Abstract
Free full text

X chromosome reactivation in oocytes of Mus caroli.
Abstract
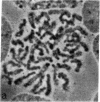
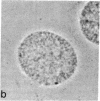
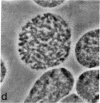
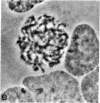
Mature mammalian oocytes have both of their X chromosomes active, while somatic cells from the same individual have one of their X chromosomes in an inactive state. We asked whether the X chromosomes of the germ cells never undergo inactivation in their ontogeny or whether inactivation of an X chromosome does occur but is followed by a subsequent reactivation event. Our approach has used an electrophoretic polymorphism for the X-linked enzyme glucose-6-phosphate dehydrogenase (G6PD) in the mouse species Mus caroli. G6PD is dimeric, and a heterodimer is produced in cells from heterozygous females if and only if both X chromosomes are active. Ovaries from heterozygous fetuses at different gestational ages were dissected and either studied cytologically or pressed between microscopy slides to obtain germ cell-rich and germ cell-poor preparations. No heterodimer band was detected on the 10th day of development in germ cell-rich preparations. On subsequent days, an increasingly intense heterodimer band was detected, which, by the 13th day, was approximately twice as intense as the corresponding homodimer bands. Consideration of (i) the G6PD activity per germ cell and per somatic cell and (ii) the percentage of germ cells in the germ cell-rich preparations indicated that a heterodimer band should have been visible on the 10th day had both X chromosomes been active. Cytological examinations showed that the earliest germ cells enter meiotic prophase on the eleventh day. These results demonstrate that oogonia have a single active X chromosome and that the inactive X chromosome is reactivated at or, more likely, shortly before entry into meiotic prophase.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
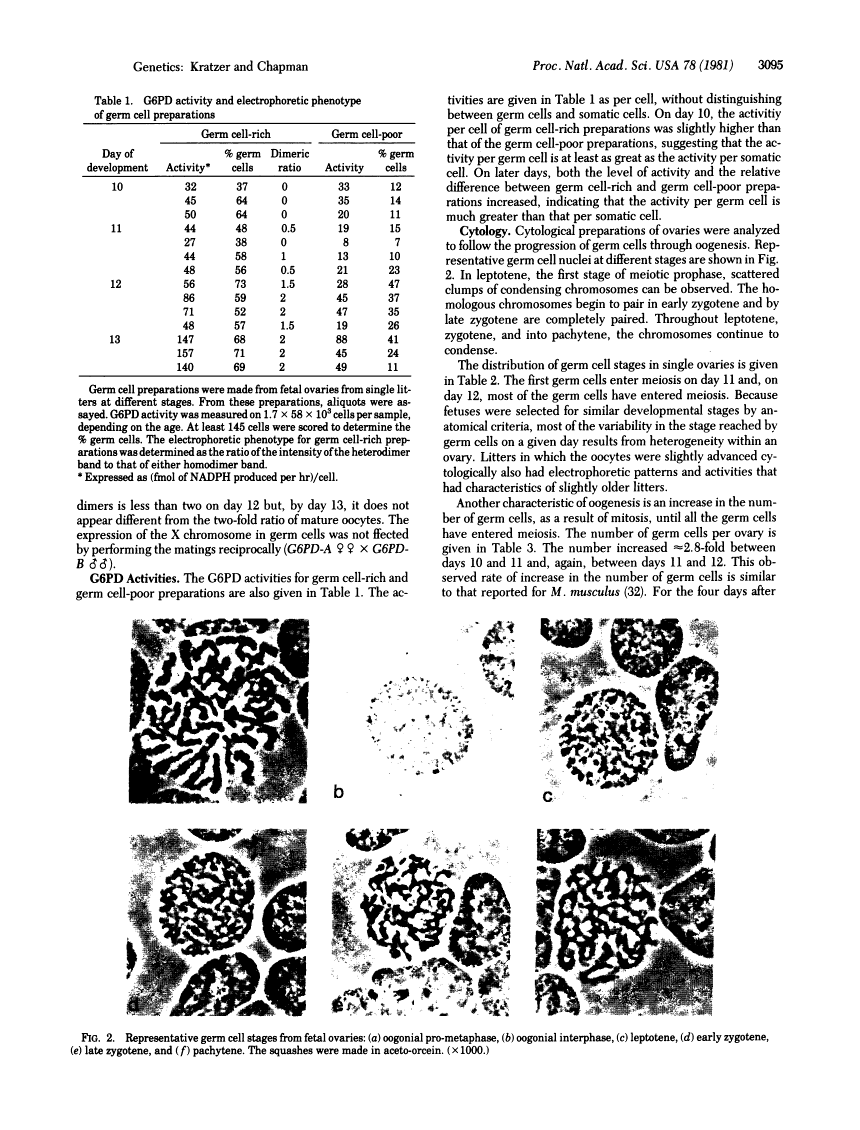
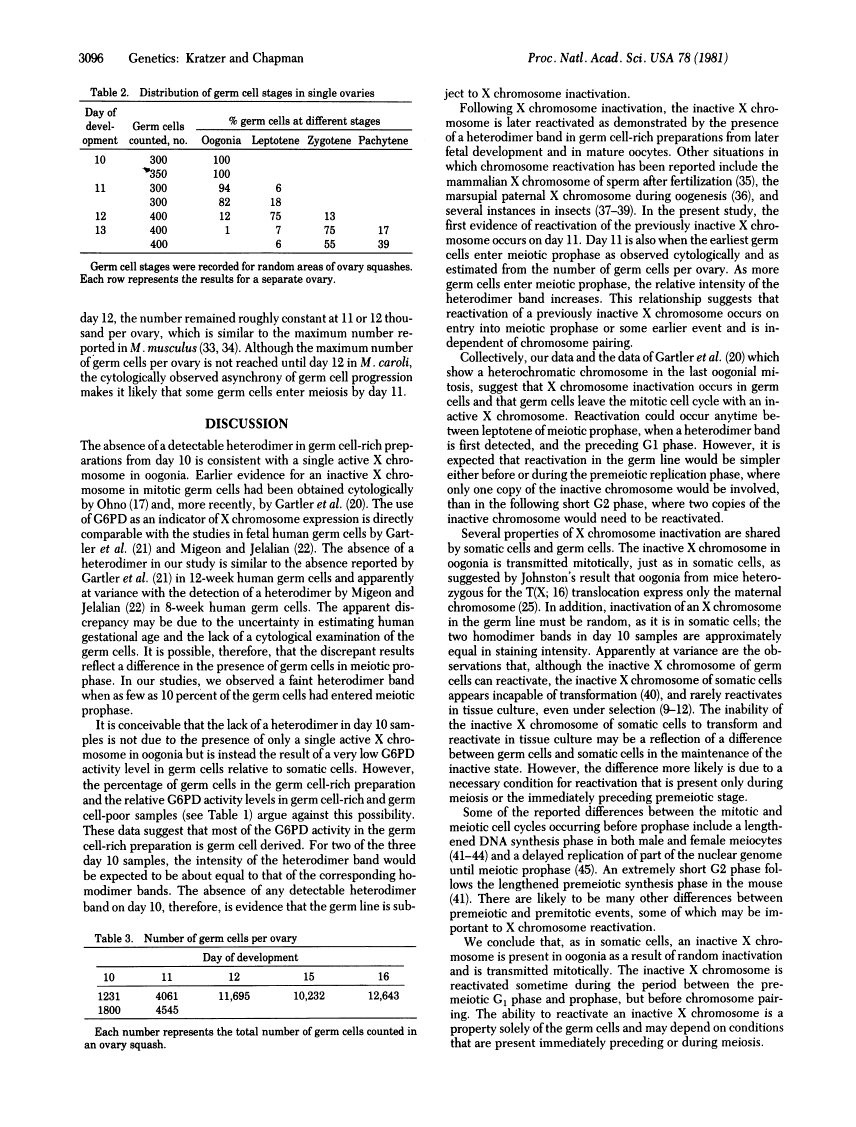
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LYON MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. [Abstract] [Google Scholar]
- Lyon MF. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. [Abstract] [Google Scholar]
- Adler DA, West JD, Chapman VM. Expression of alpha-galactosidase in preimplantation mouse embryos. Nature. 1977 Jun 30;267(5614):838–839. [Abstract] [Google Scholar]
- Epstein CJ, Smith S, Travis B, Tucker G. Both X chromosomes function before visible X-chromosome inactivation in female mouse embryos. Nature. 1978 Aug 3;274(5670):500–503. [Abstract] [Google Scholar]
- Kratzer PG, Gartler SM. HGPRT activity changes in preimplantation mouse embryos. Nature. 1978 Aug 3;274(5670):503–504. [Abstract] [Google Scholar]
- Monk M, Harper M. X-chromosome activity in preimplantation mouse embryos from XX and XO mothers. J Embryol Exp Morphol. 1978 Aug;46:53–64. [Abstract] [Google Scholar]
- Monk M, Harper MI. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature. 1979 Sep 27;281(5729):311–313. [Abstract] [Google Scholar]
- Migeon BR. Stability of X chromosomal inactivation in human somatic cells. Nature. 1972 Sep 8;239(5367):87–89. [Abstract] [Google Scholar]
- Kahan B, DeMars R. Localized Derepression on the Human Inactive X Chromosone in Mouse-Human Cell Hybrids. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1510–1514. [Europe PMC free article] [Abstract] [Google Scholar]
- Hellkuhl B, Grzeschik KH. Partial reactivation of a human inactive X chromosome in human-mouse somatic cell hybrids. Cytogenet Cell Genet. 1978;22(1-6):527–530. [Abstract] [Google Scholar]
- Mohandas T, Sparkes RS, Shapiro LJ. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. [Abstract] [Google Scholar]
- Epstein CJ. Mammalian oocytes: X chromosome activity. Science. 1969 Mar 7;163(3871):1078–1079. [Abstract] [Google Scholar]
- Epstein CJ. Expression of the mammalian X chromosome before and after fertilization. Science. 1972 Mar 31;175(4029):1467–1468. [Abstract] [Google Scholar]
- Gartler SM, Liskay RM, Campbell BK, Sparkes R, Gant N. Evidence for two functional X chromosomes in human oocytes. Cell Differ. 1972 Oct;1(4):215–218. [Abstract] [Google Scholar]
- Kozak LP, McLean GK, Eicher EM. X linkage of phosphoglycerate kinase in the mouse. Biochem Genet. 1974 Jan;11(1):41–47. [Abstract] [Google Scholar]
- OHNO S, KLINGER HP, ATKIN NB. Human oogenesis. Cytogenetics. 1962;1:42–51. [Abstract] [Google Scholar]
- Gartler SM, Rivest M, Cole RE. Cytological evidence for an inactive X chromosome in murine oogonia. Cytogenet Cell Genet. 1980;28(3):203–207. [Abstract] [Google Scholar]
- Gartler SM, Andina R, Gant N. Ontogeny of X-chromosome inactivation in the female germ line. Exp Cell Res. 1975 Mar 15;91(2):454–457. [Abstract] [Google Scholar]
- Migeon BR, Jelalian K. Evidence for two active X chromosomes in germ cells of female before meiotic entry. Nature. 1977 Sep 15;269(5625):242–243. [Abstract] [Google Scholar]
- BAKER TG. A QUANTITATIVE AND CYTOLOGICAL STUDY OF GERM CELLS IN HUMAN OVARIES. Proc R Soc Lond B Biol Sci. 1963 Oct 22;158:417–433. [Abstract] [Google Scholar]
- Andina RJ. A study of X chromosome regulation during oogenesis in the mouse. Exp Cell Res. 1978 Jan;111(1):211–218. [Abstract] [Google Scholar]
- Frels WI, Rossant J, Chapman VM. Intrinsic and extrinsic factors affecting the viability of Mus caroli x M. musculus hybrid embryos. J Reprod Fertil. 1980 Jul;59(2):387–392. [Abstract] [Google Scholar]
- BLANDAU RJ, WHITE BJ, RUMERY RE. OBSERVATIONS ON THE MOVEMENTS OF THE LIVING PRIMORDIAL GERM CELLS IN THE MOUSE. Fertil Steril. 1963 Sep-Oct;14:482–489. [Abstract] [Google Scholar]
- GOMORI G. Alkaline phosphatase of cell nuclei. J Lab Clin Med. 1951 Apr;37(4):526–531. [Abstract] [Google Scholar]
- Migeon BR, Kennedy JF. Evidence for the inactivation of an X chromosome early in the development of the human female. Am J Hum Genet. 1975 Mar;27(2):233–239. [Europe PMC free article] [Abstract] [Google Scholar]
- MINTZ B, RUSSELL ES. Gene-induced embryological modifications of primordial germ cells in the mouse. J Exp Zool. 1957 Mar;134(2):207–237. [Abstract] [Google Scholar]
- JONES EC, KROHN PL. The relationships between age, numbers of ocytes and fertility in virgin and multiparous mice. J Endocrinol. 1961 Feb;21:469–495. [Abstract] [Google Scholar]
- Peters H. Migration of gonocytes into the mammalian gonad and their differentiation. Philos Trans R Soc Lond B Biol Sci. 1970 Aug 6;259(828):91–101. [Abstract] [Google Scholar]
- Lifschytz E, Lindsley DL. The role of X-chromosome inactivation during spermatogenesis (Drosophila-allocycly-chromosome evolution-male sterility-dosage compensation). Proc Natl Acad Sci U S A. 1972 Jan;69(1):182–186. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnston PG, Robinson ES, Sharman GB. X chromosome activity in oocytes of kangaroo pouch young. Nature. 1976 Nov 25;264(5584):359–360. [Abstract] [Google Scholar]
- Nur U. Reversal of heterochromatization and the activity of the paternal chromosome set in the male mealy bug. Genetics. 1967 Jul;56(3):375–389. [Europe PMC free article] [Abstract] [Google Scholar]
- NELSON-REES WA. The effects of radiation damaged heterochromatic chromosomes on male fertility in the mealy bug, planococcus citri (risso). Genetics. 1962 Jun;47:661–683. [Europe PMC free article] [Abstract] [Google Scholar]
- Rieffel SM, Crouse HV. The elimination and differentiation of chromosomes in the germ line of sciara. Chromosoma. 1966;19(3):231–276. [Abstract] [Google Scholar]
- Liskay RM, Evans RJ. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. [Europe PMC free article] [Abstract] [Google Scholar]
- Crone M, Levy E, Peters H. The duration of the premeiotic DNA synthesis in mouse oocytes. Exp Cell Res. 1965 Sep;39(2):678–688. [Abstract] [Google Scholar]
- Callan HG. Replication of DNA in the chromosomes of eukaryotes. Proc R Soc Lond B Biol Sci. 1972 Apr 18;181(1062):19–41. [Abstract] [Google Scholar]
- Meistrich ML, Reid BO, Barcellona WJ. Meiotic DNA synthesis during mouse spermatogenesis. J Cell Biol. 1975 Jan;64(1):211–222. [Europe PMC free article] [Abstract] [Google Scholar]
- Hotta Y, Stern H. Analysis of DNA synthesis during meiotic prophase in Lilium. J Mol Biol. 1971 Feb 14;55(3):337–355. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.78.5.3093
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc319506?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/111375658
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.78.5.3093
Article citations
A method for stabilising the XX karyotype in female mESC cultures.
Development, 149(22):dev200845, 28 Nov 2022
Cited by: 2 articles | PMID: 36355065 | PMCID: PMC10112917
BAF complex-mediated chromatin relaxation is required for establishment of X chromosome inactivation.
Nat Commun, 13(1):1658, 29 Mar 2022
Cited by: 2 articles | PMID: 35351876 | PMCID: PMC8964718
New Insights into X-Chromosome Reactivation during Reprogramming to Pluripotency.
Cells, 9(12):E2706, 17 Dec 2020
Cited by: 8 articles | PMID: 33348832 | PMCID: PMC7766869
Review Free full text in Europe PMC
Mammalian Sex Chromosome Structure, Gene Content, and Function in Male Fertility.
Annu Rev Anim Biosci, 7:103-124, 09 Nov 2018
Cited by: 13 articles | PMID: 30412673
Review
Dosage compensation in the process of inactivation/reactivation during both germ cell development and early embryogenesis in mouse.
Sci Rep, 7(1):3729, 16 Jun 2017
Cited by: 10 articles | PMID: 28623283 | PMCID: PMC5473838
Go to all (46) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Glucose-6-phosphate dehydrogenase as a probe for the study of X-chromosome inactivation in hunan females.
Isozymes Curr Top Biol Med Res, 9:189-200, 01 Jan 1983
Cited by: 4 articles | PMID: 6578209
Cytological evidence for an inactive X chromosome in murine oogonia.
Cytogenet Cell Genet, 28(3):203-207, 01 Jan 1980
Cited by: 19 articles | PMID: 7438793
Evidence for two active X chromosomes in germ cells of female before meiotic entry.
Nature, 269(5625):242-243, 01 Sep 1977
Cited by: 25 articles | PMID: 593320
The role of sex chromosomes in mammalian germ cell differentiation: can the germ cells carrying X and Y chromosomes differentiate into fertile oocytes?
Asian J Androl, 17(3):360-366, 01 May 2015
Cited by: 14 articles | PMID: 25578929 | PMCID: PMC4430933
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: GM24125
Grant ID: GM07093