Abstract
Background
The syndrome of resistance to thyroid hormone (RTH) is caused by mutations in the thyroid hormone receptor β gene (THRB). The syndrome varies from asymptomatic to diffuse hypothyroidism, to pituitary-selective resistance with predominance of hyperthyroid signs and symptoms. The wide spectrum of clinical presentation is not completely attributable to specific THRB mutations. The THRB gene encodes two main isoforms, TR β1 which is widely distributed, and TR β2, whose expression is limited to the cochlea, retina, hypothalamus, and pituitary. Recent data demonstrated that in mice an intron enhancer region plays a critical role in the pituitary expression of the β2 isoform of the receptor. We thus hypothesized that polymorphisms in the human homologous region could modulate the pituitary expression of the mutated gene contributing to the clinical presentation of RTH.Methods
Screening and in vitro characterization of polymorphisms of the intron enhancer region of the THRB gene in the index case of pituitary-selective RTH.Results
The index case of pituitary-selective resistance is characterized by the missense R338W exon 9 mutation in cis with two common SNPs, rs2596623T and rs2596622C, located in the intron enhancer region of the THRB gene. Reporter gene assay experiments in GH3 pituitary-derived cells indicate that rs2596623T generates an increased pituitary cell-specific activity of the TR β2 promoter suggesting that rs2596623T leads to pituitary over-expression of the mutant allele.Conclusions
The combined coding mutation and non-coding SNP therefore generate a tissue-specific dominant-negative condition recapitulating the patient's peculiar phenotype. This case illustrates the role of regulatory regions in modifying the clinical presentation of genetic diseases.Free full text

An intronic SNP in the thyroid hormone receptor β gene is associated with pituitary cell-specific over-expression of a mutant thyroid hormone receptor β2 (R338W) in the index case of pituitary-selective resistance to thyroid hormone
Abstract
Background
The syndrome of resistance to thyroid hormone (RTH) is caused by mutations in the thyroid hormone receptor β gene (THRB). The syndrome varies from asymptomatic to diffuse hypothyroidism, to pituitary-selective resistance with predominance of hyperthyroid signs and symptoms. The wide spectrum of clinical presentation is not completely attributable to specific THRB mutations. The THRB gene encodes two main isoforms, TR β1 which is widely distributed, and TR β2, whose expression is limited to the cochlea, retina, hypothalamus, and pituitary. Recent data demonstrated that in mice an intron enhancer region plays a critical role in the pituitary expression of the β2 isoform of the receptor. We thus hypothesized that polymorphisms in the human homologous region could modulate the pituitary expression of the mutated gene contributing to the clinical presentation of RTH.
Methods
Screening and in vitro characterization of polymorphisms of the intron enhancer region of the THRB gene in the index case of pituitary-selective RTH.
Results
The index case of pituitary-selective resistance is characterized by the missense R338W exon 9 mutation in cis with two common SNPs, rs2596623T and rs2596622C, located in the intron enhancer region of the THRB gene. Reporter gene assay experiments in GH3 pituitary-derived cells indicate that rs2596623T generates an increased pituitary cell-specific activity of the TR β2 promoter suggesting that rs2596623T leads to pituitary over-expression of the mutant allele.
Conclusions
The combined coding mutation and non-coding SNP therefore generate a tissue-specific dominant-negative condition recapitulating the patient's peculiar phenotype. This case illustrates the role of regulatory regions in modifying the clinical presentation of genetic diseases.
Background
Mutations in the THRB gene, encoding thyroid hormone receptor β (TRβ), result in the syndrome of resistance to thyroid hormone (RTH)[1,2]. RTH is a rare autosomal dominant disease with two main clinical presentations. The generalized RTH (GRTH) is characterized by elevated levels of TH, inappropriately normal or elevated levels of TSH [3], and a variable clinical presentation, ranging from asymptomatic to severe hypothyroidism [4,5]. A rarer form of RTH is characterized by selective resistance mostly limited to the pituitary (PRTH), with a prevalence of hyperthyroid symptoms since peripheral tissues are relatively less resistant to TH action but are exposed to elevated TH levels [6]. Most RTH mutations are localized in "hot spots" in the C-terminal coding exons of THRB, that generate dominant negative proteins with impaired ligand binding [7]. Despite striking differences among the clinical presentations, there is a lack of strict genotype-phenotype correlation and identical THRB gene mutations have been observed in PRTH or GRTH patients [8].
The THRB gene expresses N-terminal variant TR β1 and TR β2 isoforms from specific promoters (Figure (Figure1A).1A). TR β1 is relatively widely expressed whereas TR β2 is restricted with main sites of expression in the anterior pituitary, hypothalamus, cochlea and retina [9,10]. A conserved 600 bp intron control region (ICR) directs expression of TR β2 in the pituitary and retina in transgenic mice [10]. In this study we investigate SNPs in the ICR of the human THRB gene in RTH. The screening of the ICR in our RTH cohort demonstrated the presence of a novel and four common SNPs. We also report that in the index case of PRTH a common SNP in the ICR stimulates over-activation of the TR β2 promoter in pituitary cells in vitro. Moreover, we determined that this SNP is linked in cis with the known R338W coding mutation. We propose that this genetic "double hit" of coding and non-coding changes in the same THRB allele, ultimately generates a tissue-specific dominant negative condition underlying this case of PRTH.
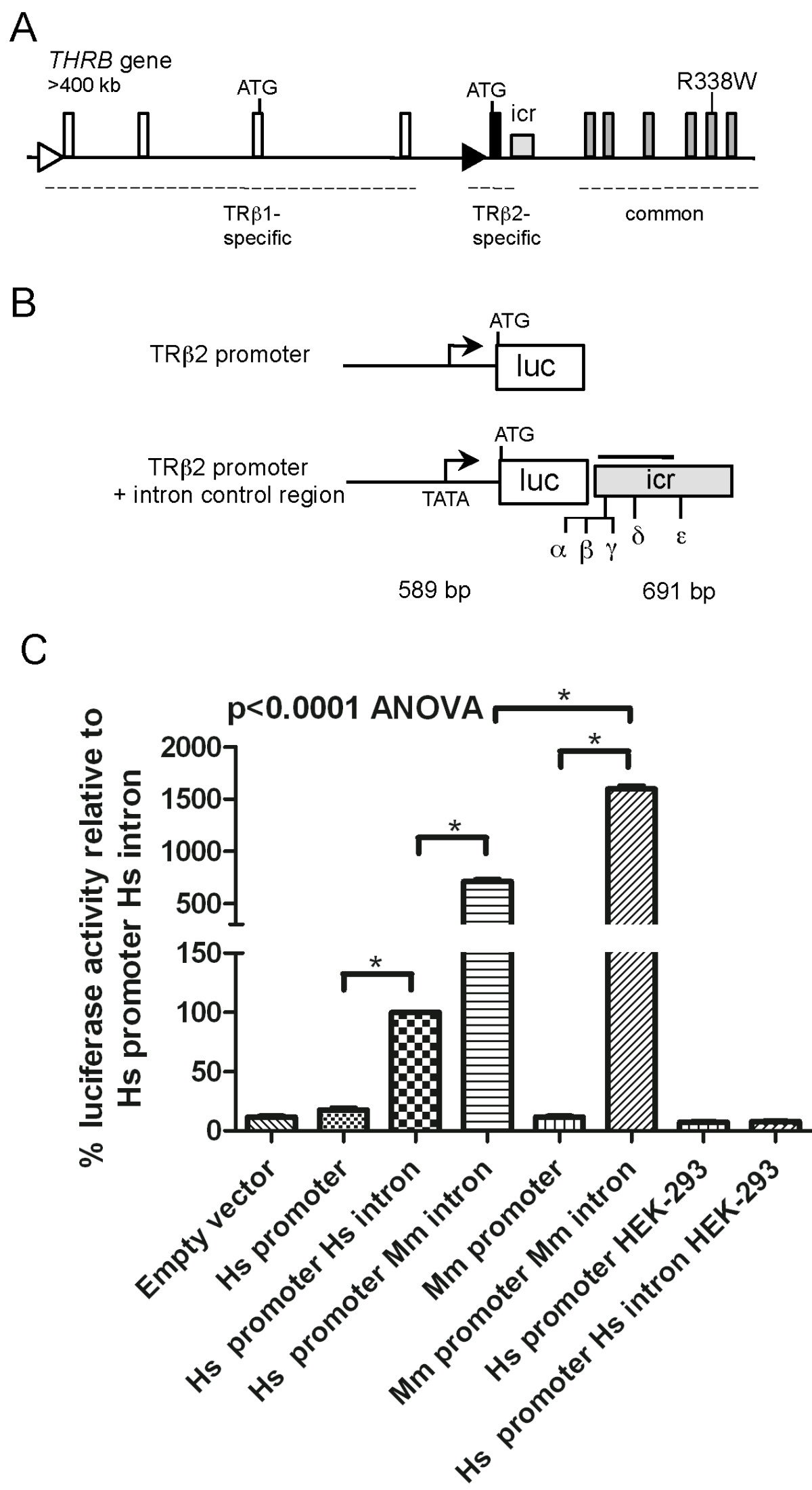
THRB gene structure, location and transcriptional activity of ICR SNPs. A. Diagram of the THRB gene showing the origins of the TR β1- and TR β2-specific transcripts. The gray square represents the conserved 600 bp TR β2-specific intron control region (ICR), homologous to the mouse sequence. The location of the exonic R338W mutation in the index case of PRTH is marked by a vertical line. Major TR β1-specific 5' exons are shown, but not all variable untranslated sequences of TR β1 transcripts are included [24]. B, Luciferase reporter gene constructs, showing in the lower half of the panel the location of SNPs found in the ICR (rs6798561 α, rs17194828 β, rs2596623 γ, rs2596622 δ, rs77624520 ε). C, Transcriptional activity of the reporter constructs; genomic sequence origin, human (Hs, Homo sapiens), mouse (Mm, Mus musculus). Compared to the human promoter alone, the human promoter plus ICR gave a 10-fold increase in pituitary cell-specific luciferase expression. Constructs containing the murine promoter and ICR are included as positive controls for ICR activity. Analysis of the chimeric reporter (human promoter + mouse ICR) indicated that the ICR activity is conserved. No activity of the ICR was observed in kidney-derived HEK-293T cells (see text for details). * = significant on Tukey's post-hoc analysis.
Methods
Patients and specimens
All patients provided an informed consent to the participation in the study and the collection of genetic material. Medical records of 45 RTH patients followed at the NIH Clinical Center since 1976, for whom DNA samples were available, were reviewed [8]. The cases were categorized as either GRTH or PRTH; the diagnosis of PRTH was made if at least two of the following findings were described in the medical records: resting tachycardia, resting energy expenditure >110% of predicted by the Harris-Benedict formula [11], hyperactive behavior, and low body mass index (<20 kg/m2 in adults or <5th percentile in children).
Genomic DNA amplification and sequencing
Exons 9, 10, and a 607 bp ICR fragment of the THRB gene of the index case of pituitary selective RTH were amplified and screened for mutations by direct sequencing. Each PCR was performed using Platinum® Taq from Invitrogen (Carlsbad, CA) using the following primers: exon 9: 5'-GAAAACCATGGGCTCAAAGA-3', and 5'-AGCGCTAGACAAGCAAAAGC-3'; exon 10: 5'-TAAAGGCCTGGAATTGGACA-3' and 5'-TCCCTCCCAACACAAAGAAA-3'; ICR: 5-TGAGGTACATTGAACATGTGC-3' and 5'-ACTGAACACCTGTTTATGGTC-3'. The sequences were analyzed and visually inspected using Sequence Scanner 1.0 (Applied Biosystems, Foster City, CA).
Reporter gene constructs
A 589 bp human TR β2 promoter fragment homologous to the mouse promoter [12] and a 691 bp ICR fragment were directionally cloned into a luciferase reporter construct in a pRep4 episomal vector [10]. Fragments were amplified by PCR from a healthy, unrelated subject using primers as follows: promoter: 5'-GGT-Nhel-TCATTACTACTGGATTTTC-3' and 5'-TGG-HindIII-GTTTCCCTGGTTCAGTTTC-3'; ICR: 5'-GTT-BamHI-TAATGGAAACATTTGAGGT-3'and 5'-GAA-SalI-AGATGAAGCAATTATGAAC-3'. This construct was used as a template to generate mutations in the ICR using QuikChange™ Site-Directed Mutagenesis (Stratagene, La Jolla, CA) (Figure (Figure1B).1B). A promoterless construct containing the ICR, and a construct lacking the ICR but containing the TR β2 promoter were generated as negative controls. A chimeric construct contained the human promoter and a mouse ICR (730bp ICR fragment of mouse Thrb gene obtained by PCR) (NC_00085.5) as described [10]. All constructs were sequenced in entirety.
Transfection and luciferase assay
Media, sera, antibiotics for cell culture, and transfection reagents were purchased from Invitrogen (Carlsbad, CA). GH3 or HEK-293T cells (6 × 103 cells/cm2) were grown in DMEM supplemented with 10% (v/v) FBS and 2 mM glutamine. Cultures were maintained at 37°C in a humidified atmosphere containing 5% (v/v) CO2.
Transfections with 200 ng of each luciferase reporter plasmid, 20 ng of pSV-RL Renilla internal control plasmid, and empty pcDNA-3 carrier DNA were performed with Lipofectamine™. Thirty-six hours after transfection, the cells were kept in serum-free medium overnight before harvesting. Luciferase activity was assayed with a dual-luciferase system and normalized to renilla activity (Promega, Madison, WI).
Statistical Analysis
The frequency of categorical variables (clinical characteristics and generalized vs. pituitary-selective RTH) was analyzed by chi-square, while continuous variables (luciferase reporter assay data) were assessed by one-way ANOVA with Tukey's post-hoc analysis. An α error of 0.05 was considered the threshold for statistical significance.
Results
Case presentation
This case was originally described by Gershengorn and Weintraub in 1975 [6]. This female patient, currently 53-years old, was at age 7 referred to the NIH Clinical Center because of goiter and hyperthyroidism. The evaluation showed increased thyroid hormone and TSH levels, with a net prevalence of hyperthyroid signs, including tachycardia, increased basal metabolic rate, and brisk reflexes. Further studies demonstrated the absence of a pituitary adenoma and the rise in TSH following the injection of TRH. The clinical picture was interpreted as "Thyrotropin-induced hyperthyroidism caused by a selective pituitary resistance to thyroid hormone" [6]. The patient was initially treated with methimazole, but the therapy was discontinued because of urticaria, and a sub-total thyroidectomy was performed. The symptoms recurred after surgery and she was treated with propylthiouracil and, at age 32, with radioactive iodine ablation. Since then, the patient has been maintained on levothyroxine and beta-blockers with the therapeutic goal of clinical euthyroidism and a TSH level in the high-normal range. The patient is doing well and has a 20-year old non-affected daughter in good health. Her therapy consists of levothyroxine 175 mcg daily (3.8 mcg/Kg), Atenolol 25 mg daily; her most recent laboratory data are: fT4 3.8 ng/dL (0.8-1.5), TSH 5.05 mcIU/mL (0.4-4.0), cholesterol 199 mg/dL (<200), triglycerides 81 mg/dL (<150), HDL 60 mg dL (>60), LDL 123 mg/dL (<100), SHBG 60 nmol/l (18-114), ACE 41 U/L (16-52). A DEXA scan showed a T-score of -0.6.
Assignment of ICR haplotype in the index case of PRTH
The index case of PRTH, affected by a previously characterized mutation in the exon 9 (R338W)[13], carried the heterozygous genotypes C/A in rs17194828, C/T in rs2596623, and T/C in rs2596622. In order to deduce the patient's haplotype, the ICR and the TR β exon 9 of her unaffected daughter were genotyped. Direct sequencing showed that the daughter is homozygous for the wild type R338 allele, heterozygous for the rs17194828 SNP (C/A genotype), and homozygous for the rs2596623 and rs2596622 SNPs (C/C and T/T genotypes, respectively). The data thus indicate that the patient's mutant W338 allele is in cis with the rs2596623T allele and rs2596622 C allele, and the unaffected daughter inherited the maternal haplotype rs2596623C-rs2596622T, and R338 (Figure (Figure2).2). Due to the unavailability of paternal DNA, we cannot assign the genotype for the rs17194828 SNP. Direct sequencing of a cohort of 45 genomic samples of RTH NIH cohort patients (Table (Table1)1) [8] revealed the presence of two additional SNPs, the previously described rs6798561G/A, and a novel one, rs77624520G/C present in a single case. A schematic representation of the ICR SNPs distribution in the THRB gene is reported in figure figure1.1. Nο statistical difference was found between GRTH and PRTH in the prevalence of each of the SNPs (Table (Table22).
Table 1
Clinical presentation and mutations in the exons 9 and 10 of the THRB gene in the NIH RTH cohort
| Exon | Amino acid change | Form | n. subjects |
|---|---|---|---|
| 9 | A317T | GRTH | 3 |
| 9 | R320L | GRTH | 1 |
| 9 | D322H | GRTH | 3 |
| 9 | G332R | GRTH | 1 |
| 9 | R338W | GRTH | 2 |
| PRTH | 2 | ||
| 9 | G345S | GRTH | 3 |
| 9 | G347A | GRTH | 3 |
| PRTH | 1 | ||
| 10 | R383H | GRTH | 1 |
| 10 | R438H | GRTH | 1 |
| 10 | M442V | GRTH | 1 |
| PRTH | 1 | ||
| 10 | M442R | GRTH | 2 |
| PRTH | 1 | ||
| 10 | FRSH 448 | PRTH | 1 |
| 10 | P453H | GRTH | 7 |
| PRTH | 2 | ||
| Not found | n/a | GRTH | 6 |
| PRTH | 3 | ||
Table 2
Prevalence of the SNPs in the intron control region of the THRB gene in the NIH RTH cohort
| SNP ID | Generalized RTH | Pituitary RTH | Odds ratio [CI] | p value | ||
|---|---|---|---|---|---|---|
| Subjects | Sex | Subjects | Sex | |||
| n = 34 | 16 M | n = 11 | 8 M | |||
| 18 F | 3 F | |||||
| rs6798561 | n | % | n | % | ||
| Genotype | ||||||
| GG | 33 | 97.0 | 11 | 100 | ||
| GA | 1 | 3 | 0 | 0 | n.a | n.a |
| AA | 0 | 0 | 0 | 0 | ||
| rs17194828 | n | % | n | % | CC vs. CA plus AA | |
| Genotype | ||||||
| CC | 28 | 82.3 | 7 | 63.6 | ||
| CA | 6 | 17.6 | 4 | 36.4 | 2.7 [0.6 to 12.1] | 0.228 |
| AA | 0 | 0 | 0 | 0 | ||
| rs2596623 | n | % | n | % | CC vs. CT plus TT | |
| Genotype | ||||||
| CC | 13 | 38.2 | 3 | 27.3 | ||
| CT | 18 | 52.9 | 7 | 63.6 | 1.7 [0.4 to 7.4] | 0.720 |
| TT | 3 | 8.82 | 1 | 9.1 | ||
| rs2596622 | n | % | n | % | TT vs. TC plus CC | |
| Genotype | ||||||
| TT | 15 | 44.1 | 3 | 27.3 | ||
| TC | 16 | 47.0 | 8 | 72.7 | 2.1 [0.5 to 9.3] | 0.482 |
| CC | 3 | 8.8 | 0 | 0 | ||
| rs77624520 | n | % | n | % | ||
| Genotype | ||||||
| GG | 33 | 97.0 | 11 | 100 | ||
| GC | 1 | 3 | 0 | 0 | n.a | n.a |
| CC | 0 | 0 | 0 | 0 | ||
The SNPs are listed according to the location on the chromosome as reported on Ensembl database.

Haplotype assignment of the index case of PRTH. Direct sequencing the ICR and exon 9 of the THRB demonstrated that the patient carried the heterozygous genotypes C/A in rs17194828, C/T in rs2596623, and T/C in rs2596622, and the R338W mutation. Conversely, the unaffected daughter carries the heterozygous genotype C/A in rs17194828. The data thus indicate that the patient's mutant W338 allele is in cis with the rs2596623 T and rs2596622 C allele and the unaffected daughter inherited the maternal haplotype rs2596623C-rs2596622T, and R338.
Transcriptional enhancer function of the human ICR
Analyses of a luciferase reporter construct containing the human TR β2 promoter demonstrated that in pituitary-derived GH3 cells, the human ICR stimulated a 10-fold increase in luciferase expression compared to a reporter carrying the promoter alone (p < 0.0001). In a chimeric reporter, the murine ICR also stimulated the human promoter indicating that ICR function was conserved between species, consistent with the high sequence conservation. Compared to the human ICR, the murine ICR stimulated a greater degree of enhancement of luciferase activity (p < 0.0001) under the assay conditions used (Figure (Figure1C).1C). Similarly to previous observations with murine sequences, no enhancer activity was observed in kidney-derived HEK-293T cells, demonstrating that the activity of the human ICR is cell type-specific.
Enhancer function of ICR with different SNPs
To evaluate the effects of ICR SNPs on TR β2 promoter activity, we generated luciferase reporter constructs containing the SNPs observed in the NIH RTH cohort. We also generated a rs2596623T-rs2596622C construct replicating the ICR haplotype of the PRTH index case. Compared to the ancestral allele the rs2596623T produced a significant 30% increase in luciferase expression (p < 0.0001), suggesting that the rs2596623T increased transcriptional activity of the TR β2 promoter. Compared to rs2596623T alone, the rs2596623T-rs2596622C haplotype resulted in a marginal, non-significant increase in transcriptional activity (p = 0.543), indicating that the increase in transcriptional activity is solely due to the rs2596623T (Figure (Figure3).3). The other SNPs did not significantly change expression levels of luciferase.

Modulatory effects of SNPs on the expression of the TR β2 promoter-luciferase-ICR reporter construct. The SNPs detected in the index case of PRTH were introduced in the reporter gene construct by site-directed mutagenesis. As compared to the "wild type" sequence, rs2596623T gave a significant 30% increase in transcriptional activity. A similar increase in transcriptional activity was observed when the cells were transfected with the rs2596623T/rs2596622C naturally occurring haplotype. Transfections of the rs2596622C or rs17194828A SNPs alone did not result in a significant change in the transcriptional activity of the reporter gene construct. No difference in transcriptional activity was observed after transfection with SNPs rs6798561A and rs77624520C (data not shown) (see text for details). The results were confirmed by three independent preparations of reporter plasmids that were tested at least thrice with triplicate points determined for each assay * = significant on Tukey's post-hoc analysis.
Discussion
Since the original description of RTH [1], and the subsequent description of the PRTH form [6], many investigators have attempted to demonstrate a correlation between genotype and phenotype. Although some mutations in the THRB gene may associate preferentially with PRTH, and among them R338W appears particularly frequent, patients sharing the same mutation can present the entire range of symptoms associated with the various forms of RTH [5,13]. Furthermore, although the clinical presentation tends to segregate within the kindred, there is no absolute concordance, and even among siblings the phenotype differences can be remarkable [14]. In vitro experiments demonstrated that the R338W mutant receptor has reduced affinity for TH and a dominant negative activity typical of RTH syndrome [13,15]. Some in vitro data suggest that R338W selectively impairs TR β2 rather than TR β1 isoform function [16], but this finding does not explain completely the clinical presentation since R338W is also associated with GRTH. Machado and co-workers reported that a naturally occurring mutation (R429Q) associated with PRTH produces a mutant TR β product that is specifically defective as a transcriptional repressor, while retaining relatively normal activator function [17]. This particular mutation is thus in keeping with a selective PRTH, since a main action of thyroid hormone in the thyrotroph is the inhibition of TSH β-subunit gene transcription. Taken together, these clinical and laboratory observations suggest that various factors, regardless of the particular THRB mutation, contribute to the clinical presentation of RTH.
Our hypothesis that ICR mutations play a role in the RTH presentation was further supported by the growing recognition that sequence variations in cis-acting control regions determine differences in gene expression levels. Furthermore, association studies have identified non-coding sequences as a risk factor for various diseases [18,19]. We have shown that the human ICR, like the murine ICR, stimulates TR β2 promoter activity in pituitary- but not in kidney-derived cell lines. The somewhat weaker activity of the human ICR than the murine ICR may reflect bias in the assay which was performed in rodent GH3 cells. Alternatively, there may be real species differences in the magnitude of the response since thyroid homeostasis parameters differ in degree between rodents and humans, as indicated for example by their different serum ratios of T3/T4 [20], or by different patterns of expression of deiodinase type-2 [21].
Our study of the NIH RTH cohort demonstrated four known SNPs and one novel SNP in the human ICR. The data indicate that in the index case of PRTH a specific ICR haplotype resides in cis to the R338W THRB gene pathogenic mutation. Further, when we tested the activity of each individual SNP, only rs2596623T gave a significant increase in reporter gene expression. rs2596623 resides near the 5' boundary of a highly conserved 380 bp sequence in the ICR that is sufficient to direct TR β2 promoter activation in the pituitary but not retina in vivo in transgenic mice [10]. We speculate that rs2596623T enhances the recruitment or binding stability of a transcription factor complex that activates the TR β2 promoter in the pituitary.
Our finding tend to support the hypothesis that rs2596623T stimulates pituitary over-expression of the pathogenic R338W TR β2 protein, tilting the balance of expression between the wild type and R338W mutant THRB alleles in a tissue-specific fashion. This dominant negative condition would result in a relatively increased resistance in the pituitary as compared to the peripheral tissues, recapitulating the patient's phenotype. Indeed, of the four unrelated RTH patients who carry the R338W mutation in the NIH RTH cohort (Table (Table1),1), the PRTH phenotype is limited to the two cases heterozygous for rs2596623 (C/T). For the second heterozygous case, due to lack of genetic material from relatives, we are unable to indicate whether rs2596623T resides in cis with the pathogenic R338W mutation. Nonetheless one could speculate that in the two R338W GRTH cases the homozygosity at rs2596623 (C/C or T/T) results in "balanced" expression of the wild type and mutant receptors in the pituitary, not dissimilar from peripheral tissues.
No statistical association was found between any SNP and the phenotypes. This is not surprising because of the relatively small number of subjects and the lack of genetic material that would allow us to determine haplotypes in most cases.
Gene expression levels are heritable and several human diseases have been associated with changes in enhancer sequences rather than coding exons. For example, a polymorphic intron sequence that increases COL1A1 transcription has been associated with changes in bone mineral density and increased incidence of osteoporotic bone fractures [22]. Another mutation that decreases activity of an intronic enhancer in the RET gene and has been associated with some cases of Hirschsprung disease [23]. The case of PRTH we describe is unique in suggesting a genetic "double hit" that combines an enhancer SNP and a coding exon change on a single THRB allele.
Conclusions
The molecular characterization of the index case of PRTH is unique in illustrating a genetic "double hit" that combines an enhancer SNP and a coding exon mutation on a single THRB allele resulting in the modulation of the clinical presentation.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ATA, carried out the in vitro studies, and wrote the manuscript draft. VC, contributed to the in vitro studies and analyzed the clinical data. HL, designed the reporter gene assay and contributed to the in vitro studies. CSC, MCS, contributed to the collection and analysis of the clinical data. DF contributed in the design of the study and in the interpretation of the data, and contributed to the writing of the manuscript. FSC, conceived the study, analyzed the data and supervised the writing of the manuscript.
All authors read and approved the final manuscript.
Acknowledgements
This research could have not been accomplished without the selfless participation of the patients enrolled in the NIH RTH cohort. This work was supported by the Intramural Research Program of the NIDDK, programs Z01-DK047057-01, and DK 047037-04.
References
- Refetoff S, DeWind LT, DeGroot LJ. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967;27:279–294. 10.1210/jcem-27-2-279. [Abstract] [CrossRef] [Google Scholar]
- Sakurai A, Takeda K, Ain K, Ceccarelli P, Nakai A, Seino S, Bell GI, Refetoff S, DeGroot LJ. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor beta. Proc Natl Acad Sci USA. 1989;86:8977–8981. 10.1073/pnas.86.22.8977. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chatterjee VK. Resistance to thyroid hormone--an uncommon cause of thyroxine excess and inappropriate TSH secretion. Acta Med Austriaca. 1994;21:56–60. [Abstract] [Google Scholar]
- Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399. [Abstract] [Google Scholar]
- Weiss RE, Marcocci C, Bruno-Bossio G, Refetoff S. Multiple genetic factors in the heterogeneity of thyroid hormone resistance. J Clin Endocrinol Metab. 1993;76:257–259. 10.1210/jc.76.1.257. [Abstract] [CrossRef] [Google Scholar]
- Gershengorn MC, Weintraub BD. Thyrotropin-induced hyperthyroidism caused by selective pituitary resistance to thyroid hormone. A new syndrome of "inappropriate secretion of TSH". J Clin Invest. 1975;56:633–642. 10.1172/JCI108133. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Adams M, Matthews C, Collingwood TN, Tone Y, Beck-Peccoz P, Chatterjee KK. Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone. Identification of thirteen novel mutations in the thyroid hormone receptor beta gene. J Clin Invest. 1994;94:506–515. 10.1172/JCI117362. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brucker-Davis F, Skarulis MC, Grace MB, Benichou J, Hauser P, Wiggs E, Weintraub BD. Genetic and clinical features of 42 kindreds with resistance to thyroid hormone. The National Institutes of Health Prospective Study. Ann Intern Med. 1995;123:572–583. [Abstract] [Google Scholar]
- Hodin RA, Lazar MA, Wintman BI, Darling DS, Koenig RJ, Larsen PR, Moore DD, Chin WW. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989;244:76–79. 10.1126/science.2539642. [Abstract] [CrossRef] [Google Scholar]
- Jones I, Ng L, Liu H, Forrest D. An intron control region differentially regulates expression of thyroid hormone receptor beta2 in the cochlea, pituitary, and cone photoreceptors. Mol Endocrinol. 2007;21:1108–1119. 10.1210/me.2007-0037. [Abstract] [CrossRef] [Google Scholar]
- Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie Institute of Washington; 1919. [Google Scholar]
- Wood WM, Dowding JM, Bright TM, McDermott MT, Haugen BR, Gordon DF, Ridgway EC. Thyroid hormone receptor beta2 promoter activity in pituitary cells is regulated by Pit-1. J Biol Chem. 1996;271:24213–24220. 10.1074/jbc.271.39.24213. [Abstract] [CrossRef] [Google Scholar]
- Sasaki S, Nakamura H, Tagami T, Miyoshi Y, Nogimori T, Mitsuma T, Imura H. Pituitary resistance to thyroid hormone associated with a base mutation in the hormone-binding domain of the human 3,5,3'-triiodothyronine receptor-beta. J Clin Endocrinol Metab. 1993;76:1254–1258. 10.1210/jc.76.5.1254. [Abstract] [CrossRef] [Google Scholar]
- Beck-Peccoz P, Chatterjee VK. The variable clinical phenotype in thyroid hormone resistance syndrome. Thyroid. 1994;4:225–232. 10.1089/thy.1994.4.225. [Abstract] [CrossRef] [Google Scholar]
- Sasaki S, Nakamura H, Tagami T, Miyoshi Y, Nakao K. Functional properties of a mutant T3 receptor beta (R338W) identified in a subject with pituitary resistance to thyroid hormone. Mol Cell Endocrinol. 1995;113:109–117. 10.1016/0303-7207(95)03621-D. [Abstract] [CrossRef] [Google Scholar]
- Safer JD, Langlois MF, Cohen R, Monden T, John-Hope D, Madura J, Hollenberg AN, Wondisford FE. Isoform variable action among thyroid hormone receptor mutants provides insight into pituitary resistance to thyroid hormone. Mol Endocrinol. 1997;11:16–26. 10.1210/me.11.1.16. [Abstract] [CrossRef] [Google Scholar]
- Machado DS, Sabet A, Santiago LA, Sidhaye AR, Chiamolera MI, Ortiga-Carvalho TM, Wondisford FE. A thyroid hormone receptor mutation that dissociates thyroid hormone regulation of gene expression in vivo. Proc Natl Acad Sci USA. 2009;106:9441–9446. 10.1073/pnas.0903227106. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Epstein DJ. Cis-regulatory mutations in human disease. Brief Funct Genomic Proteomic. 2009;8:310–316. 10.1093/bfgp/elp021. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Visel A, Rubin EM, Pennacchio LA. Genomic views of distant-acting enhancers. Nature. 2009;461:199–205. 10.1038/nature08451. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Segal J, Troen BR, Ingbar SH. Influence of age and sex on the concentrations of thyroid hormone in serum in the rat. J Endocrinol. 1982;93:177–181. 10.1677/joe.0.0930177. [Abstract] [CrossRef] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. 10.1210/er.23.1.38. [Abstract] [CrossRef] [Google Scholar]
- Mann V, Hobson EE, Li B, Stewart TL, Grant SF, Robins SP, Aspden RM, Ralston SH. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest. 2001;107:899–907. 10.1172/JCI10347. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Emison ES, McCallion AS, Kashuk CS, Bush RT, Grice E, Lin S, Portnoy ME, Cutler DJ, Green ED, Chakravarti A. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature. 2005;434:857–863. 10.1038/nature03467. [Abstract] [CrossRef] [Google Scholar]
- Frankton S, Harvey CB, Gleason LM, Fadel A, Williams GR. Multiple messenger ribonucleic acid variants regulate cell-specific expression of human thyroid hormone receptor beta1. Mol Endocrinol. 2004;18:1631–1642. 10.1210/me.2003-0346. [Abstract] [CrossRef] [Google Scholar]
Articles from Journal of Translational Medicine are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/1479-5876-9-144
Read article for free, from open access legal sources, via Unpaywall:
https://translational-medicine.biomedcentral.com/counter/pdf/10.1186/1479-5876-9-144
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/104870509
Article citations
Expanding the phenotype of THRB: a range of macular dystrophies as the major clinical manifestations in patients with a dominant splicing variant.
Front Cell Dev Biol, 11:1197744, 21 Jul 2023
Cited by: 3 articles | PMID: 37547476 | PMCID: PMC10401274
Noncoding Mutations in a Thyroid Hormone Receptor Gene That Impair Cone Photoreceptor Function.
Endocrinology, 164(3):bqad006, 01 Jan 2023
Cited by: 3 articles | PMID: 36631163 | PMCID: PMC10091487
Severe Resistance to Thyroid Hormone Beta in a Patient with Athyreosis.
Thyroid, 32(3):336-339, 01 Mar 2022
Cited by: 6 articles | PMID: 34969265 | PMCID: PMC8971974
Uncovering Evidence for Endocrine-Disrupting Chemicals That Elicit Differential Susceptibility through Gene-Environment Interactions.
Toxics, 9(4):77, 06 Apr 2021
Cited by: 4 articles | PMID: 33917455 | PMCID: PMC8067468
Review Free full text in Europe PMC
Resistance to Thyroid Hormone Beta: A Focused Review.
Front Endocrinol (Lausanne), 12:656551, 31 Mar 2021
Cited by: 35 articles | PMID: 33868182 | PMCID: PMC8044682
Review Free full text in Europe PMC
Go to all (17) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
SNPs (5)
- (7 citations) dbSNP - rs17194828
- (7 citations) dbSNP - rs2596622
- (4 citations) dbSNP - rs2596623
- (2 citations) dbSNP - rs77624520
- (2 citations) dbSNP - rs6798561
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Introducing a point mutation identified in a patient with pituitary resistance to thyroid hormone (Arg 338 to Trp) into other mutant thyroid hormone receptors weakens their dominant negative activities.
J Endocrinol, 151(2):293-300, 01 Nov 1996
Cited by: 4 articles | PMID: 8958790
Pituitary resistance to thyroid hormone syndrome is associated with T3 receptor mutants that selectively impair beta2 isoform function.
Mol Endocrinol, 19(6):1529-1542, 31 Mar 2005
Cited by: 35 articles | PMID: 15802373
Variable Clinical Characteristics and Molecular Spectrum of Patients with Syndromes of Reduced Sensitivity to Thyroid Hormone: Genetic Defects in the THRB and SLC16A2 Genes.
Horm Res Paediatr, 90(5):283-290, 29 Nov 2018
Cited by: 4 articles | PMID: 30497070
Homozygous thyroid hormone receptor β-gene mutations in resistance to thyroid hormone: three new cases and review of the literature.
J Clin Endocrinol Metab, 97(4):1328-1336, 08 Feb 2012
Cited by: 49 articles | PMID: 22319036 | PMCID: PMC3319181
Review Free full text in Europe PMC
Funding
Funders who supported this work.

 1
1


