Jagged2 expression was significantly up-regulated in highly metastatic cell line, MDA231LM, which is derived from MDA231 cells while it is significantly lower in non-tumorigenic MCF10A cells. These results suggest an increasing chance of Notch signaling being activated in MDA231LM cells due to the large amount of Jagged2 expression, and this property of MDA231LM cells may be the result of metastatic phenotype selection driven by hypoxia. Next, we examined the role of Notch signaling in promoting cell survival under hypoxia. MDA231, MDA231LM and MDA231 LM-DNMAML which constitutively expresses the dominant negative Mastermind to suppress Notch signaling, were cultured under hypoxia and cell viability was measured at different time points by MTT assay. We found that the cell viability of MDA231LM was not affected by hypoxia; however, MDA231 and MDA231LM-DNMAML cells showed decreased cell viability after 48hr exposure to hypoxia, indicating that hypoxia induced cell survival requires Notch signaling (Fig. 4B). To further investigate the role of Jagged2 in promoting cancer cell survival under hypoxia, we knocked down the Jagged2 expression in MDA231LM and also ectopically expressed Jagged2 in MDA231 by infecting these cells with sh-Jagged2 and pSin-EF2-Jagged2 lenti viruses, respectively. As shown in Fig4 C, the knock-down of Jagged2 significantly attenuated cell survival in MDA231LM and MDA231cells under hypoxia, while the ectopic expression of this gene significantly augmented cell survival. Because the activation of Akt signaling is known to be a key mediator of cell survival under hypoxia, and Akt was reported to be a potential target of Notch signaling in melanoma and lung cancer (Bedogni et al., 2008; Zhao et al., 2010; Eliasz et al., 2010), we evaluated the effect of Notch signaling on Akt by treating MDA231LM cells with DAPT, a γ-secretase inhibitor which prevents the release of activated Notch intracellular domain. We found that the amount of phospho-Akt-1 was strongly decreased by the DAPT treatment under hypoxia, while the total amount of Akt was not affected by the suppression of Notch signaling (Fig. 4D). These results suggest that Notch signaling promotes cell survival under hypoxia through the activation of Akt pathway. Furthermore, to examine whether Notch signaling is involved in hypoxia-induced invasion and EMT in breast cancer cells, we first measured the invasive ability of MDA231LM cells using Matrigel invasion chamber under hypoxia in the presence or absence of DAPT for 24hrs. We found that the DAPT treatment significantly decreased the number of invading cells, suggesting that inhibition of Notch signaling abrogates the invasive ability of cancer cells under hypoxia (Fig. 4E). To further understand the molecular basis of Notch mediated cell invasion, we examined mRNA level of the snail gene, which is known to be involved in EMT by decreasing E-cadherin expression, in MCF7, MDA231 and MDA231LM cells. As shown in Fig 4F, the snail gene was highly expressed in MDA231LM which is the most invasive among the tested cell lines with highest level of expression of Jagged2. Hypoxia also induced the morphological change of MDA231LM cells to become a more mesenchymal shape which was reversed by DAPT treatment (Fig. 4G). Next, we examined the level of E-cad mRNA in MDA231LM and MCF7 cells under normoxia and hypoxia in the presence or absence of DAPT. We found that E-cad mRNA was significantly down-regulated in both MCF7 and MDA231LM cells under hypoxia, and this hypoxia effect was significantly abrogated by DAPT (Fig. 4H). To further examine the role of Jagged2 in promoting snail expression through activating Notch signaling, we cultured MDA231LM cells under normoxia and hypoxia with or without the infection of sh-Jagged2 lentivirus followed by measuring the mRNA levels of snail as well as Hes1 which is a typical downstream target of Notch signaling. As shown in Fig 2I, we found that the knock-down of Jagged2 significantly decreases the expression of both Hes1 and snail mRNA. Taken together, our results strongly suggest that the activation of Notch signaling mediated by hypoxia-induced Jagged2 expression leads to cell survival via Akt activation and cell invasion via promotion of EMT.
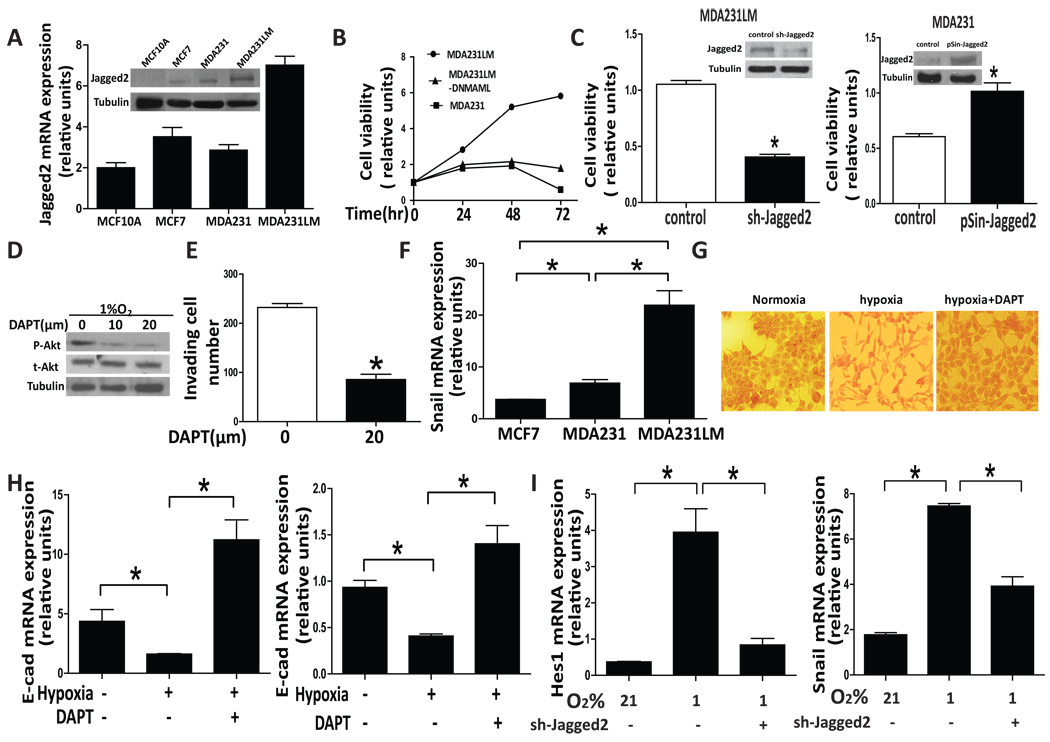
(A) mRNA and whole cell lysates were prepared from the non-tumorigenic cell line, MCF10A and three human breast cancer cell lines, MCF7, MDA231 and MDA231LM, and quantitative RT-PCR and Western blot were performed to evaluate the amount of Jagged2 expression. (B) MDA231, MDA231LM and MDA231LM-DNMAML were cultured in 96-well plates under hypoxic condition (1%O2). Cell viability was measured at different time point by MTT assay. (C) MDA231 and MDA231LM cells were infected with sh-Jagged2 and pSin-Jagged2 lentivirus, respectively. Cells were also infected with control lentiviruses. These cells were incubated for 12 hours under normoxic condition and they were transferred to hypoxic condition (1%O2). After 72 hrs, cell viability was measured by MTT assay. Knock-down and ectopic expression of Jagged2 were also examined by Western blot (inserted photos). (D) MDA231LM cells were also cultured in the presence or absence of DAPT under hypoxia for 48 hrs and the expression of phospho-Akt was examined by Western blot. (E) MDA231LM cells were seeded in Matrigel invasion chambers in the presence or absence of DAPT (20 µM) under hypoxia. After 24hrs incubation, the number of invading cells was counted. (F) mRNA were prepared from MCF7, MDA231 and MDA231LM, and qRT-PCR was performed to evaluate the snail expression.(G) MDA231LM cells were cultured in the presence or absence of DAPT under normoxia or hypoxia for 48 hrs and cell morphology was observed under microscope. (H) mRNA levels of E-cadherin expression were examined in MDA231LM (left panel) and MCF7 cells (right panel) that were cultured under normoxia or hypoxia with or without the treatment of DAPT (20 µm). (I) MDA231LM cells were infected with sh-Jagged2 or control lentivirus for 12hrs under normoxia, and they were transferred to hypoxic condition (1%O2). After 48 hrs of incubation, mRNA levels of Hes1 and snail were measured by qRT-PCR. Values are means ± SD of triplicate measurements. *, P<0.01.
Hypoxia up-regulates Jagged2 in bone marrow stromal cells
Bone is the major metastatic site of breast cancer. Several lines of evidence indicate that bone marrow is generally hypoxic, suggesting that hypoxic pre-metastatic niche may contribute to the progression of bone metastasis in breast cancer. Therefore, to examine the role of Notch signaling in bone metastasis with hypoxic microenvironment, we evaluated Jagged2 expression in immortalized human bone marrow stromal cells HS5 under hypoxic condition. We found that mRNA and protein levels of Jagged2 were significantly elevated by either 1% O2 culture or hypoxia-mimetic compound DFO treatment in a time dependent manner (Fig. 5A). In addition, we also performed immunocytochemical staining of HS5 and HS27cells for Jagged2 expression under hypoxia. We found that the Jagged2 protein was strongly expressed in both HS5 and HS27 cells after being exposed to hypoxia for 48hrs (Supplemental Fig. S4). Next, we used MDA231BoM cell line, which has a strong tendency to metastasize to bones to examine whether interaction between bone marrow stromal cells and cancer cells indeed activates Notch signaling under hypoxia. A plasmid containing the Notch reporter gene was stably cloned into MDA231BoM cells, and the Notch reporter activity was measured after 48hr of co-culture with HS5 under hypoxia or DFO treatment (Fig. 5B). We found that the Notch reporter activity was significantly up-regulated in both hypoxia and DFO treated groups, implying that hypoxic bone microenvironment plays a pivotal role in activating Notch signaling in cancer cells and that such activation is contributed by the increased amount of Notch ligands, particularly Jagged2.
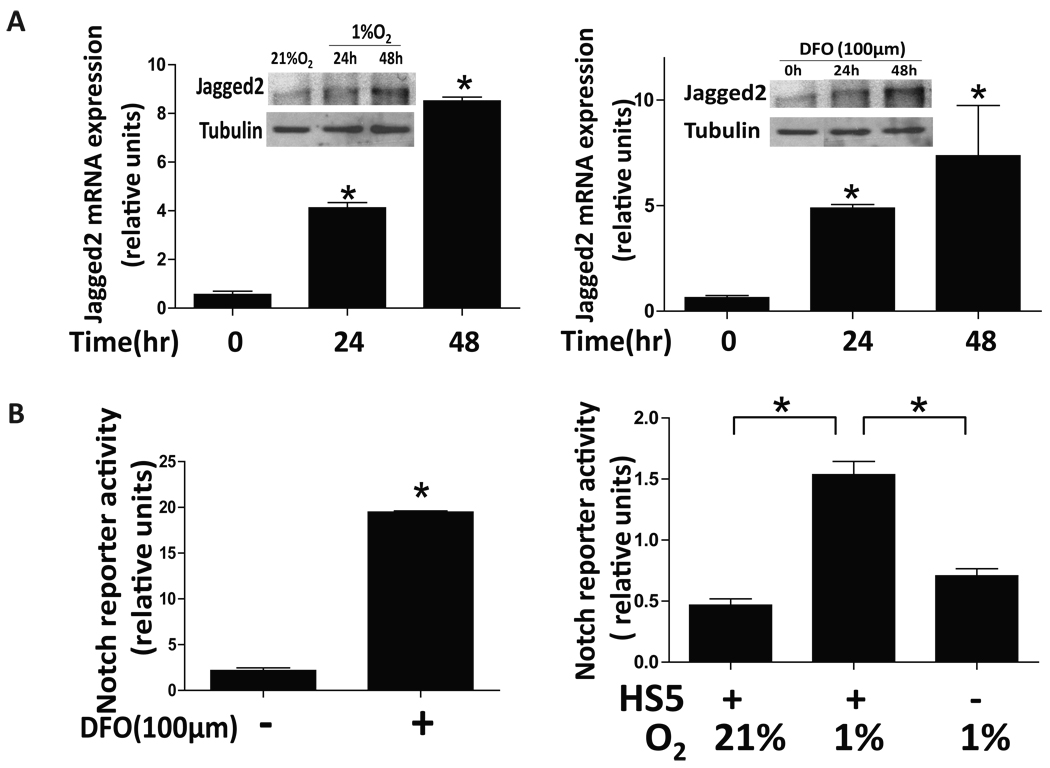
(A) Human bone marrow stromal cell line, HS5, was cultured in two sets of 24-well plates under normoxic (21%O2) or hypoxic (1%O2) conditions for up to 48hrs. One set of cells was collected and RNA was prepared. The samples were then subjected to qRT-PCR using primers for the Jagged2 and β-actin genes. Another set of cells was collected, and the cell lysates were subjected to Western blot analysis using anti-Jagged2 and anti-tubulin antibodies (inserted figure). Similar experiments were performed to examine the mRNA and protein levels of Jagged2 in HS5 after DFO treatment by qRT-PCR and Western blot (right panel). (B) MDA231BoM cells which stably expressed the Notch reporter gene were seeded on monolayer of HS5 cells followed by DFO (left panel) or hypoxia (right panel) treatment. Luciferase activity was measured after 48hrs of co-culture. Values are means ± SD of triplicate measurements. *, P<0.01.
Notch signaling promotes cancer stem-like cell self renewal under hypoxia
Because Notch signaling is known to be involved in stem cell renewal and hypoxic condition generates a favorable niche to these cells, we sought a possibility that the hypoxia – induced Notch ligand expression in bone stromal cell plays a role in self-renewal of cancer stem-like cells. To address this question, we first isolated cancer stem-like cell population (CD24− CD44+ ESA+) from MDA231BoM cells by MACS, and the tumor-initiating ability of this cell population was examined in nude mice (Supplemental Fig. S5A). We then cultured the bone stromal cells (HS5) in monolayer and seeded the stem-like cells of MDA231BoM which was labeled with GFP on top of the HS5 cells, and they were further cultured under hypoxia or normoxia for 48 hrs followed by Immunocytochemical analysis using anti-NICD antibody. As shown in Fig. 6A, Notch signaling was highly activated in the cancer stem-like cells that were co-cultured with HS5 under hypoxia compared to the cells that were co-cultured under normoxia or those that were cultured alone under hypoxia. In addition, we co-cultured the cancer stem-like cells with HS5 under normoxia or hypoxia in the presence or absence of DAPT followed by FACS analysis to measure the percentage of NICD+ cells in overall stem cell population (Fig. 6B). Our result indicates that Notch signaling is significantly activated in cancer stem-like cells that were co-cultured with the HS5 cells under hypoxia. In a parallel experiment, we measured the number of CD24− CD44+ ESA+ cells by FACS analysis and found that the number of cancer stem-like cell population was indeed significantly increased when co-cultured with HS5 under hypoxia and that this increase was blocked by DAPT (Fig. 6C). To further verify our results, we co-cultured HS5 and MDA231BoM stem-like cells that were labeled with the Cell Tracker dye under hypoxia for 48 hrs. Cells were then subjected to ALDH activity assay which is a recently developed method for identifying cancer stem cells. As shown in Supplemental Fig S5B–C, we found that hypoxia or ectopic expression of Jagged2 in HS5 cells significantly increased the number of ALDH-positive cells while DAPT treatment significantly abrogated such effect. We also generated a 231BoM/Tet NICD cell line which inducibly expresses the active form of Notch by tetracycline, and CD24− CD44+ ESA+ cell population was isolated from this cell line. The sorted cells were then cultured in mammosphere medium in the presence or absence of tetracycline, and the number of stem-like cells was measured by FACS analysis (Fig. 6D). We found that the number of cancer stem-like cells was significantly increased upon tetracycline induction. Taken together, our results indicate that hypoxic condition induces Notch ligand, particularly Jagged2, in bone stromal cells and that the interaction of stromal cells with cancer stem-like cells activates their Notch signaling which leads to self renewal of these cells and promotion of further tumor progression.
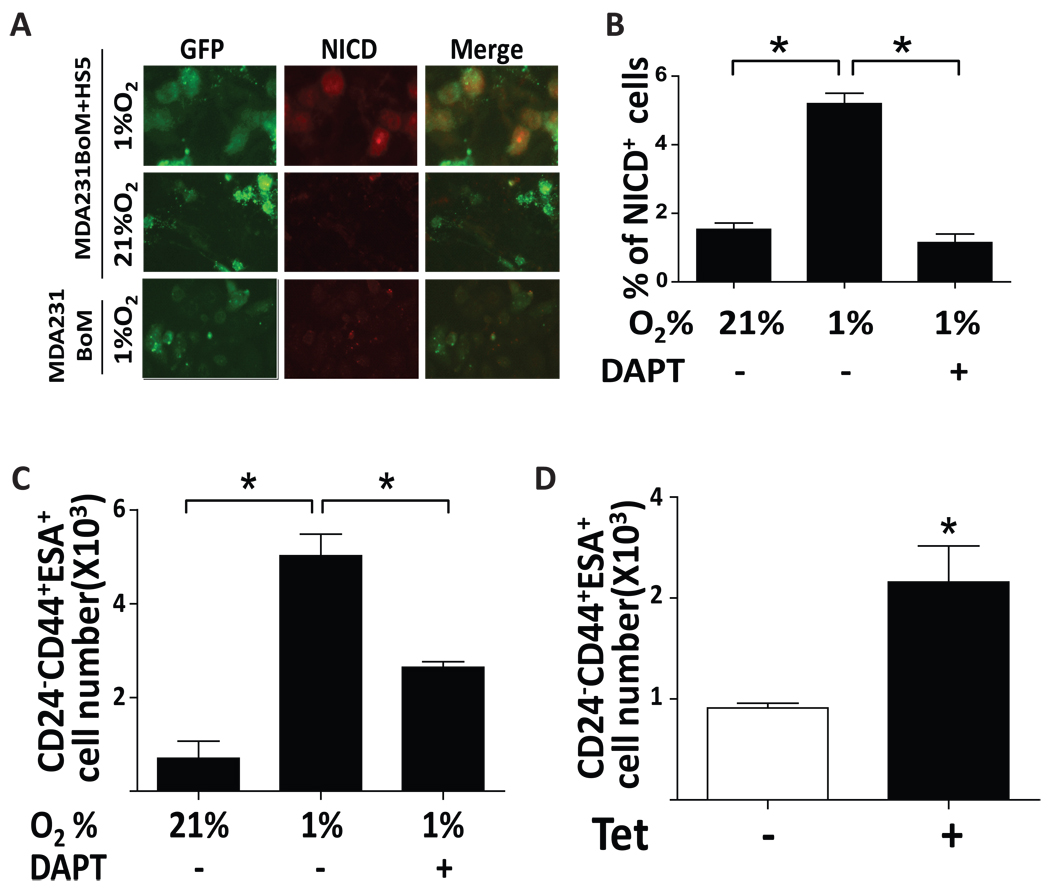
(A) Stem-like cells (CD24−CD44+ESA+) were isolated from MDA231BoM/GFP and they were co-cultured with HS5 monolayer under normoxia and hypoxia for 48hrs. Immunocytochemistry was performed using anti-NICD antibody. (B) MDA231BoM/GFP stem-like cells were co-cultured with HS5 monolayer for 48hrs under normoxia and hypoxia in the presence or absence of DAPT (20 µm). Cells were then subjected to FACS analysis by using anti-NICD antibody. (C) 231BoM/GFP stem-like cells were co-cultured with HS5 monolayer under normoxia and hypoxia in the presence or absence of DAPT (20 µm) for 72hrs. The number of CD24−CD44+ESA+ cells was measured by incubating cells with anti-CD24, anti-CD44 and anti-ESA antibodies followed by FACS analysis. (D) MDA231BoM-Tet/NICD cells were cultured in the presence or absence of tetracycline for 48 hrs and cells were subjected to FACS analysis by using anti-CD24, anti-CD44 and anti-ESA antibodies. Values are means ± SD of triplicate measurements. *, P<0.01.
Discussion
Notch signaling is a highly conserved pathway which plays a vital role in normal embryonic development and morphogenesis as well as in stem cell maintenance (Yu et al., 2008). However, aberrant expression of Notch signaling is also frequently observed in many solid tumors and leukemia, suggesting a critical role of this pathway in tumor pathology and cancer progression (Ellisen et al., 1991; Koch & Radtke, 2007). The results of our immunohistochemical analysis also indicate that Notch signaling is strongly activated in normal glands of breast tissue and generally down-regulated in DCIS and advanced tumor cells; however, it is significantly up-regulated at the invasive front. These results illustrate an intricate role of Notch signaling in tumor progression. A hallmark of Notch signaling is the requirement of the ligand-receptor interaction through direct cell-cell contact, which may occur between tumor cells or tumor cell-stroma interactions (Felli et al., 1999). Therefore, dysregulation of Notch signaling in cancer cells is likely caused by either alteration of the receptor expression in cancer cell and/or abnormal expression of ligand in tumor-surrounding cells. In mammalian cells, there are four Notch receptors, Notch 1 to 4, and they are cleaved by γ-secretase upon ligand binding to become the activated form, NICD (Artavanis-Tsakonas et al., 1999). On the other hand, less is known about the expression status of Notch ligand in tumor and tumor-associated stroma. The results of our existing microarray data analysis indicate that Jagged2 is the ligand, and in some cases the only ligand, whose expression is significantly correlated with metastasis-free survival in many patient cohorts. It should be noted that Reedijk et al (Reedijk et al., 2005) and Dickson et al (Dickson et al., 2007) previously reported that mRNA expression of Jagged1 had significant correlation to patient survival. The reason for this apparent contradiction is not clear; however, there are several differences between these analyses and our approach. First, their analysis is based on in situ hybridization, while our analysis is based on existing data set of microarray analysis which used gene chip arrays. Although in situ hybridization is more accurate for localization, it is less quantitative. Secondly, the design and location of probes for Jagged1 gene in these analyses are different. The gene chip generally uses multiple probes. Thirdly, the numbers of patients are different. We analyzed all together more than 600 patients in three different cohorts while they examined about 100 patients in each of their experiments. These differences may have contributed to the different outcome of the results of mRNA expression of Jagged1 in breast cancer patients.
We also demonstrated that high expression of Jagged2 and concomitant activation of Notch signaling often coincides with the hypoxic regions of invasive front in breast cancer and that hypoxic condition indeed significantly up-regulates the Jagged2 expression in both tumor and stromal cells in vitro. Although there is a general concern whether the cell lines that have been adapted for years to grow in 21% oxygen reflects in vivo hypoxic condition, which may be indeed a limitation of the in vitro experiment, our results using multiple cell lines clearly indicate that Jagged2 specifically responds to hypoxia which is also consistent with the result of immunohistochemical analysis for clinical sample using hypoxic markers. Hypoxia is a hallmark of tumor, which contributes to tumor cell survival, angiogenesis and chemo-resistance (Maynard & Ohh, 2007). The central area of tumor mass is often hypoxic due to necrotic cell death; however, it is well established that invasive front of tumor is also strongly hypoxic because of the rapid rate of proliferation of tumor cells (Horree et al., 2007). It has been reported that hypoxia increases the transcriptional activity of NICD by inhibiting its degradation in neuronal stem cells and myogenic cells (Gustafsson et al., 2005). Similar results were also observed in NSCL (Chen et al., 2007; Eliasz et al., 2010). It was also found that HIF1 alpha can directly bind to NICD and stabilize this protein and activity (Cejudo-Martin & Johnson, 2005). This protein stabilization is certainly considered as one of the mechanisms which contribute to the Notch activation at the invasive front; however, our results indicate that the major factor in the Notch activation is the hypoxia-induced expression of Jagged2 through cell-cell interaction of tumor cells. In fact, we have shown that hypoxia can significantly activate Jagged2 expression as well as the Notch signaling in breast tumor cell lines, but this activation is strongly cell-density dependent and the clustered cells showed much higher sensitivity to Notch activation by hypoxia than a single cell in the culture (Fig. 3C). How hypoxia promotes invasiveness of tumor cells through activation of Notch signaling is an intriguing question. Our results indicate that inhibition of hypoxia-induced Notch signaling significantly decreased cell survival and invasiveness by blocking Akt pathway and also by suppressing EMT. It should be noted that Akt was previously found to be activated by Notch signaling in melanoma and lung cancers (Zhao et al., 2010; Bedogni et al., 2008). In addition, Notch signaling has been known to be associated with chemo-resistance and cell survival (Wang et al., 2010; Eliasz et al., 2010). Therefore, hypoxia-induced Notch activation may render tumor cells to become more resistant to cell death through activation of Akt. Taken together, the aggressive nature of tumor cells at the invasive front appears to be mediated by Akt/EMT induction through up-regulation of Jagged2 followed by the activation of Notch signaling.
Bone is one of the most common sites of breast cancer metastasis (Kominsky & Davidson, 2006). It is becoming clear that there are many regions that are hypoxic in the bone, and these areas play an important role in the self-renewal ability of hematopoietic stem cells by providing stem cell niche (Yin & Li, 2006). Because metastatic cells must have stem-like characteristics, it is highly plausible that cancer stem-like cells take advantage of these niches once they reach the bone. In fact, our results indicate that bone marrow stromal cells significantly over-expressed Jagged2 under hypoxic condition and that co-culturing the stromal cells with the cancer stem-like cells significantly promoted proliferation of cancer stem-like cells by activating Notch signaling. Therefore, Jagged2 expression in bone marrow stromal cells at the hypoxic regions in the bone is considered to provide appropriate niche for metastatic cancer stem-like cells and promote their self-renewal.
Collectively, our results indicate that the expression of Jagged2 is significantly augmented by hypoxia at the invasive front and the interaction between breast cancer cells and the Jagged2-expressing cells significantly enhanced the invasiveness and survival of cancer cells through the activation of Akt/EMT pathways. Furthermore, hypoxic regions in the bone marrow provide the niche for cancer stem-like cells by expressing the hypoxia-induced Jagged2. Therefore, Jagged2 is considered to be a valuable marker to predict the outcome in patients with metastatic disease, and more importantly, intervention in Jagged2 expression and cell-cell interaction may serve as a novel therapeutic target for metastatic breast cancer.
Materials and Methods
Cell culture and reagents
Human breast carcinoma cell lines, MCF7 and MDA231were purchased from American Type Culture Collection. MDA231LM and MDA231BoM were kind gifts from Dr. Massague (Memorial Sloan-Kettering Cancer Center). Cells were maintained in RPMI 1640 supplemented with 10% FBS and grown at 37 °C in a 5% CO2 atmosphere. Cells grown in hypoxia were maintained in hypoxia chamber filled with mixture of gases (1%O2, 5%CO2, 94%N2) at 37°C. N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) was purchased from Sigma Chemical Co.
Plasmids construction
PGL2pro-4XCBF Notch reporter was a kind gift from Dr. Hayward (Johns Hopkins University). NICD cDNA with a Myc-tag was provided by Dr. Bresnick (University of Wisconsin). The tetracycline-inducible system T-Rex (Invitrogen) was used to create a cell line with inducible NICD expression. First, the Myc-NICD cDNA was amplified by PCR and cloned into pcDNA5/TO (Invitrogen). The human breast cancer cell line MDA231BoM was transfected with pcDNA6/TR encoding the Tet repressor, and a stable cell line (MDA231BoM/Tet) was generated. Then, the pcDNA5/TO/Myc-NICD expression plasmid was stably transfected into the MDA231BoM/Tet cell, and the resultant clones were designated as MDA231BoM Tet-Myc-NICD. To generate the Jagged2 reporter plasmid, the promoter of Jagged2 (from +15 to −1,352 bp) was amplified by PCR and the product was cloned into the PGL3-basic plasmid. The resultant clones were designated as PGL3-Jagged2. pCDNA3-Jagged2-myc was a kind gift from Dr Sisodia (University of Chicago) and Jagged2 sequence was sub cloned into pSin-EF2-Pur vector between EcoRI and SpeI sites.
Western blot
Western blot analysis was performed as described previously using antibodies against cleaved Notch1 (1:200; Val1744; Cell Signaling Technology), Jagged2 (1:200; R&D Systems Inc.), α -tubulin (1:1,000 Cell Signaling Technology), HIF1α (1:200; BD Bioscience), phospho-Akt1 (1:500; Ser473; Cell Signaling Technology), and Akt1 (1:500; Cell Signaling Technology) (Furuta et al., 2008).
Quantitative real-time PCR
Total RNA was isolated from the cells and reverse transcribed. The cDNA was then amplified with a pair of forward and reverse primers for the following genes: Jagged1(5’-TCGCTGTATCTGTCCACCTG - 3’ and 5’-AGTCACTGGCACGGTTGTAG - 3’), Jagged2 (5’- CTCCTCATTCGGGGTGGTAT - 3’ and 5’- GTCGTCATCCCCTTCCAGT -3’), DLL1 (5’- CAGGGTTGCACATTTCTCC -3’ and 5’- GCACGGACCTCAAGTACTCC-3’), DLL3 (5’- CCTGCGCGCTGAATGTC-3’ and 5’- CATCGAAACCTGGAGAGAGG-3’), DLL4(5’-CACACACTGGACTATAATCTGG -3’ and 5’- ACACATTCGTTCCTCTCTTCTG-3’)and β-actin (5’-TGAGACCTTCAACACCCCAGCCATG-3’ and 5’-CGTAGATGGGCACAGTGTGGGTG-3’). PCR reactions were performed using DNA engine opticon 2 system (MJ Research) and the Maxima® SYBR Green qPCR Master Mix (Fermentas Life Science). The thermal cycling conditions composed of an initial denaturation step at 95°C for 5 min followed by 40 cycles of PCR using the following profile: 94 °C, 30 s; 58 °C, 30 s; and 72 °C, 30 s.
Reporter assay
PGL3-Jagged2 plasmid was transfected to breast cancer cell line MCF7 by using Lipofectamine 2000 (Invitrogen). After 48 h, the cells were collected and the luciferase activities were then measured by using Dual-Luciferase Reporter Assay System (Promega). The Renilla expression plasmid phRG-TK (Promega) was used as an internal control.
MTT cell viability assay
Cells were seeded in 96-well plates and cultured under hypoxia (1%O2) for the indicated time, and the viability was assessed by MTT assay (Promega).
Immunohistochemistry
Human breast cancer specimens were obtained from surgical pathology archives of the Akita Red Cross Hospital and Iwate Medical School. Human breast cancer tissue array was purchased from Biomax (BR1503). The sections from paraffin-embedded tissue specimens were deparaffinized and antigens were retrieved by heating the slides in 10 mM sodium citrate (pH 6.0) at 85 °C for 30min. The slides were treated with 3% H2O2 and then incubated overnight at 4 °C with anti-NICD rabbit polyclonal antibody (1:500; Abcam 8387 lot#329981.), anti-Jagged1 rabbit polyclonal antibody (1:200; Cell Signaling Technology), anti-Jagged2 goat polyclonal antibody (1:200; R&D Systems, Inc.), anti-DLL1 mouse monoclonal antibody (1:100; R&D Systems, Inc.), anti-DLL3 goat polyclonal antibody (1:200; Santa Cruz Biotechnology), anti-DLL4 rabbit polyclonal antibody(1:200; Santa Cruz Biotechnology), anti-glut1 antibody (1:100; Santa Cruz Biotechnology) and anti-CA9 mouse monoclonal antibody (1:100; R&D Systems, Inc.). The sections were then incubated with secondary antibodies and visualized using Envision-plus kit (Dako Corp.) or ABC staining system (Santa Cruz Biotechnology). Results of the immunohistochemistry were judged based on the intensity of staining, comparing the tumor cells and the normal glands on the same slide. Grading of the NICD expression levels was done by two independent persons without prior knowledge of the patient data.
Cancer stem-like cells isolation
Magnetic activated cell sorting (MACS) method was used to isolate cancer stem-like cells. Briefly, 5×107 cells were suspended in 1ml MACS buffer and treated with DNase for 10mins at 37 °C. Cells were then suspended in 100µl of MACS buffer and further incubated with biotin-conjugated anti-CD24, APC-conjugated anti-CD44 and biotin-conjugated anti-ESA antibodies followed by incubation with anti-biotin antibody and anti-APC antibodies at room temperature for 10 min. CD24−, CD44+ and ESA+ fractions were collected by serial passages through magnetic columns according to the manufacturer’s protocol.
Immunocytochemistry
Cells fixed with 70% ethanol were washed with PBS and blocked by 2%BSA for 1hr. Cells were then washed again with PBS and incubated with primary anti-NICD (Val1744; Cell Signaling Technology) and anti-Jagged2 (1:200; R&D Systems, Inc) antibodies overnight at 4 °C. Cells were further incubated with anti-rabbit IgG Alexa Fluor (R) 555molecular probe (Cell Signaling Technology) and anti-goat FITC (Invitrogen) for 1hr at room temperature. Fluorescence images were taken by a fluorescent microscope.
In vitro invasion assay
Cell invasion assay was performed as described previously using Matrigel invasion chamber (Beckton Dickinson, Bedford, MA) (Bandyopadhyay et al., 2006). The cells that invaded through the membrane were stained with tetrazolium dye and their numbers were counted under microscope.
FACS (Fluorescence-activated cell sorting)
104 Cells were suspended in FACS buffer (PBS with 0.1% BSA and 0.1% Triton X100) followed by incubation with FITC conjugated anti-CD24, APC conjugated anti-CD44 and PE conjugated anti-ESA for 15mins at room temperature. Cells were then washed with PBS and re-suspended in PBS for FACS analysis. For NICD expression analysis, cells were incubated with anti-NICD antibody (1:200, Val1744; Cell Signaling Technology) for 15 min at room temperature followed by incubation with anti-rabbit IgG Alexa Fluor (R) 555molecular probe for 10 mins at room temperature. Cells were then suspended in PBS and subjected to FACS analysis.
ALDH (Aldehyde dehydrogenase) staining and FACS analysis
104 MDA231BoM stem-like cells were first labeled with Cell Tracker Dye (CellVue Burgundy cell labeling kit, eBioscience) and seeded on the monolayer of bone marrow stromal cells and they were co-cultured for 48 hrs. Cells were then collected and ALDH staining was performed according to the manufacturer’s instructions (ALDEFLUOR® stem cell technologies), followed by analyzing the cells by FACS to measure the number of cancer stem-like cells.
Supplementary Material
1
Supplemental Fig. 1. Expression of Notch related genes in breast cancer patients:(A)Kaplan-Meier analyses were performed for the expression of various Notch ligands in breast cancer patients using existing GEO data base (GSE7390, GSE2034, NKI-295). (B) Kaplan-Meier analyses were performed for the expression of various Notch receptors in the patient of GSE7390 cohort. (C) Immunohistochemical analysis was performed for Jagged 2 expression using a tissue microarray containing 71 breast cancer patients. Chi-square tests for Jagged2 expression and different subtypes as well as lymph node metastasis status were performed. (D) Chi-square test for Jagged2 expression and estrogen receptor status was performed for patients in GSE7390 cohort.
2
Supplemental Fig. 2. Expression of Notch signal and ligands in breast cancer patients:(A) Immunohistochemical analysis for NICD was performed for 71 specimens from breast cancer patients. Chi-square test for NICD expression and status of various clinical parameters were performed. (B) Chi-square test of NICD and Jagged2 expression was performed for the same set of patient samples in (A). (C) Representative photos of immunohistochemical analysis for various Notch ligands and control IgG using consecutively sliced slides.
3
Supplemental Fig. 3 Expression of Notch ligands under hypoxic condition:(A and B). MDA231 (A) and MCF7 (B) were cultured under normoxia (21% O2) or hypoxia (1%O2) for 48 hrs. Cells were then collected and subjected to qRT-PCR analysis for various Notch ligands.
4
Supplemental Fig. 4. Expression of Jagged2 in bone stromal cells under hypoxic condition:Human bone marrow stroma cells, HS5 and HS27, were cultured under normoxic (21% O2) or hypoxic (1%O2) condition for 48hrs followed by immunocytochemical analysis for Jagged2.
5
Supplemental Fig. 5. Isolation of cancer stem-like cells and the effect of hypoxia:Limiting dilution assay of cancer stem-like cells. Cancer stem-like cells (CD24−CD44+ESA+) or non-stem-like cells (CD24+CD44−ESA−) were isolated from MDA231BoM as described in Materials and Methods. The tumor-initiating abilities of these cells were examined by inoculating the isolated cells into nude mice followed by measuring tumor formation. (B) The cancer stem-like cells (CD24−CD44+ESA+) isolated from MDA231BoM cells were labeled with Cell Tracker Dye and co-cultured with HS5 under normoxia or hypoxia with or without the treatment of DAPT(20 µm) for 48 hours. Cells were then collected and subjected to ALDH activity assay. (C) HS5 cells were infected with control or pSin-Jagged2 lentivirus, respectively, and they were incubated for 12hrs. Isolated cancer stem-like cells from MDA231BoM cells were labeled with Cell Tracker Dye and then seeded on top of the HS5 cells with or without the treatment of DAPT(20 µm) for 72 hours. Cells were then collected and subjected to ALDH activity assay.
Acknowledgments
This work was supported by NIH (R01CA124650, R01CA129000 to KW), the US Department of Defense (BC085424, BC085590 to KW) and Susan Komen Foundation (KG080477 to KW).
Footnotes
Conflict of interest.
The authors declare no conflict of interest.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Science. 1999;284:770–776. [Abstract] [Google Scholar]
- Bandyopadhyay S, Wang Y, Zhan R, Pai SK, Watabe M, Iiizumi M, Furuta E, Mohinta S, Liu W, Hirota S, Hosobe S, Tsukada T, Miura K, Takano Y, Saito K, Commes T, Piquemal D, Hai T, Watabe K. Cancer Res. 2006;66:11983–11990. [Abstract] [Google Scholar]
- Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. J Clin Invest. 2008;118:3660–3670. [Abstract] [Google Scholar]
- Bertout JA, Patel SA, Fryer BH, Durham AC, Covello KL, Olive KP, Goldschmidt MH, Simon MC. Cancer Res. 2009;69:3213–3220. [Europe PMC free article] [Abstract] [Google Scholar]
- Cejudo-Martin P, Johnson RS. Dev Cell. 2005;9:575–576. [Abstract] [Google Scholar]
- Chen Y, De Marco MA, Graziani I, Gazdar AF, Strack PR, Miele L, Bocchetta M. Cancer Res. 2007;67:7954–7959. [Abstract] [Google Scholar]
- Choi K, Ahn YH, Gibbons DL, Tran HT, Creighton CJ, Girard L, Minna JD, Qin FX, Kurie JM. J Biol Chem. 2009;284:17766–17774. [Europe PMC free article] [Abstract] [Google Scholar]
- D'Souza B, Meloty-Kapella L, Weinmaster G. Curr Top Dev Biol. 2010;92:73–129. [Europe PMC free article] [Abstract] [Google Scholar]
- Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Stem Cells. 2008;26:1818–1830. [Abstract] [Google Scholar]
- Dickson BC, Mulligan AM, Zhang H, Lockwood G, O'Malley FP, Egan SE, Reedijk M. Mod Pathol. 2007;20:685–693. [Abstract] [Google Scholar]
- Eliasz S, Liang S, Chen Y, De Marco MA, Machek O, Skucha S, Miele L, Bocchetta M. Oncogene. 2010;29:2488–2498. [Europe PMC free article] [Abstract] [Google Scholar]
- Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J. Cell. 1991;66:649–661. [Abstract] [Google Scholar]
- Felli MP, Maroder M, Mitsiadis TA, Campese AF, Bellavia D, Vacca A, Mann RS, Frati L, Lendahl U, Gulino A, Screpanti I. Int Immunol. 1999;11:1017–1025. [Abstract] [Google Scholar]
- Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, Kamada S, Saito K, Iiizumi M, Liu W, Ericsson J, Watabe K. Cancer Res. 2008;68:1003–1011. [Abstract] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Dev Cell. 2005;9:617–628. [Abstract] [Google Scholar]
- Haase VH. Kidney Int. 2009;76:492–499. [Europe PMC free article] [Abstract] [Google Scholar]
- Horree N, van Diest PJ, Sie-Go DM, Heintz AP. Hum Pathol. 2007;38:1232–1238. [Abstract] [Google Scholar]
- Kiaris H, Politi K, Grimm LM, Szabolcs M, Fisher P, Efstratiadis A, Artavanis-Tsakonas S. Am J Pathol. 2004;165:695–705. [Europe PMC free article] [Abstract] [Google Scholar]
- Klinakis A, Szabolcs M, Politi K, Kiaris H, Artavanis-Tsakonas S, Efstratiadis A. Proc Natl Acad Sci U S A. 2006;103:9262–9267. [Europe PMC free article] [Abstract] [Google Scholar]
- Koch U, Radtke F. Cell Mol Life Sci. 2007;64:2746–2762. [Abstract] [Google Scholar]
- Kominsky SL, Davidson NE. J Clin Oncol. 2006;24:2227–2229. [Abstract] [Google Scholar]
- Lambin P, Theys J, Landuyt W, Rijken P, van der Kogel A, van der Schueren E, Hodgkiss R, Fowler J, Nuyts S, de Bruijn E, Van Mellaert L, Anne J. Anaerobe. 1998;4:183–188. [Abstract] [Google Scholar]
- Lee SH, Jeong EG, Yoo NJ, Lee SH. Apmis. 2007;115:1357–1363. [Abstract] [Google Scholar]
- Leong KG, Karsan A. Blood. 2006;107:2223–2233. [Abstract] [Google Scholar]
- Main H, Lee KL, Yang H, Haapa-Paananen S, Edgren H, Jin S, Sahlgren C, Kallioniemi O, Poellinger L, Lim B, Lendahl U. Exp Cell Res. 2010;316:1610–1624. [Abstract] [Google Scholar]
- Maynard MA, Ohh M. Cell Mol Life Sci. 2007;64:2170–2180. [Abstract] [Google Scholar]
- Mullendore ME, Koorstra JB, Li YM, Offerhaus GJ, Fan X, Henderson CM, Matsui W, Eberhart CG, Maitra A, Feldmann G. Clin Cancer Res. 2009;15:2291–2301. [Europe PMC free article] [Abstract] [Google Scholar]
- Nam DH, Jeon HM, Kim S, Kim MH, Lee YJ, Lee MS, Kim H, Joo KM, Lee DS, Price JE, Bang SI, Park WY. Clin Cancer Res. 2008;14:4059–4066. [Abstract] [Google Scholar]
- Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Proc Natl Acad Sci U S A. 2007;104:5431–5436. [Europe PMC free article] [Abstract] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Nature. 2006;441:437–443. [Abstract] [Google Scholar]
- Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE. Cancer Res. 2005;65:8530–8537. [Abstract] [Google Scholar]
- Roodman GD. N Engl J Med. 2004;350:1655–1664. [Abstract] [Google Scholar]
- Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Proc Natl Acad Sci U S A. 2008;105:6392–6397. [Europe PMC free article] [Abstract] [Google Scholar]
- Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P, Oberhuber G. Clin Cancer Res. 2002;8:1831–1837. [Abstract] [Google Scholar]
- Stylianou S, Clarke RB, Brennan K. Cancer Res. 2006;66:1517–1525. [Abstract] [Google Scholar]
- Tomes L, Emberley E, Niu Y, Troup S, Pastorek J, Strange K, Harris A, Watson PH. Breast Cancer Res Treat. 2003;81:61–69. [Abstract] [Google Scholar]
- Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee S, Kong D, Sarkar FH. Biochim Biophys Acta. 2010;1806:258–267. [Europe PMC free article] [Abstract] [Google Scholar]
- Yin T, Li L. J Clin Invest. 2006;116:1195–1201. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu X, Zou J, Ye Z, Hammond H, Chen G, Tokunaga A, Mali P, Li YM, Civin C, Gaiano N, Cheng L. Cell Stem Cell. 2008;2:461–471. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhao N, Guo Y, Zhang M, Lin L, Zheng Z. Oncol Rep. 2010;23:1443–1447. [Abstract] [Google Scholar]
- Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Cancer Res. 1999;59:5830–5835. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1038/onc.2011.122
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3145824?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Effects and Mechanisms of Luteolin, a Plant-Based Flavonoid, in the Prevention of Cancers via Modulation of Inflammation and Cell Signaling Molecules.
Molecules, 29(5):1093, 29 Feb 2024
Cited by: 1 article | PMID: 38474604 | PMCID: PMC10934766
Review Free full text in Europe PMC
Metastasis and cancer associated fibroblasts: taking it up a NOTCH.
Front Cell Dev Biol, 11:1277076, 10 Jan 2024
Cited by: 0 articles | PMID: 38269089 | PMCID: PMC10806909
Review Free full text in Europe PMC
Hu-Qi-Zheng-Xiao Decoction Inhibits the Metastasis of Hepatocellular Carcinoma Cells by Suppressing the HIF-1α Signaling Pathway to Inhibit EMT, LCSC, and Angiogenic Process.
Integr Cancer Ther, 23:15347354231226126, 01 Jan 2024
Cited by: 0 articles | PMID: 38385348 | PMCID: PMC10893843
The Role of Breast Cancer Cells in Bone Metastasis: Suitable Seeds for Nourishing Soil.
Curr Osteoporos Rep, 22(1):28-43, 11 Jan 2024
Cited by: 0 articles | PMID: 38206556
Review
Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer.
Mol Cancer, 22(1):172, 18 Oct 2023
Cited by: 14 articles | PMID: 37853437 | PMCID: PMC10583419
Review Free full text in Europe PMC
Go to all (142) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
GEO - Gene Expression Omnibus (2)
- (2 citations) GEO - GSE2034
- (2 citations) GEO - GSE7390
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The NOTCH ligand JAGGED2 promotes pancreatic cancer metastasis independent of NOTCH signaling activation.
Mol Cancer Ther, 14(1):289-297, 28 Oct 2014
Cited by: 20 articles | PMID: 25351917
Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain.
EMBO Mol Med, 5(3):384-396, 01 Mar 2013
Cited by: 108 articles | PMID: 23495140 | PMCID: PMC3598079
Estrogen-dependent DLL1-mediated Notch signaling promotes luminal breast cancer.
Oncogene, 38(12):2092-2107, 15 Nov 2018
Cited by: 50 articles | PMID: 30442981 | PMCID: PMC6756232
Notch Signaling in Breast Cancer: A Role in Drug Resistance.
Cells, 9(10):E2204, 29 Sep 2020
Cited by: 46 articles | PMID: 33003540 | PMCID: PMC7601482
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (6)
Grant ID: R01CA129000
Grant ID: R01 CA129000-04
Grant ID: R01 CA129000
Grant ID: R01CA124650
Grant ID: R01 CA124650-04
Grant ID: R01 CA124650





