Abstract
Free full text

Molecular cloning and comparative analyses of the genomes of simian sarcoma virus and its associated helper virus.
Abstract
Closed circular viral DNA of simian sarcoma virus (SSV) and simian sarcoma-associated virus (SSAV) obtained from acutely infected dog cells was purified on preparative agarose gels, cleaved with EcoRI, and cloned in the phage lambda vector Charon 21A. The cloned 9-kilobase SSAV genome (B11) has the same restriction map as the bulk of the unintegrated linear SSAV DNA intermediate. Heteroduplex analysis between an SSV clone (lambda-C60) and an SSAV clone (lambda-B11) showed two substitution loops and one deletion loop. By using detailed restriction enzyme mapping and electron microscopic analysis, we showed that one of the substitution loops corresponds to an inversion of one of the two long terminal repeat units and adjacent cellular sequences in C60. The other substitution loop mapped close to the 3' long terminal repeat. At least part of this region was shown to contain SSV-specific sequences not shared by SSAV. The 1.9-kilobase deletion mapped at 3.5-5.5 kilobases of the linear SSAV genome, corresponding to most, if not all, of the pol gene.
Full text
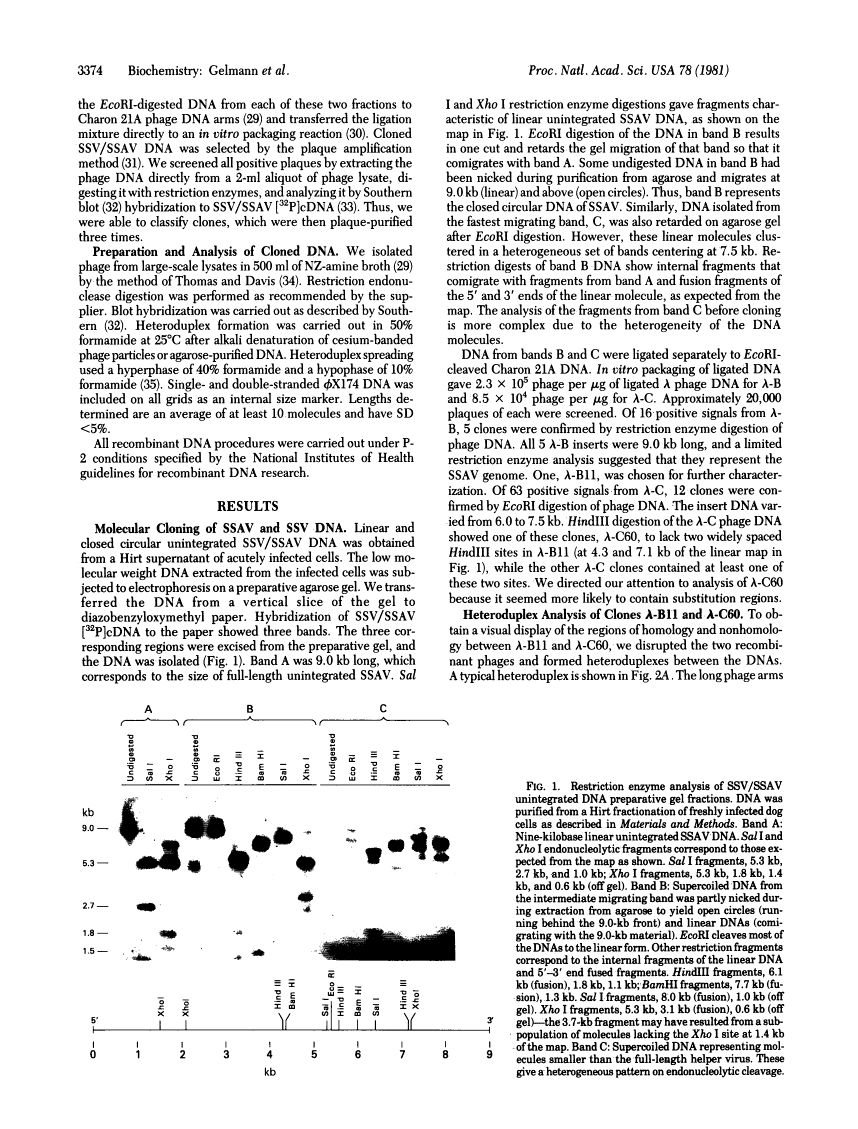
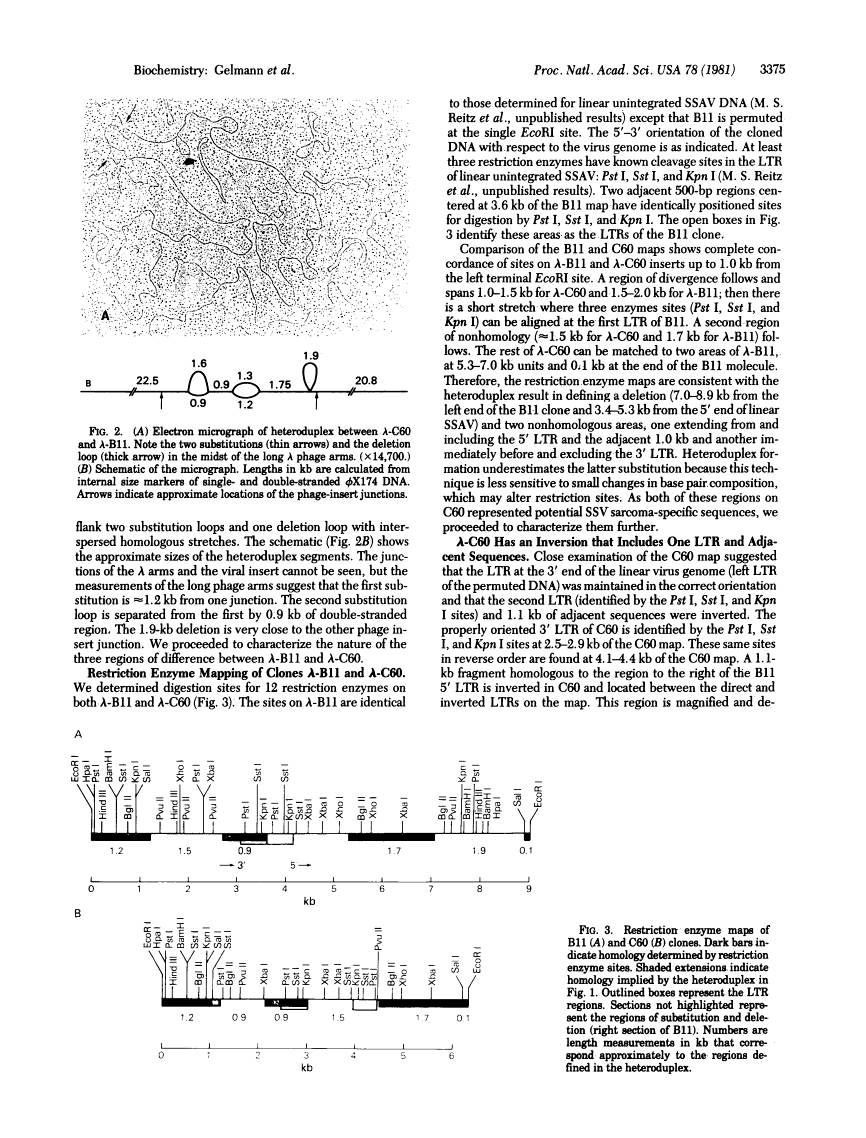
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Theilen GH, Gould D, Fowler M, Dungworth DL. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [Abstract] [Google Scholar]
- Wolfe LG, Deinhardt F, Theilen GH, Rabin H, Kawakami T, Bustad LK. Induction of tumors in marmoset monkeys by simian sarcoma virus, type 1 (Lagothrix): a preliminary report. J Natl Cancer Inst. 1971 Nov;47(5):1115–1120. [Abstract] [Google Scholar]
- Wolfe LG, Smith RK, Deinhardt F. Simian sarcoma virus, type 1 (Lagothrix): focus assay and demonstration of nontransforming associated virus. J Natl Cancer Inst. 1972 Jun;48(6):1905–1908. [Abstract] [Google Scholar]
- Todaro GJ, Lieber MM, Benveniste RE, Sherr CJ. Infectious primate type C viruses: Three isolates belonging to a new subgroup from the brains of normal gibbons. Virology. 1975 Oct;67(2):335–343. [Abstract] [Google Scholar]
- Wong-Staal F, Gallo RC, Gillespie D. Genetic relationship of a primate RNA tumour virus genome to genes in normal mice. Nature. 1975 Aug 21;256(5519):670–672. [Abstract] [Google Scholar]
- Reitz MS, Jr, Luczak JC, Gallo RC. Mapping of related and nonrelated sequences of RNA from wooly monkey virus and gibbon ape leukemia virus. Virology. 1979 Feb;93(1):48–56. [Abstract] [Google Scholar]
- Sherr CJ, Lieber MM, Benveniste RE, Todaro GJ. Endogenous baboon type C virus (M7): biochemical and immunologic characterization. Virology. 1974 Apr;58(2):492–503. [Abstract] [Google Scholar]
- Benveniste RE, Todaro GJ. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974 Dec 6;252(5483):456–459. [Abstract] [Google Scholar]
- Lieber MM, Sherr CJ, Todaro GJ, Benveniste RE, Callahan R, Coon HG. Isolation from the asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2315–2319. [Europe PMC free article] [Abstract] [Google Scholar]
- Callahan R, Meade C, Todaro GJ. Isolation of an endogenous type C virus related to the infectious primate type C viruses from the Asian rodent Vandeleuria oleracea. J Virol. 1979 Apr;30(1):124–131. [Europe PMC free article] [Abstract] [Google Scholar]
- Bishop JM. Retroviruses. Annu Rev Biochem. 1978;47:35–88. [Abstract] [Google Scholar]
- Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. [Abstract] [Google Scholar]
- Frankel AE, Fischinger PJ. Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3705–3709. [Europe PMC free article] [Abstract] [Google Scholar]
- Frankel AE, Gilbert JH, Porzig KJ, Scolnick EM, Aaronson SA. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. [Europe PMC free article] [Abstract] [Google Scholar]
- Hsu TW, Sabran JL, Mark GE, Guntaka RV, Taylor JM. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. [Europe PMC free article] [Abstract] [Google Scholar]
- Hughes SH, Shank PR, Spector DH, Kung HJ, Bishop JM, Varmus HE, Vogt PK, Breitman ML. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. [Abstract] [Google Scholar]
- Gilboa E, Goff S, Shields A, Yoshimura F, Mitra S, Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. [Abstract] [Google Scholar]
- Benz EW, Jr, Dina D. Moloney murine sarcoma virions synthesize full-genome-length double-stranded DNA in vitro. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3294–3298. [Europe PMC free article] [Abstract] [Google Scholar]
- Vande Woude GF, Oskarsson M, Enquist LW, Nomura S, Sullivan M, Fischinger PJ. Cloning of integrated Moloney sarcoma proviral DNA sequences in bacteriophage lambda. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4464–4468. [Europe PMC free article] [Abstract] [Google Scholar]
- Dhar R, McClements WL, Enquist LW, Vande Woude GF. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. [Europe PMC free article] [Abstract] [Google Scholar]
- Shoemaker C, Goff S, Gilboa E, Paskind M, Mitra SW, Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. [Europe PMC free article] [Abstract] [Google Scholar]
- Tronick SR, Robbins KC, Canaani E, Devare SG, Andersen PR, Aaronson SA. Molecular cloning of Moloney murine sarcoma virus: arrangement of virus-related sequences within the normal mouse genome. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6314–6318. [Europe PMC free article] [Abstract] [Google Scholar]
- McClements WL, Enquist LW, Oskarsson M, Sullivan M, Vande Woude GF. Frequent site-specific deletion of coliphage lambda murine sarcoma virus recombinants and its use in the identification of a retrovirus integration site. J Virol. 1980 Aug;35(2):488–497. [Europe PMC free article] [Abstract] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. [Abstract] [Google Scholar]
- Wahl GM, Stern M, Stark GR. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. [Europe PMC free article] [Abstract] [Google Scholar]
- Vogelstein B, Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. [Europe PMC free article] [Abstract] [Google Scholar]
- Williams BG, Blattner FR. Construction and characterization of the hybrid bacteriophage lambda Charon vectors for DNA cloning. J Virol. 1979 Feb;29(2):555–575. [Europe PMC free article] [Abstract] [Google Scholar]
- Sternberg N, Tiemeier D, Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. [Abstract] [Google Scholar]
- Woo SL. A sensitive and rapid method for recombinant phage screening. Methods Enzymol. 1979;68:389–395. [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Taylor JM, Illmensee R, Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. [Abstract] [Google Scholar]
- Thomas M, Davis RW. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. [Abstract] [Google Scholar]
- Rigby PW, Dieckmann M, Rhodes C, Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. [Abstract] [Google Scholar]
- Duesberg PH. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. [Abstract] [Google Scholar]
- Calos MP, Miller JH. Transposable elements. Cell. 1980 Jul;20(3):579–595. [Abstract] [Google Scholar]
- Sherr CJ, Fedele LA, Oskarsson M, Maizel J, Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. [Europe PMC free article] [Abstract] [Google Scholar]
- Sutcliffe JG, Shinnick TM, Verma IM, Lerner RA. Nucleotide sequence of Moloney leukemia virus: 3' end reveals details of replications, analogy to bacterial transposons, and an unexpected gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3302–3306. [Europe PMC free article] [Abstract] [Google Scholar]
- Donoghue DJ, Sharp PA, Weinberg RA. Comparative study of different isolates of murine sarcoma virus. J Virol. 1979 Dec;32(3):1015–1027. [Europe PMC free article] [Abstract] [Google Scholar]
- Shields A, Goff S, Paskind M, Otto G, Baltimore D. Structure of the Abelson murine leukemia virus genome. Cell. 1979 Dec;18(4):955–962. [Abstract] [Google Scholar]
- Bister K, Hayman MJ, Vogt PK. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. [Abstract] [Google Scholar]
- Bister K, Löliger HC, Duesberg PH. Oligoribonucleotide map and protein of CMII: detection of conserved and nonconserved genetic elements in avian acute leukemia viruses CMII, MC29, and MH2. J Virol. 1979 Oct;32(1):208–219. [Europe PMC free article] [Abstract] [Google Scholar]
- Mellon P, Pawson A, Bister K, Martin GS, Duesberg PH. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5874–5878. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.78.6.3373
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/78/6/3373.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.78.6.3373
Article citations
Simian sarcoma-associated virus fails to infect Chinese hamster cells despite the presence of functional gibbon ape leukemia virus receptors.
J Virol, 72(12):9453-9458, 01 Dec 1998
Cited by: 30 articles | PMID: 9811678 | PMCID: PMC110436
Syrian hamster embryo cell lines useful for detecting transforming genes in mouse tumours: detection of transforming genes in X-ray-related mouse tumours.
Br J Cancer, 67(2):262-267, 01 Feb 1993
Cited by: 3 articles | PMID: 8431357 | PMCID: PMC1968199
Crystal structure of human platelet-derived growth factor BB.
EMBO J, 11(11):3921-3926, 01 Nov 1992
Cited by: 110 articles | PMID: 1396586 | PMCID: PMC556902
Expression of growth factors during the differentiation of embryonic stem cells in monolayer.
Dev Biol, 142(2):406-413, 01 Dec 1990
Cited by: 26 articles | PMID: 2257974
Effects of fractionated x-irradiation on the Ly-6--Ril-1--Pol-5 region.
Immunogenetics, 32(4):252-262, 01 Jan 1990
Cited by: 2 articles | PMID: 1700761
Go to all (36) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Deletions of specific regions of the simian sarcoma-associated virus genome are found in defective viruses and in the simian sarcoma virus.
J Virol, 41(2):593-604, 01 Feb 1982
Cited by: 11 articles | PMID: 6281470 | PMCID: PMC256788
Molecular cloning of integrated simian sarcoma virus: genome organization of infectious DNA clones.
Proc Natl Acad Sci U S A, 78(5):2918-2922, 01 May 1981
Cited by: 64 articles | PMID: 6265924 | PMCID: PMC319470
Molecular cloning of circular unintegrated DNA of two types of the SEATO strain of gibbon ape leukemia virus.
J Virol, 44(1):269-275, 01 Oct 1982
Cited by: 9 articles | PMID: 6292490 | PMCID: PMC256262
Nucleotide sequence of the transforming gene of simian sarcoma virus.
Proc Natl Acad Sci U S A, 79(10):3179-3182, 01 May 1982
Cited by: 15 articles | PMID: 6285340 | PMCID: PMC346378