Abstract
Free full text

Characterization of a membrane-associated receptor from bovine liver that binds phosphomannosyl residues of bovine testicular beta-galactosidase.
Abstract
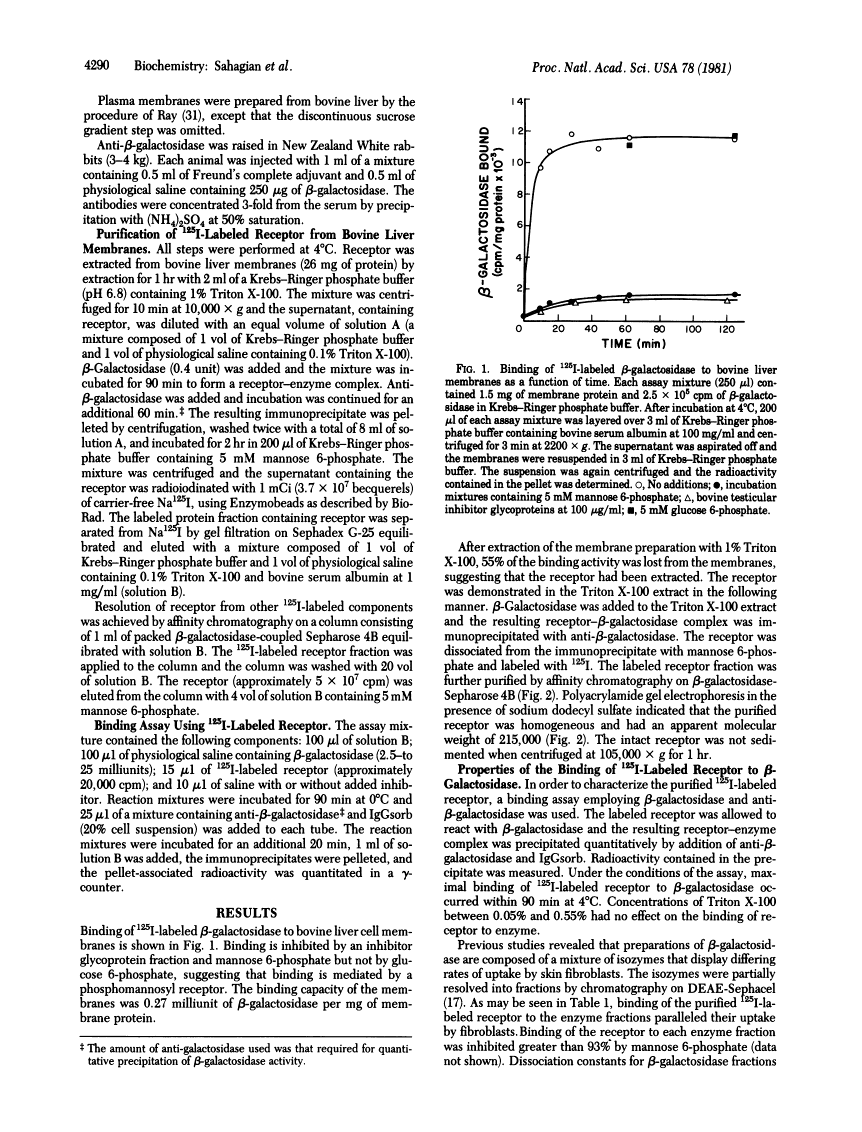
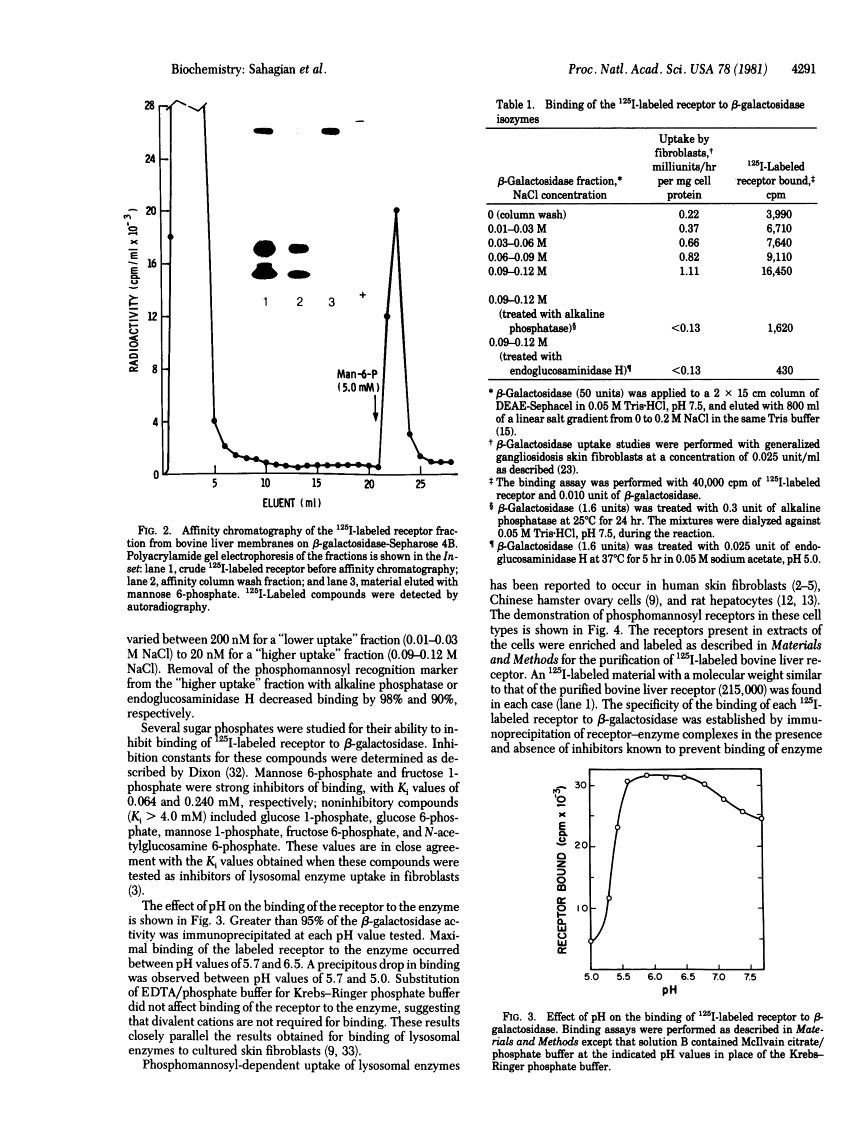
A receptor that binds the phosphomannosyl recognition marker of bovine testicular beta-galactosidase (beta-D-galactoside galactohydrolase, EC 3.2.1.23) was isolated from bovine liver membranes. The receptor was extracted from crude plasma membrane preparations with Triton X-100 and immunoprecipitated as a receptor--beta-galactosidase complex with anti-beta-galactosidase. The receptor was dissociated from the precipitate with mannose 6-phosphate, labeled with 125I, and purified on a beta-galactosidase-Sepharose 4B affinity matrix. A quantitative binding assay employing anti-beta-galactosidase and IgGsorb (formalin-fixed Staphylococcus aureus) was devised to study the binding of 125I-labeled receptor to beta-galactosidase. Maximal binding of receptor to enzyme occurred at pH values between 5.7 and 6.5. Divalent cations were not required for binding. The values of the dissociation constant obtained for beta-galactosidase varied between 200 nM observed with "lower uptake" forms and 20 nM for "higher uptake" forms of the enzyme. A number of phosphorylated monosaccharides were tested as inhibitors of binding of enzyme to receptor; mannose 6-phosphate and fructose 1-phosphate served as inhibitors and exhibited Ki values of 0.064 mM and 0.24 mM, respectively. The receptor has a subunit molecular weight of 215,000. Similar receptors were also demonstrated in Triton X-100 extracts of human skin fibroblasts, Chinese hamster ovary cells, and rat hepatocytes. These cell types are known to assimilate lysosomal enzymes containing covalently bound mannose 6-phosphate residues.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Neufeld EF, Lim TW, Shapiro LJ. Inherited disorders of lysosomal metabolism. Annu Rev Biochem. 1975;44:357–376. [Abstract] [Google Scholar]
- Kaplan A, Fischer D, Achord D, Sly W. Phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases by human fibroblasts. J Clin Invest. 1977 Nov;60(5):1088–1093. [Europe PMC free article] [Abstract] [Google Scholar]
- Sando GN, Neufeld EF. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. [Abstract] [Google Scholar]
- Ullrich K, Mersmann G, Weber E, Von Figura K. Evidence for lysosomal enzyme recognition by human fibroblasts via a phosphorylated carbohydrate moiety. Biochem J. 1978 Mar 15;170(3):643–650. [Europe PMC free article] [Abstract] [Google Scholar]
- Neufeld EF, Sando GN, Garvin AJ, Rome LH. The transport of lysosomal enzymes. J Supramol Struct. 1977;6(1):95–101. [Abstract] [Google Scholar]
- von Figura K, Weber E. An alternative hypothesis of cellular transport of lysosomal enzymes in fibroblasts. Effect of inhibitors of lysosomal enzyme endocytosis on intra- and extra-cellular lysosomal enzyme activities. Biochem J. 1978 Dec 15;176(3):943–950. [Europe PMC free article] [Abstract] [Google Scholar]
- Rome LH, Weissmann B, Neufeld EF. Direct demonstration of binding of a lysosomal enzyme, alpha-L-iduronidase, to receptors on cultured fibroblasts. Proc Natl Acad Sci U S A. 1979 May;76(5):2331–2334. [Europe PMC free article] [Abstract] [Google Scholar]
- Fischer HD, Gonzalez-Noriega A, Sly WS. Beta-glucuronidase binding to human fibroblast membrane receptors. J Biol Chem. 1980 Jun 10;255(11):5069–5074. [Abstract] [Google Scholar]
- Robbins AR. Isolation of lysosomal alpha-mannosidase mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1911–1915. [Europe PMC free article] [Abstract] [Google Scholar]
- Ullrich K, Mersmann G, Fleischer M, von Figura K. Epithelial rat liver cells have cell surface receptors recognizing a phosphorylated carbohydrate on lysosomal enzymes. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1591–1598. [Abstract] [Google Scholar]
- Ullrich K, Basner R, Gieselmann V, Von Figura K. Recognition of human urine alpha-N-acetylglucosaminidase by rat hepatocytes. Involvement of receptors specific for galactose, mannose 6-phosphate and mannose. Biochem J. 1979 May 15;180(2):413–419. [Europe PMC free article] [Abstract] [Google Scholar]
- Rome LH, Miller J. Butanedione treatment reduces receptor binding of a lysosomal enzyme to cells and membranes. Biochem Biophys Res Commun. 1980 Feb 12;92(3):986–993. [Abstract] [Google Scholar]
- Fischer HD, Gonzalez-Noriega A, Sly WS, Morré DJ. Phosphomannosyl-enzyme receptors in rat liver. Subcellular distribution and role in intracellular transport of lysosomal enzymes. J Biol Chem. 1980 Oct 25;255(20):9608–9615. [Abstract] [Google Scholar]
- Distler J, Hieber V, Sahagian G, Schmickel R, Jourdian GW. Identification of mannose 6-phosphate in glycoproteins that inhibit the assimilation of beta-galactosidase by fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4235–4239. [Europe PMC free article] [Abstract] [Google Scholar]
- DISTLER JJ, MERRICK JM, ROSEMAN S. Glucosamine metabolism. III. Preparation and N-acetylation of crystalline D-glucosamine- and D-galactosamine-6-phosphoric acids. J Biol Chem. 1958 Jan;230(1):497–509. [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Swanstrom R, Shank PR. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. [Abstract] [Google Scholar]
- Stanners CP, Eliceiri GL, Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. [Abstract] [Google Scholar]
- Hieber V, Distler J, Myerowitz R, Schmickel RD, Jourdian GW. Selective noncompetitive assimilation of bovine testicular beta-galactosidase and bovine liver beta-glucuronidase by generalized gangliosidosis fibroblasts. J Clin Invest. 1980 Apr;65(4):879–884. [Europe PMC free article] [Abstract] [Google Scholar]
- Kilberg MS, Handlogten ME, Christensen HN. Characteristics of an amino acid transport system in rat liver for glutamine, asparagine, histidine, and closely related analogs. J Biol Chem. 1980 May 10;255(9):4011–4019. [Abstract] [Google Scholar]
- Distler JJ, Jourdian GW. beta-Galactosidase from bovine testes. Methods Enzymol. 1978;50:514–520. [Abstract] [Google Scholar]
- Bolton AE, Hunter WM. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. [Europe PMC free article] [Abstract] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [Abstract] [Google Scholar]
- Ray TK. A modified method for the isolation of the plasma membrane from rat liver. Biochim Biophys Acta. 1970 Jan 6;196(1):1–9. [Abstract] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. [Europe PMC free article] [Abstract] [Google Scholar]
- Gonzalez-Noriega A, Grubb JH, Talkad V, Sly WS. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. [Europe PMC free article] [Abstract] [Google Scholar]
- Natowicz MR, Chi MM, Lowry OH, Sly WS. Enzymatic identification of mannose 6-phosphate on the recognition marker for receptor-mediated pinocytosis of beta-glucuronidase by human fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4322–4326. [Europe PMC free article] [Abstract] [Google Scholar]
- von Figura K, Klein U. Isolation and characterization of phosphorylated oligosaccharides from alpha-N-acetylglucosaminidase that are recognized by cell-surface receptors. Eur J Biochem. 1979 Mar;94(2):347–354. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.78.7.4289
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc319775?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
A Lifetime of Adventures in Glycobiology.
Annu Rev Biochem, 87:1-21, 01 Jun 2018
Cited by: 4 articles | PMID: 29925256
Bidirectional apical-basal traffic of the cation-independent mannose-6-phosphate receptor in brain endothelial cells.
J Cereb Blood Flow Metab, 37(7):2598-2613, 24 Mar 2017
Cited by: 7 articles | PMID: 28337939 | PMCID: PMC5531359
Intramembrane proteolysis within lysosomes.
Ageing Res Rev, 32:51-64, 30 Apr 2016
Cited by: 10 articles | PMID: 27143694
Review
Retromer-Mediated Trafficking of Transmembrane Receptors and Transporters.
Membranes (Basel), 5(3):288-306, 06 Jul 2015
Cited by: 19 articles | PMID: 26154780 | PMCID: PMC4584283
Review Free full text in Europe PMC
Efficient hepatic delivery of drugs: novel strategies and their significance.
Biomed Res Int, 2013:382184, 28 Oct 2013
Cited by: 50 articles | PMID: 24286077 | PMCID: PMC3826320
Review Free full text in Europe PMC
Go to all (75) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Beta-galactosidase decreases the binding affinity of the insulin-like-growth-factor-II/mannose-6-phosphate receptor for insulin-like-growth-factor II.
Eur J Biochem, 190(1):71-77, 01 May 1990
Cited by: 17 articles | PMID: 2163834
Insulin-like growth factor-II (IGF-II) inhibits both the cellular uptake of beta-galactosidase and the binding of beta-galactosidase to purified IGF-II/mannose 6-phosphate receptor.
J Biol Chem, 264(8):4710-4714, 01 Mar 1989
Cited by: 45 articles | PMID: 2538455
Bovine testicular beta-galactosidase: purification of enzyme fractions that exhibit high affinity for phosphomannosyl receptors.
Anal Biochem, 182(2):432-437, 01 Nov 1989
Cited by: 4 articles | PMID: 2558593
Morphologic study of the internalization of a lysosomal enzyme by the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells.
Proc Natl Acad Sci U S A, 78(11):6967-6971, 01 Nov 1981
Cited by: 54 articles | PMID: 6273898 | PMCID: PMC349174
Funding
Funders who supported this work.
NIADDK NIH HHS (2)
Grant ID: AM-10531
Grant ID: AM-5026