Abstract
Free full text

Evaluation of Mammary Gland Development and Function in Mouse Models
Abstract
The human mammary gland is composed of 15-20 lobes that secrete milk into a branching duct system opening at the nipple. Those lobes are themselves composed of a number of terminal duct lobular units made of secretory alveoli and converging ducts1. In mice, a similar architecture is observed at pregnancy in which ducts and alveoli are interspersed within the connective tissue stroma. The mouse mammary gland epithelium is a tree like system of ducts composed of two layers of cells, an inner layer of luminal cells surrounded by an outer layer of myoepithelial cells denoted by the confines of a basement membrane2. At birth, only a rudimental ductal tree is present, composed of a primary duct and 15-20 branches. Branch elongation and amplification start at the beginning of puberty, around 4 weeks old, under the influence of hormones3,4,5. At 10 weeks, most of the stroma is invaded by a complex system of ducts that will undergo cycles of branching and regression in each estrous cycle until pregnancy2. At the onset of pregnancy, a second phase of development begins, with the proliferation and differentiation of the epithelium to form grape-shaped milk secretory structures called alveoli6,7. Following parturition and throughout lactation, milk is produced by luminal secretory cells and stored within the lumen of alveoli. Oxytocin release, stimulated by a neural reflex induced by suckling of pups, induces synchronized contractions of the myoepithelial cells around the alveoli and along the ducts, allowing milk to be transported through the ducts to the nipple where it becomes available to the pups 8. Mammary gland development, differentiation and function are tightly orchestrated and require, not only interactions between the stroma and the epithelium, but also between myoepithelial and luminal cells within the epithelium9,10,11. Thereby, mutations in many genes implicated in these interactions may impair either ductal elongation during puberty or alveoli formation during early pregnancy, differentiation during late pregnancy and secretory activation leading to lactation12,13. In this article, we describe how to dissect mouse mammary glands and assess their development using whole mounts. We also demonstrate how to evaluate myoepithelial contractions and milk ejection using an ex-vivo oxytocin-based functional assay. The effect of a gene mutation on mammary gland development and function can thus be determined in situ by performing these two techniques in mutant and wild-type control mice.

Protocol
1. Mammary gland dissection
Euthanize the mouse using CO2 inhalation. Avoid cervical dislocation if possible since it may damage major blood vessels in the neck and result in accumulation of blood around mammary glands, rendering the dissection more difficult. However, if other criteria are to be evaluated in the same mouse, such as blood levels of oxytocin, alternative euthanasia methods may be necessary, though this must be evaluated by your IACUC.
On a polystyrene foam wrapped in foil, place the mouse on its back and spread the four limbs. Pin firmly all four limbs using 16 to 20 gauge needles (Figure 1A).
Spray the mouse generously with 70% ethanol.
Using forceps, pull up the abdominal skin at the midline and make a small incision with sharp scissors.
Starting from the incision, cut the skin up to the neck of the animal while avoiding puncturing the abdominal or thoracic cavities (Figure 1B).
Cut the skin on the front legs to the midline incision, resulting in a "Y shape" (Figure 1B). Cut the skin on the rear limbs the same way (Figure 1B).
Using hemostatic forceps, gently peel the abdominal and thoracic skin to the side of the mouse. If needed, gently cut conjunctive tissue.
Fix the skin to the polystyrene foam using 16 to 20 gauge needles, exposing the mammary glands attached to the underside of the skin. Proceed and complete one side at a time (Figure 1C).
Using forceps, gently lift the mammary gland of interest and cut between the gland and the skin with small sharp scissors, starting from the outside towards the spine of the animal. Make sure you cut carefully, so no pieces of skin or muscle remain on the gland. All mammary glands (Figure 1) can be removed using this protocol, but abdominal mammary glands (#4) are best for whole mounting. The 2nd and 3rd glands have some interdigitated muscles and while they can be used for whole mount and histology, they might not be appropriate for all analyses.
2. Whole mount
At least one day prior to dissection, prepare carmine alum solution by dissolving 1g of carmine and 2.5g of aluminum potassium sulfate in 500 ml of distilled water and boil for 20 minutes in a 1 liter Erlenmeyer flask. Adjust the final volume to 500 ml with water. Filter through a Whatman paper and add a small amount of thymol (few grains) as a preservative. Refrigerate. After staining, carmine alum solution can be poured back to the stock bottle and reused many times over several weeks. Make a fresh solution when color becomes faint.
After excising the mammary gland from the mouse (step 1), spread it directly on a glass slide. Try to flatten it as much as possible, keeping the original in situ shape. The gland will stick on the slide nicely when properly stretched.
Fix the gland by immersing it, on the slide, in Carnoy's fixative (100% EtOH, chloroform, glacial acetic acid; 6:3:1) for 4 hours in the fume hood at room temperature in a jar. Note that others have used 2 hours which seems to be sufficient. Alternatively, glands can also be fixed at 4°C overnight.
Wash in 70% ethanol for 15 min. Then, rehydrate gradually in water by removing half of the ethanol solution from the jar and replacing it with ddH2O. Incubate for 5 min. Repeat 3 times. Alternatively, use 70%-, 35%-, 15%- ethanol and water baths for 5 minutes each.
Rinse in distilled water for 5 minutes.
Stain in carmine alum overnight at room temperature. It should be noted that staining might take longer depending on the thickness of the gland.
Remove carmine alum stain to reuse and gradually dehydrate stained tissue through serial ethanol baths (50%, 70%, 95%, 100%) for 5 min each and clear in xylene, or in a non-toxic alternative such as Histo-clear, overnight. Both dehydration and clearing might also require longer incubations with thicker samples.
Immerse mammary gland in methyl salicylate. Mammary glands can be kept in methyl salicylate in a fume hood until images are ready to be taken.
Images can be taken in a fume hood with a regular digital camera positioned on a tripod (use "macro" function if available), by placing the slides on a light table. Use a dissecting microscope for higher magnification. Alternatively, mammary glands can be mounted in media such as Permount (after step 2.7) allowing for pictures to be taken outside a chemical fume hood on any imaging platform providing the mounts are sufficiently thin.
In order to quantify mammary gland development using whole mount, measurements such as the number of ramifications (or side-branches) along portions of the ducts, the length of the primary ducts, or a ratio of epithelial to adipose tissue area could be assessed. Finally, after photographic documentation, mammary gland whole mount can be embedded in paraffin for sectioning and conventional histological staining.
3. Oxytocin-induced milk ejection
Separate the pups from the dam just after they have been fed. Wait 1 hour before performing the assay.
Sacrifice the mother and expose the mammary glands as described before (steps 1.1 to 1.8), without removing them from the animal.
Start the assay by evenly dropping either PBS or the oxytocin solution directly on the mammary gland of interest, using a transfer pipette. A large spectrum of doses can be used; ˜8 pg/ml (physiological dose) is typically used. However, high non-physiological doses (up to 1 mg/ml) can be used to ensure the absence of response in genetically modified mouse models. For this procedure, it is recommended to use thoracic (#2-3) glands, using one side as a control (exposed to PBS) and the other side to test milk ejection (oxytocin exposure).
Incubate for 1 minute and remove the solution by carefully aspirating it with a transfer pipette. Milk entry into the ducts can be monitored by video tape recording, or by taking images before and after PBS or oxytocin exposure.
4. Representative Results:
In a good whole mount, the mammary gland is nicely spread and the epithelium ducts are easily observable (Figure 2A). If the gland is not well-spread, it can lift up in part or totally from the slide when placed in the fixative solution. The gland will then become hard and unusable (Figure 2B). Similarly, if the gland is not well dissected, either some part of the epithelium can be missing (Figure 2C) or remaining parts of the skin or abdominal muscle will interfere with the analysis of the gland (Figure 2D).
For the oxytocin assay, it is important to separate the suckling pups from the dam for the same amount of time on every occasion. In this way, you will make sure that no milk or small amounts of milk is present in the ducts prior to oxytocin exposure, but also that enough milk is present in the alveoli that can be ejected into the ducts by myoepithelial contractions (Figure 3A). If pups are removed too soon, milk can accumulate in the alveoli and in the ducts rendering the effect of oxytocin difficult to monitor (Figure 3B). Alternatively, if the experiment is performed too early after pup removal, milk won't have time to accumulate in the alveoli and oxytocin-induced contractions of myoepithelial cells won't trigger enough milk release into the ducts to be observed (Figure 3C).
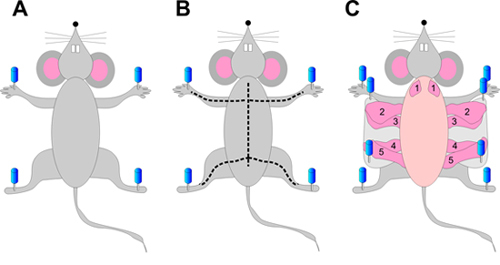
Figure 1. Mouse mammary gland dissection. Pin the mouse on its back by the four limbs (A). Cut the skin first from the belly to the neck. Then make perpendicular incisions from the center of the belly to each limb, as shown by the dotted line (B). Gently peel the skin on the side of the animal and pin it, exposing the mammary glands (C).
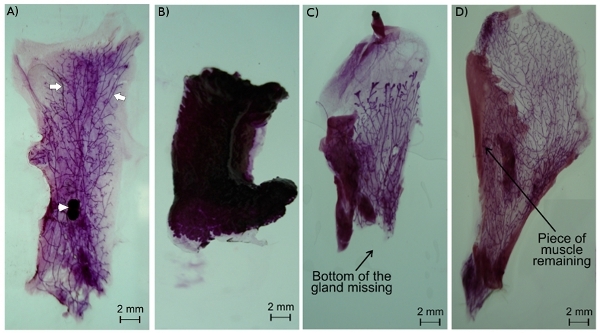
Figure 2. Mouse mammary gland whole mount. Epithelium ducts (arrows) and the lymph node (arrowhead) are easily observed in a nicely spread mammary gland whole mount of gland #4 (A). A poorly spread mammary gland may become detached from the slide and collapsed, rendering it unusable (B). An improperly excised mammary gland can either have missing parts (C) or pieces of muscle or skin remaining (D). Note that the 2nd and 3rd glands have interdigitated muscle, but can still be used for whole mount or histology.
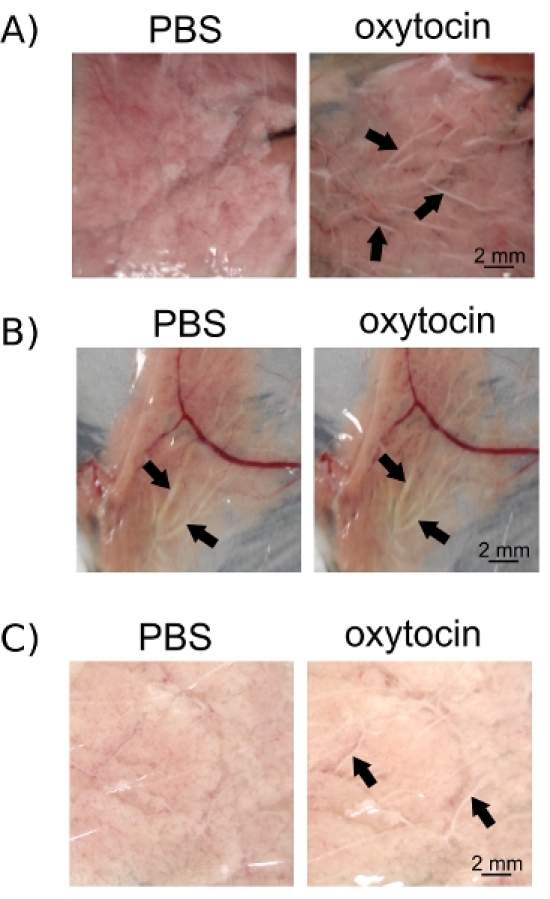
Figure 3. Oxytocin-induced milk ejection can be observed in epithelium ducts (arrows) following oxytocin exposure (A). However, if ducts are already filled with milk (arrows) at the beginning of the assay due to an inappropriately long period of latency after the last suckling of the pups, further accumulation of milk in the ducts (arrows) after oxytocin exposure is hard to observe (B). Alternatively, a short latency period after pup suckling will not allow for enough accumulation of milk into the alveoli to be properly observed in the ducts (arrows) after oxytocin exposure (C). Images were taken before and after oxytocin exposure using a numeric camera (Cybershot, Sony).
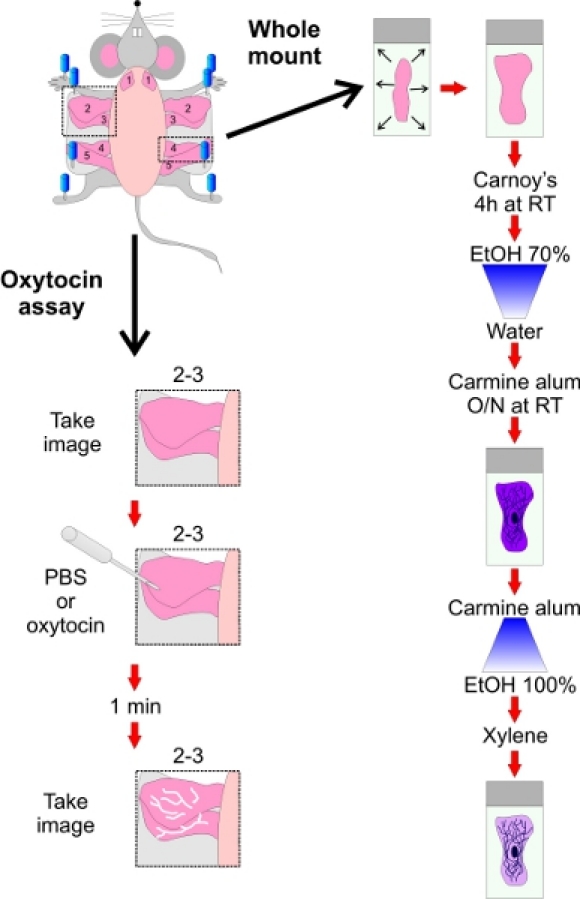
Figure 4. Schematic representations of whole mounting and oxytocin treatment assays.
Discussion
Mammary gland development and function are tightly orchestrated. Mutant mice may harbor gene mutations that can impair mammary gland development and function highlighting the need to evaluate gland architecture13,12. Whole mounting can be performed as described in this article at all stages of mammary gland development. Typically, development of the epithelium is assessed at the beginning of puberty ( ˜4 weeks old), in the middle of puberty ( ˜6 weeks old) and after puberty ( ˜ 10-12 weeks old)2,10. At these stages, the amount of duct branching, the number of terminal end buds and length of the ducts can be determined in order to quantify the degree of gland development14,15. Similarly, alveologenesis (development of the alveoli during pregnancy) is usually determined at 2-3 points during pregnancy; the structural changes that occur during lactation and involution can also be observed and quantified in whole mount gland preparations16. However, quantification of whole mounts from late pregnant and lactating mice can be difficult due to the density of the tissue. It is recommended to combine whole mount with histological analyses, especially for those stages 12. For more detailed histological evaluation, mammary glands can be removed using the same protocol noted in this report, fixed in formalin and subjected to hematoxylin and eosin staining. Whole mount can also be paraffin embedded and used for histology once imaging has been performed. For both techniques, proper and delicate dissection of the mammary glands is crucial. If mutant mice are being assessed, it is important to compare these mice to the closest control mice possible, since different mouse strains can show variable side- or tertiary-ramifications, or alveologenesis 17. For instance, when compared to BALB/c mice, C57BL/6 mice exhibit reduced side branching and alveologenesis 17,18. Therefore, BALB/c mice should not be used as a control for genetically modified mice generated on a C57BL/6 mouse background. While whole mounting provides insight into gross developmental defects, the function of the mammary gland may also need to be evaluated using other complementary techniques.
The main function of the mammary gland is to produce and deliver milk to pups. These processes require not only proper differentiation of secretory luminal cells during pregnancy, but also coordinate and efficient contractions of the myoepithelial cells around the alveoli and along the ducts for milk transport8. While milk production and quality can be evaluated by analytical assessment of milk composition12, among others, the causes of impaired milk delivery to the pups is harder to evaluate. Indeed, the observed absence of milk in the stomachs of newborn pups can be linked to many defects such as impaired milk production, inability of the pups to suckle, behavioral defects of the mother or milk ejection defects. One way of evaluating milk transport and ejection defects from the alveoli into the ducts is to induce myoepithelial contractions using oxytocin, as described in this article. However, quantification of milk transport and ejection is difficult since the effect of oxytocin ex vivo is rapid (usually milk can be observed in the ducts within 1 minute after exposure), even when using low physiological doses of oxytocin10. Thus, a slight difference in the efficiency of milk ejection between two mouse lines might not be observable using this technique. Quantifying this response is difficult but one semi-quantitative way of assessing oxytocin-induced milk ejection is to examine the response under a variety of dosages and to determine the treatment time necessary to fill ducts with milk. . In all cases, consistency of the wait period between the last pup suckling to the beginning of the assay is critical. For a more accurate quantification of lactation or identification of a milk ejection defect, this method can be complemented by the quantification of milk proteins and signaling pathways, such as β-casein and Stat5, respectively, or in vitro myoepithelial cell contraction upon oxytocin exposure19.
In summary, transgenic mouse models are important and widely used for studying mammary gland function and development, and to assess the role of key genes in these processes. The architecture of the mammary gland epithelium can be easily observed in properly excised glands using whole mount at all stages of the development. While lactation defects leading to pups malnutrition can have many origins, a deficiency in milk ejection can be assessed by oxytocin-induced synchronized contraction of myoepithelial cells in situ. Together, these two techniques (Figure 4) can give important insights into the effect of specific gene mutations or deletions on mammary gland development and function.
Disclosures
No conflicts of interest declared.
Acknowledgments
These studies were funded by CIHR and CBCRA grants to DWL. IP was funded by fellowships from CIHR-STP, FRSQ and CIHR. MKGS was funded by OGS and CIHR-STP scholarships. The authors thank Kevin Barr for his assistance with mouse breeding.
References
- Geddes DT. Inside the lactating breast: the latest anatomy research. J Midwifery Womens Health. 2007;52:556–556. [Abstract] [Google Scholar]
- Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8:201–201. [Europe PMC free article] [Abstract] [Google Scholar]
- Silberstein GB, Flanders KC, Roberts AB, Daniel CW. Regulation of mammary morphogenesis: evidence for extracellular matrix-mediated inhibition of ductal budding by transforming growth factor-beta 1. Dev Biol. 1992;152:354–354. [Abstract] [Google Scholar]
- Daniel CW, Robinson S, Silberstein GB. The role of TGF-beta in patterning and growth of the mammary ductal tree. J Mammary Gland Biol Neoplasia. 1996;1:331–331. [Abstract] [Google Scholar]
- Neville MC, Daniel CW. The Mammary gland : development, regulation, and function. New York: Plenum Press; 1987. [Google Scholar]
- Oakes SR, Hilton HN, Ormandy CJ. The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res. 2006;8:207–207. [Europe PMC free article] [Abstract] [Google Scholar]
- Anderson SM, Rudolph MC, McManaman JL, Neville MC. Key stages in mammary gland development. Secretory activation in the mammary gland: it's not just about milk protein synthesis! Breast Cancer Res. 2007;9:204–204. [Europe PMC free article] [Abstract] [Google Scholar]
- Reversi A, Cassoni P, Chini B. Oxytocin receptor signaling in myoepithelial and cancer cells. J Mammary Gland Biol Neoplasia. 2005;10:221–221. [Abstract] [Google Scholar]
- Haslam SZ. Cell to cell interactions and normal mammary gland function. J Dairy Sci. 1988;71:2843–2843. [Abstract] [Google Scholar]
- Plante I, Laird DW. Decreased levels of connexin43 result in impaired development of the mammary gland in a mouse model of oculodentodigital dysplasia. Dev Biol. 2008;318:312–312. [Abstract] [Google Scholar]
- Talhouk RS. Heterocellular interaction enhances recruitment of alpha and beta-catenins and ZO-2 into functional gap-junction complexes and induces gap junction-dependant differentiation of mammary epithelial cells. Exp Cell Res. 2008;314:3275–3275. [Abstract] [Google Scholar]
- Palmer CA, Neville MC, Anderson SM, McManaman JL. Analysis of lactation defects in transgenic mice. J Mammary Gland Biol Neoplasia. 2006;11:269–269. [Abstract] [Google Scholar]
- Howlin J, McBryan J, Martin F. Pubertal mammary gland development: insights from mouse models. J Mammary Gland Biol Neoplasia. 2006;11:283–283. [Abstract] [Google Scholar]
- You L. Modulation of mammary gland development in prepubertal male rats exposed to genistein and methoxychlor. Toxicol Sci. 2002;66:216–216. [Abstract] [Google Scholar]
- Grill CJ, Cohick WS, Sherman AR. Postpubertal development of the rat mammary gland is preserved during iron deficiency. J Nutr. 2001;131:1444–1444. [Abstract] [Google Scholar]
- Hennighausen L, Robinson GW. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12:449–449. [Abstract] [Google Scholar]
- Aupperlee Strain-specific differences in the mechanisms of progesterone regulation of murine mammary gland development. Endocrinology. 2009;150:1485–1485. [Europe PMC free article] [Abstract] [Google Scholar]
- Montero Girard G. Association of estrogen receptor-alpha and progesterone receptor A expression with hormonal mammary carcinogenesis: role of the host microenvironment. Breast Cancer Res. 2007;9:R22–R22. [Europe PMC free article] [Abstract] [Google Scholar]
- Moore DM, Vogl AW, Baimbridge K, Emerman JT. Effect of calcium on oxytocin-induced contraction of mammary gland myoepithelium as visualized by NBD-phallacidin. J Cell Sci. 1987;88:563–563. [Abstract] [Google Scholar]
Articles from Journal of Visualized Experiments : JoVE are provided here courtesy of MyJoVE Corporation
Full text links
Read article at publisher's site: https://doi.org/10.3791/2828
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3196158?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Loss of tyrosine 211 phosphorylation of proliferating cell nuclear antigen (PCNA) enhances postnatal mammary gland development.
Biomedicine (Taipei), 14(3):40-48, 01 Sep 2024
Cited by: 0 articles | PMID: 39386182 | PMCID: PMC11460574
Accelerating whole-sample polarization-resolved second harmonic generation imaging in mammary gland tissue via generative adversarial networks.
Biomed Opt Express, 15(9):5251-5271, 15 Aug 2024
Cited by: 0 articles | PMID: 39296390 | PMCID: PMC11407270
Inhibitory Effects of Alpha-Connexin Carboxyl-Terminal Peptide on Canine Mammary Epithelial Cells: A Study on Benign and Malignant Phenotypes.
Cancers (Basel), 16(4):820, 18 Feb 2024
Cited by: 1 article | PMID: 38398211 | PMCID: PMC10887206
Subfunctionalized expression drives evolutionary retention of ribosomal protein paralogs Rps27 and Rps27l in vertebrates.
Elife, 12:e78695, 12 Jun 2023
Cited by: 3 articles | PMID: 37306301 | PMCID: PMC10313321
Adolescent- and adult-initiated alcohol exposure in mice differentially promotes tumorigenesis and metastasis of breast cancer.
Alcohol Clin Exp Res (Hoboken), 47(2):251-262, 10 Dec 2022
Cited by: 3 articles | PMID: 36462938 | PMCID: PMC10906809
Go to all (51) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Review: Mammary gland development in swine: embryo to early lactation.
Animal, 13(s1):s11-s19, 01 Jul 2019
Cited by: 17 articles | PMID: 31280748
Review
Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland.
J Histochem Cytochem, 30(7):667-676, 01 Jul 1982
Cited by: 122 articles | PMID: 6179984
Asymmetric expression of connexins between luminal epithelial- and myoepithelial- cells is essential for contractile function of the mammary gland.
Dev Biol, 399(1):15-26, 11 Dec 2014
Cited by: 19 articles | PMID: 25500615 | PMCID: PMC4996272
Mex3c mutation affects lactation through impairing milk ejection in female mice.
Biosci Rep, 40(12):BSR20201285, 01 Dec 2020
Cited by: 0 articles | PMID: 33180120 | PMCID: PMC7729293
Funding
Funders who supported this work.




