Abstract
Free full text

Isolation of Brain-infiltrating Leukocytes
Abstract
We describe a method for preparing brain infiltrating leukocytes (BILs) from mice. We demonstrate how to infect mice with Theiler's murine encephalomyelitis virus (TMEV) via a rapid intracranial injection technique and how to purify a leukocyte-enriched population of infiltrating cells from whole brain. Briefly, mice are anesthetized with isoflurane in a closed chamber and are free-hand injected with a Hamilton syringe into the frontal cortex. Mice are then killed at various times after infection by isoflurane overdose and whole brains are extracted and homogenized in RPMI with a Tenbroeck tissue grinder. Brain homogenates are centrifuged through a continuous 30% Percoll gradient to remove the myelin and other cell debris. The cell suspension is then strained at 40 μm, washed and centrifuged on a discontinuous Ficoll-Paque Plus gradient to select and purify the leukocytes. The leukocytes are then washed and resuspended in appropriate buffers for immunophenotyping by flow cytometry. Flow cytometry reveals a population of innate immune cells at the early stages of infection in C57BL/6 mice. At 24 hours post infection, multiple subsets of immune cells are present in the BILs, with an enriched population of Gr1+, CD11b+ and F4/80+cells. Therefore, this method is useful in characterizing the immune response to acute infection in the brain.
Protocol
1. Intracranial virus injection:
The following technique has been modified and utilized extensively by our lab and colleagues. Briefly, intracranial injection of the Daniel's strain of Theiler's murine encephalomyelitis virus (TMEV) or sham-infection (1, 10) is performed on young mice (preferably 5-6 weeks of age) to elicit brain infiltrating leukocytes (BILs). Please note that results will differ between the Daniel's strain, the BeAN strain, and the GDVII strain. For the purpose of harvesting BILs, mice receive 2x105 PFU of TMEV and are infected for 24 hours.
Attach the injection needle (27 gauge, ¼ in, Kendall), to an automatic 1 ml Hamilton syringe and set to deliver 10 μl of virus.
Draw up TMEV in 10 μL DMEM into the syringe with careful attention to air bubbles. When appropriate, sham infected mice receive 10 μl of virus-free DMEM.
Just prior to injection, pour approximately 1 mL of isoflurane into a bell jar that contains a wire grid with cotton substrate underneath.
Place mice direclty on wire grid in the bell jar until they become lightly anesthetized, insensitive to toe-pinch and immobilized by the inhalant anesthetic, approximately 10-15 seconds. Mice should not come in direct contact with isoflurane, as substance can be irritating and caustic.
While under anesthesia, prepare mice for injection by quickly locating appropriate coordinates in the frontal cortex region on the skull using shaving and inking. The general location for injection is 1 mm anterior to bregma and 1 mm lateral to the sagittal suture on the right side (Figure 1). While stereotactic injection may be appropriate for some experimental situations, we have found that free-hand injection without shaving or inking is suitable for the majority of experiments. Parting the fur with the needle point along both axes is appropriate for injection.
To make an injection, orient the needle perpendicular to the skull and press through the bone to approximately a 3 mm depth.
Express virus into the brain by pressing the Hamilton syringe button down and waiting 2 seconds for the virus to enter the brain before withdrawing.
Carefully remove the needle and place the mouse in a clean, dry cage. Mice awaken from anesthesia quickly and resume normal function within minutes. Give proper attention to mice that exhibit abnormal symptoms, such as excessive bleeding from the injection site, as euthanasia of the animal may be appropriate. Proper animal handling 6 and adherence to the National Institutes of Health and Institutional Animal Care and Use Committee guidelines must be followed.
2. Brain-infiltrating leukocyte cell preparation:
Prepare round-bottom Oak Ridge centrifuge tubes which can hold a capacity of 30 ml with 10 ml RPMI 1640, 9 ml Percoll and 1 ml 10X PBS and tubes should sit at RT on the bench during the brain removal process.
Bring Ficoll-Paque Plus to room temperature (RT).
Euthanize animal by modified isoflurane overdose 5 and quickly remove the brain using a stainless steel tissue spatula and place in a 15 ml conical tube containing 5 ml RPMI on ice. Under some circumstances, PBS-perfusion may be appropriate to remove circulating peripheral blood cells from the BILs preparation. Mice should be fully and properly anesthetized prior to PBS-perfusion.
Transfer brain and RPMI solution to a 7 ml glass Pyrex brand Tenbroeck tissue grinder and gently homogenize with 10-15 strokes.
Pipet an additional 5 ml RPMI into the homogenizer and pipet up and down twice. Transfer brain homogenate (approx. 10 ml) to previously prepared round-bottom tube and gently invert 2-3 times to mix.
Spin brain homogenate at 7800gave for 30 min at RT in a F0360 fixed-angle rotor in a Beckman Allegra X-22R clinical centrifuge.
After centrifugation, remove myelin debris that has floated to the top of the gradient. Collect the leukocyte layer that floats above the red blood cell pellet (Figure 2).
Strain leukocyte layer through a 40 μm cell strainer into a 50 ml conical. Bring solution up to 50 ml volume with RMPI.
Spin tubes containing diluted cell suspension in a clinical centrifuge at 1500 rpm (600gave) for 5 min at RT.
Aspirate the supernatant and collect the cell pellet. Resuspend the pellet in 1 ml FACS Buffer (1% bovine serum albumin, 0.02% sodium azide in calcium and magnesium-free PBS) and transfer the cell suspension to 5 ml round bottom FACS tubes.
Carefully underlay suspension with 1 ml Ficoll-Paque Plus.
Spin tube at 2500 rpm (1400 gave) in clinical centrifuge for 25 min at RT with no brake.
Remove white, fluffy layer at the interface (Figure 2) with 1 ml pipette and transfer to new 5 ml FACS tube. Wash cells with 4 ml FACS buffer.
Spin tube at 1500 rpm (600gave) for 5 min at RT in clinical centrifuge to pellet cells.
Aspirate supernatant and collect cell pellet. Resuspend the leukocyte pellet in appropriate buffer and keep on ice in new FACS tube.
Count cells, assess cell viability by Trypan blue exclusion and analyze by flow cytometry. In general, investigators can expect to acquire approximately 5 x 105 cells/animal.
3. Flow cytometric immunophenotyping
After isolating and counting the leukocytes, spin the cells for 3 minutes at 1500 rpm (600 gave) in a clinical centrifuge.
Resuspend the pellet in blocking buffer containing the following: FACS buffer, supernatant from 2.4G2 hybridoma (Fc block; anti-CD16/32) and fetal bovine serum at a ratio of 10:5:1 and incubate for 30 minutes at 4°C.
Prepare and add conjugated antibodies against extracellular antigens to the blocked cells at a concentration of 1:200 and incubate for 30 minutes at 4°C in the dark. Stain and incubate cells in 200 μl in a 96-well V-bottom plate. Appropriate controls include unstained cells and fluorochrome compensation samples, as necessary.
Spin the stained cells at RT for 3 minutes at 1500 rpm (600gave) in a clinical centrifuge using a rotor appropriate for 96-well plates.
Aspirate the supernatant and wash the cells with 200 μl FACS buffer by gently pipetting up and down 3 times.
Repeat the above washing technique twice.
After washing, resuspend the cells in 2% paraformaldehyde and transfer to FACS tubes. Fixation is required for flow cytometric analysis of cells infected of Biosafety Level 2 reagents.
Run the fixed cells on a BD FACS Calibur following a modified flow cytometry method 8 and analyze files offline using FlowJo, WinMDI or other available flow cytometry analysis software.
The analysis of 50-100,000 events per sample is generally required for adequate immunophenotyping.
Directly conjugated antibodies used in this experiment:
CD45 was detected with clone 30-F11. Ly6C/G was detected with clone Gr1, RB6-8C5. CD11b was detected with clone M1/70. F4/80 was detected with clone BM8.
4. Representative Results:
We show cell phenotyping results for mouse BILs at 24 hours post infection (Figure 3). Leukocytes were stained with PerCP-conjugated anti-mouse CD45 to detect immune cells, PE-conjugated anti-mouse Ly6C/G to detect inflammatory monocytes, APC-conjugated anti-mouse CD11b and APC-conjugated anti-mouse F4/80 to detect cells of monocyte lineage. The analysis was performed with a BD FACS Calibur instrument.
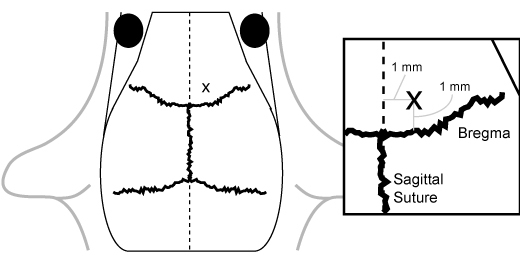
Figure 1. Anatomical localization of the injection site. The injection site is located 1 mm anterior to bregma and 1 mm lateral to the sagittal suture on the right side.
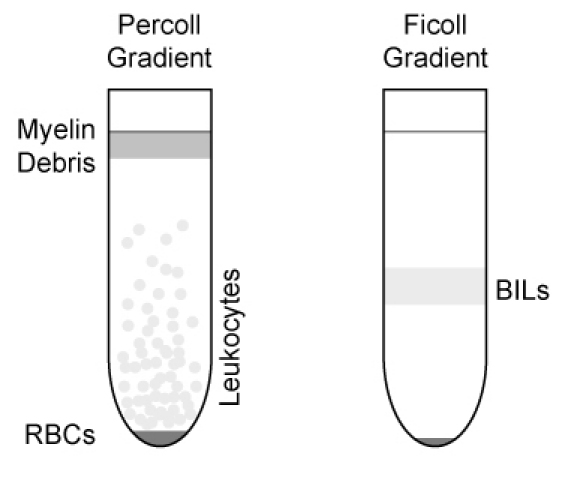
Figure 2. Illustration of gradient separation of leukocytes and BILs. Leukocytes are initially collected directly below the myelin debris layer and above the RBC pellet in the Percoll gradient. The white, fluffy layer (BILs) at the interface is collected from the Ficoll gradient.
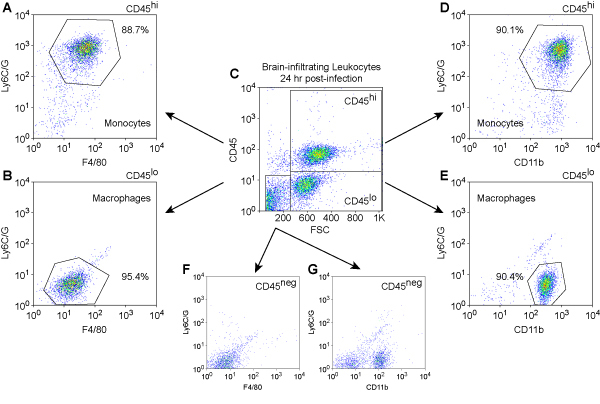
Figure 3. Immunophenotype of brain-infilatrating leukocytes at 24 hr post-infection.Mouse leukocytes were isolated from the brain by differential density centrifugation of tissue homogenates prepared from animals at 24 hr after intracranial infection. Cells were stained with fluorescently-conjugated antibodies against mouse CD45, Ly6C/G, CD11b, and F4/80. Initial gating was performed by plotting CD45, a marker for immune cells, against forward scatter (FSC), an indicator of cell size (C). The CD45hi (A, D), CD45lo (B, E), and CD45neg (F, G) populations were then analyzed for relative expression levels of the monocyte and macrophage markers Ly6C/G, F4/80 (A, B, F), and CD11b (D, E, G). Ly6C/G+F4/80+CD11b+ inflammatory monocytes were found almost exclusively in the CD45hi population (A, D), consistent with the infiltration of these cells from the periphery. In contrast, Ly6C/G-F4/80loCD11b+ macrophages were found almost exclusively in the CD45lo (B, E) and CD45neg (G) populations, consistent with a resident phenotype. In numerous experiments, we have never observed CD45hi cells or Ly6C/G+F4/80+CD11b+ inflammatory monocytes in the brain of uninfected or sham-infected mice (data not shown).
Discussion
We routinely use flow cytometry to determine both the quality of the brain infiltrating cell preparation, and to distinguish different populations of immune cells 2, 9. At acute time-points, our BILs method yields high percentages of inflammatory monocytes within the CD45hi population as well as high percentages of macrophages in the CD45lo population. This indicates that a reproducible immune response within the brain can robustly be characterized by our method.
This technique is of particular importance for experiments that require a well-characterized population of immune cells from the mouse brain. We have previously performed adoptive transfer experiments by injecting our brain infiltrating leukocytes into host mice via tail vein injection 7, and we have characterized the BILs via various in vitro experiments 9. Our BILs preparation is particularly beneficial in experiments that must distinguish different populations of immune cells from resident cells of the brain, including resident macrophages and microglia. Another application of interest includes real-time RT-PCR analysis performed on total RNA isolated from BILs at 7 days post infection to identify effector functions 3. For example, we measured GAPDH, granzyme B and perforin RNA in BILs collected at 7 days post infection from perforin-competent and -deficient animals infected with TMEV. We have also utilized our BILs method to determine how NKG2D contributes to clearing TMEV from the brain of acutely infected mice 4.
There are several critical aspects of the method. It is essential to bring all solutions to room temperature prior to preparation to avoid undue stress to the leukocytes and to ensure proper densitometric separation. Stress on the leukocytes contributes to cell death, which makes them especially unreliable for in vivo experiments where a live population of cells is adoptively transferred into a host animal. We have also determined that the use of cold Percoll not only changes the density of the discontinuous gradient, but also affects the quality of the leukocytes. Another critical element is sufficient and gentle homogenization of brain tissue. We have found that inadequate and rough homogenization of brain tissue results in copious amounts of cell debris seen by microscopy and flow cytometry. Cell debris may also result from not straining the brain homogenate with the appropriately sized cell strainer. Proper attention to detail is key in preparing ample and sufficient BILs for further experimentation and analysis.
Acknowledgments
This work was supported by grant NS64571 from the NINDS (CLH), by an early career development award from the Mayo Clinic (CLH), and by a generous gift from Donald and Frances Herdrich (CLH). We would like to thank the Mayo Clinic Flow Cytometry Core for assistance.
References
- Buenz EJ, Howe CL. Picornaviruses and cell death. Trends Microbiol. 2006;14:28–36. [Abstract] [Google Scholar]
- Buenz EJ, Limberg PJ, Howe CL. A high-throughput 3-parameter flow cytometry-based cell death assay. Cytometry A. 2007;71:170–173. [Europe PMC free article] [Abstract] [Google Scholar]
- Deb C, Lafrance-Corey RG, Zoecklein L, Papke L, Rodriguez M, Howe CL. Demyelinated axons and motor function are protected by genetic deletion of perforin in a mouse model of multiple sclerosis. J. Neuropath. Exp. Neurol. 2009;68:1037–1048. [Europe PMC free article] [Abstract] [Google Scholar]
- Deb C, Howe CL. NKG2D contributes to efficient clearance of picornavirus from the acutely infected murine brain. J. Neurovirol. 2008;14:261–266. [Europe PMC free article] [Abstract] [Google Scholar]
- Donovan J, Brown P. Euthanasia. Current Protocols in Neuroscience. 2005:A.4H.1–A.4H.4. [Abstract] [Google Scholar]
- Donovan J, Brown P. Handling & restraint. Current Protocols in Immunology. 2006;73:1.3.1–1.3.6. [Abstract] [Google Scholar]
- Donovan J, Brown P. Parenteral injections. Current Protocols in Neuroscience. 2005:A.4F.1–A.4F.9. [Abstract] [Google Scholar]
- Holmes KL, Otten G, Yokoyama WM. Flow cytometry analysis using Becton Dickinson FACS Calibur. Current Protocols in Immunology. 2002:5.4.1–5.4.22. [Abstract] [Google Scholar]
- Howe CL, Ure D, Adelson JD, LaFrance-Corey R, Johnson A, Rodriguez M. CD8+ T cells directed against a viral peptide contribute to loss of motor function by disrupting axonal transport in a viral model of fulminant demyelination. J Neuroimmunol. 2007;188:13–21. [Europe PMC free article] [Abstract] [Google Scholar]
- Lipton HL. Theiler's virus infection in mice: An unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from Journal of Visualized Experiments : JoVE are provided here courtesy of MyJoVE Corporation
Full text links
Read article at publisher's site: https://doi.org/10.3791/2747
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3178654
Citations & impact
Impact metrics
Citations of article over time
Article citations
Inhibiting leukocyte-endothelial cell interactions by Chinese medicine Tongxinluo capsule alleviates no-reflow after arterial recanalization in ischemic stroke.
CNS Neurosci Ther, 29(10):3014-3030, 30 Apr 2023
Cited by: 3 articles | PMID: 37122157 | PMCID: PMC10493667
FGL2-targeting T cells exhibit antitumor effects on glioblastoma and recruit tumor-specific brain-resident memory T cells.
Nat Commun, 14(1):735, 10 Feb 2023
Cited by: 7 articles | PMID: 36759517 | PMCID: PMC9911733
Inflammatory monocytes and microglia play independent roles in inflammatory ictogenesis.
J Neuroinflammation, 19(1):22, 29 Jan 2022
Cited by: 12 articles | PMID: 35093106 | PMCID: PMC8800194
The survival and function of IL-10-producing regulatory B cells are negatively controlled by SLAMF5.
Nat Commun, 12(1):1893, 25 Mar 2021
Cited by: 20 articles | PMID: 33767202 | PMCID: PMC7994628
Transcriptional Diversity and Niche-Specific Distribution of Leukocyte Populations during Staphylococcus aureus Craniotomy-Associated Biofilm Infection.
J Immunol, 206(4):751-765, 08 Jan 2021
Cited by: 13 articles | PMID: 33419769 | PMCID: PMC7854520
Go to all (25) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Superior isolation of antigen-specific brain infiltrating T cells using manual homogenization technique.
J Immunol Methods, 439:23-28, 10 Sep 2016
Cited by: 20 articles | PMID: 27623324 | PMCID: PMC5310589
Inflammatory monocytes damage the hippocampus during acute picornavirus infection of the brain.
J Neuroinflammation, 9:50, 09 Mar 2012
Cited by: 50 articles | PMID: 22405261 | PMCID: PMC3368782
Interleukin-10 expression during the acute phase is a putative prerequisite for delayed viral elimination in a murine model for multiple sclerosis.
J Neuroimmunol, 249(1-2):27-39, 14 May 2012
Cited by: 26 articles | PMID: 22591945
Neuropathogenesis of Theiler's murine encephalomyelitis virus infection, an animal model for multiple sclerosis.
J Neuroimmune Pharmacol, 5(3):355-369, 06 Nov 2009
Cited by: 68 articles | PMID: 19894121 | PMCID: PMC2888670
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NINDS NIH HHS (3)
Grant ID: R01 NS064571-02
Grant ID: R01 NS064571
Grant ID: NS64571




