Abstract
Background & aims
The identification of the cellular and molecular pathways that mediate the development of non-alcoholic steatohepatitis is of crucial importance. Cytokines produced by liver-resident and infiltrating inflammatory cells, play a pivotal role in liver inflammation. The role of the proinflammatory cytokines IL-1α and IL-1β in steatohepatitis remains elusive.Methods
We employed IL-1α and IL-1β-deficient mice and transplanted marrow cells to study the role of liver-resident and bone marrow-derived IL-1 in steatosis and its progression to steatohepatitis.Results
Atherogenic diet-induced steatohepatitis in wild-type mice was associated with 16 and 4.6 fold-elevations in mRNA levels of hepatic IL-1α and IL-1β, respectively. In mice deficient in either IL-1α or IL-1β the transformation of steatosis to steatohepatitis and liver fibrosis was markedly reduced. This protective effect in IL-1α-deficient mice was noted despite increased liver cholesterol levels. Deficiency of IL-1α markedly reduced plasma serum amyloid A and steady-state levels of mRNA coding for inflammatory genes (P-selectin, CXCL1, IL-6, and TNFα) as well as pro-fibrotic genes (MMP-9 and Collagen) and particularly a 50% decrease in TGFβ levels (p = 0.004). IL-1α mRNA levels were two-folds lower in IL-1β-deficient mice, and IL-1β transcripts were three-folds lower in IL-1α-deficient compared to wild-type mice. Hepatic cell derived IL-1α rather than from recruited bone marrow-derived cells was required for steatohepatitis development.Conclusions
These data demonstrate the critical role of IL-1α and IL-1β in the transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. Therefore, the potential of neutralizing IL-1α and/or IL-1β to inhibit the development of steatohepatitis should be explored.Free full text

Lack of Interleukin-1α or Interleukin-1β Inhibits Transformation of Steatosis to Steatohepatitis and Liver Fibrosis in Hypercholesterolemic Mice
Abstract
Background
The identification of the cellular and molecular pathways that mediate the development of non-alcoholic steatohepatitis is of crucial importance. Cytokines produced by liver-resident and infiltrating inflammatory cells, play a pivotal role in liver inflammation. The role of the proinflammatory cytokines IL-1α and IL-1β in steatohepatitis remains elusive.
Aims & Methods
We employed IL-1α and IL-1β-deficient mice and transplanted marrow cells to study the role of liver-resident and bone marrow-derived IL-1 in steatosis and its progression to steatohepatitis.
Results
Atherogenic diet-induced steatohepatitis in wild-type mice was associated with 16 and 4.6 folds-elevations in mRNA levels of hepatic IL-1α and IL-1β, respectively. In mice deficient in either IL-1α or IL-1β the transformation of steatosis to steatohepatitis and liver fibrosis was markedly reduced. This protective effect in IL-1α-deficient mice was noted despite increased liver cholesterol levels. Deficiency of IL-1α markedly reduced plasma serum amyloid A and steady-state levels of mRNA coding for inflammatory genes (P-selectin, CXCL1, IL-6, TNFα) as well as pro-fibrotic genes (MMP-9 and Collagen) and particularly a 50% decrease in TGFβ (p=0.004). IL-1α mRNA levels were 2 folds lower in IL-1β-deficient mice, and IL-1β transcripts were 3 folds lower in IL-1α-deficient compared to wild-type mice. Hepatic cell derived IL-1α rather than from recruited bone marrow-derived cells is required for steatohepatitis development.
Conclusions
These data demonstrate the critical role of IL-1α and IL-1β in the transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. Therefore, the potential of neutralizing IL-1α and/or IL-1β to inhibit development of steatohepatitis should be explored.
Introduction
Non-alcoholic fatty liver disease (NAFLD) encompasses steatosis, non-alcoholic steatohepatitis (NASH) and cirrhosis, in the absence of alcohol abuse. Our understanding of the key factors that mediate the transformation of steatosis to steatohepatitis and liver fibrosis remains incomplete.[1] According to the "two-hit" hypothesis, "first hit" steatosis sensitizes the liver to induction of inflammation that may lead to the development of steatohepatitis.[2] There is increasing evidence supporting an important role for cytokines, including interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α in various aspects of inflammatory liver diseases.[3, 4] These cytokines are produced in the liver by Kupffer cells and hepatocytes, playing roles in lipid metabolism and hepatic inflammation.[5–7] Other cell types, including invading bone marrow-derived macrophages and neutrophils, can also produce inflammatory cytokines that promote liver damage.[8, 9] IL-1α and IL-1β and the specific receptor antagonist (IL-1Ra) exert their effect upon binding to IL-1 receptor type I (IL-1RI).[10] Binding of IL-1α or IL-1β to IL-1RI, induces expression of a variety of pro-inflammatory genes, including autocrine amplification of IL-1 production.[10] IL-1α and IL-1β are synthesized as precursors and their processing to mature forms requires specific cellular proteases. The precursor of IL-1β (preIL-1β) is only active as a mature secreted molecule after cleavage by caspase-1.[5],[11] In contrast, IL-1α exerts its effects both in the mature and the precursor (preIL-1α) forms when binding to its receptor.[12] PreIL-1α is also active as a nucleus associated protein and in its membrane-associated form.[10] We have hypothesized that the different sub-cellular compartments in which active IL-1α and IL-1β are present dictate different biological functions of these molecules.[13, 14] IL-1α belongs to a newly recognized group of dual-function cytokines that play a role in the nucleus apart from their extracellular, receptor-mediated effects.[15, 16] When released from dying cells, IL-1α initiates sterile inflammation, acting as a damage-associated molecular pattern molecule.[8, 13] While the role of IL-1Ra in lipid metabolism and fatty liver has been studied in vivo with conflicting results [17, 18], the specific role of IL-1α and IL-1β in fatty liver has not been determined yet. We have recently shown that IL-1α from non-bone marrow derived cells plays a role in lipid metabolism.[19] In the present work, we conducted studies employing IL-1α and IL-1β deficient mice to determine the effect of IL-1 deficiency on development of steatosis and steatohepatitis.
Methods
Animals and experimental procedures
The generation of IL-1α and IL-1β KO mice has been described previously.[20, 21] Wild type (WT) C57BL/6 mice were purchased from Harlan, Israel. Mice were maintained on a 12-h light/12-h dark cycle. The study protocols were approved by the Sheba Medical Center Board for Studies in Experimental Animals. To induce fatty liver, 8–10 week-old male mice were fed a high fat/cholesterol and cholate diet containing 17% total fat, 1.25% cholesterol and 0.5% sodium-cholate (Teklad Premier Laboratory Diet No. TD 90221) hereafter referred to as the atherogenic diet.
Experiment 1
The IL-1α, IL-1β knockout (KO) and WT mice (n=5 in each group) were characterized before and after consumption of the atherogenic diet for 2, 5, 10, and 18 weeks.
Experiment 2
We used the bone marrow transplantation method in order to asses the role of bone marrow-derived versus hepatic IL-1α or IL-1β in a diet-induced mouse model of steatohepatitis. At the age of 10 weeks, irradiated male WT mice were reconstituted with bone marrow cells from either WT (n=15), IL-1β KO (n=12), or IL-1α KO (n=12) mice. In order to induce steatohepatitis, two weeks after the bone marrow transplantation procedure, mice were given the atherogenic diet for 9 weeks. A control group of WT mice, who received WT bone marrow cells, was maintained on a regular chow diet throughout the experiment (Chow WT to WT, n=6). In addition, we used IL-1β−/− mice that received either WT (n=8) or IL-1β−/− (n=8) bone marrow cells, and IL-1α−/− mice that received WT (n=7) or IL-1α−/− (n=7) bone marrow cells.
Chemical analysis of serum and tissue
Animals were sacrificed by inhaling CO2, blood was collected after a 16-hour fast, by a puncture of the inferior vena cava or the retro orbital sinus. Analysis of total plasma cholesterol and triglyceride levels, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, albumin, bilirubin and glucose levels was performed using an automated enzymatic technique (Boehringer Mannheim, Germany). Plasma lipoproteins were separated by size exclusion chromatography using a Superose-6 column (1×30 cm) on an FPLC system (Pharmacia).[22]
Tissue preparation and histology
Livers were fixed in 4% paraformaldehyde and embedded in paraffin or frozen in liquid nitrogen and stored at −80°C. All samples were routinely stained for general morphology with hematoxylin-eosin, and for collagen with Masson’s trichrome. Liver immunohistochemistry staining was performed on 5-µm-thick paraffin embedded sections. Sections were deparaffinized and stained with goat anti-mouse IL-1α (1:20; R&D Systems, Minneapolis, MN) as described.[23]
Bone marrow transplantation
Mice in experiment 2 were exposed to a single dose of lethal irradiation (1000Rad) from a Clinical Linear Accelerator- Varian 2110C (6MV, 240mU/min), one day before the transplantation. Bone marrow (BM) cell suspensions were isolated from femurs and tibias of female donor mice by flushing with RPMI medium 1640 (GIBCO). Bone marrow cells were washed, resuspended in PBS, counted and then injected into the mouse tail vein in a final volume of 300µl. Each recipient mouse received 5×106 bone marrow cells.
Hepatic lipids
Hepatic lipids were extracted according to the method of Folch et al.[24] The extract was dissolved in 2-propanol and subsequently analyzed for total cholesterol, and triglycerides using an automated enzymatic technique (Boehringer Mannheim, Germany).
Assessment of serum amyloid A (SAA) levels
SAA levels in mouse plasma were determined by enzyme-linked immunosorbent assay according to the manufacturer's instructions (Immunology Consultants Laboratory, USA).
Analysis of gene expression by quantitative real-time PCR
Total RNA from mouse tissues was isolated using Rneasy Mini Kit (Qiagen, Hilden, Germany). Total RNA was reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Expression of 44 target and 4 reference genes was done with a TaqMan low-density array based on an Applied Biosystems 7900HT Micro Fluidic Card (Applied Biosystems). Real-time PCR for individual genes was carried out using a 7500 sequence detection System (Applied Biosystems) using GAPDH for normalization. PCR primers for Taqman/Probe Library assays were designed with the Probe Library Assay Design Center (http://www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp). Detailed methods, assay names, and primers used for each gene are listed in Supplementary methods.
Statistical analyses
The values reported are the mean±SE. Student’s t-test was used, where applicable, and an ANOVA analysis was applied to assess interactions between groups and differences between means. p<0.05 is accepted as statistically significant.
Results
IL-1α deficiency resulted in higher plasma non-HDL cholesterol levels
At baseline and 2 weeks, total cholesterol levels were similar in all three groups. At 5, 10 and 18 weeks plasma cholesterol levels were significantly higher in IL-1α KO mice compared with IL-1β KO and WT mice (Fig. 1A) due to increased cholesterol in the VLDL fraction (Fig. 1, C and D). Plasma triglyceride (TG) levels were similar in all groups (Fig. 1B).
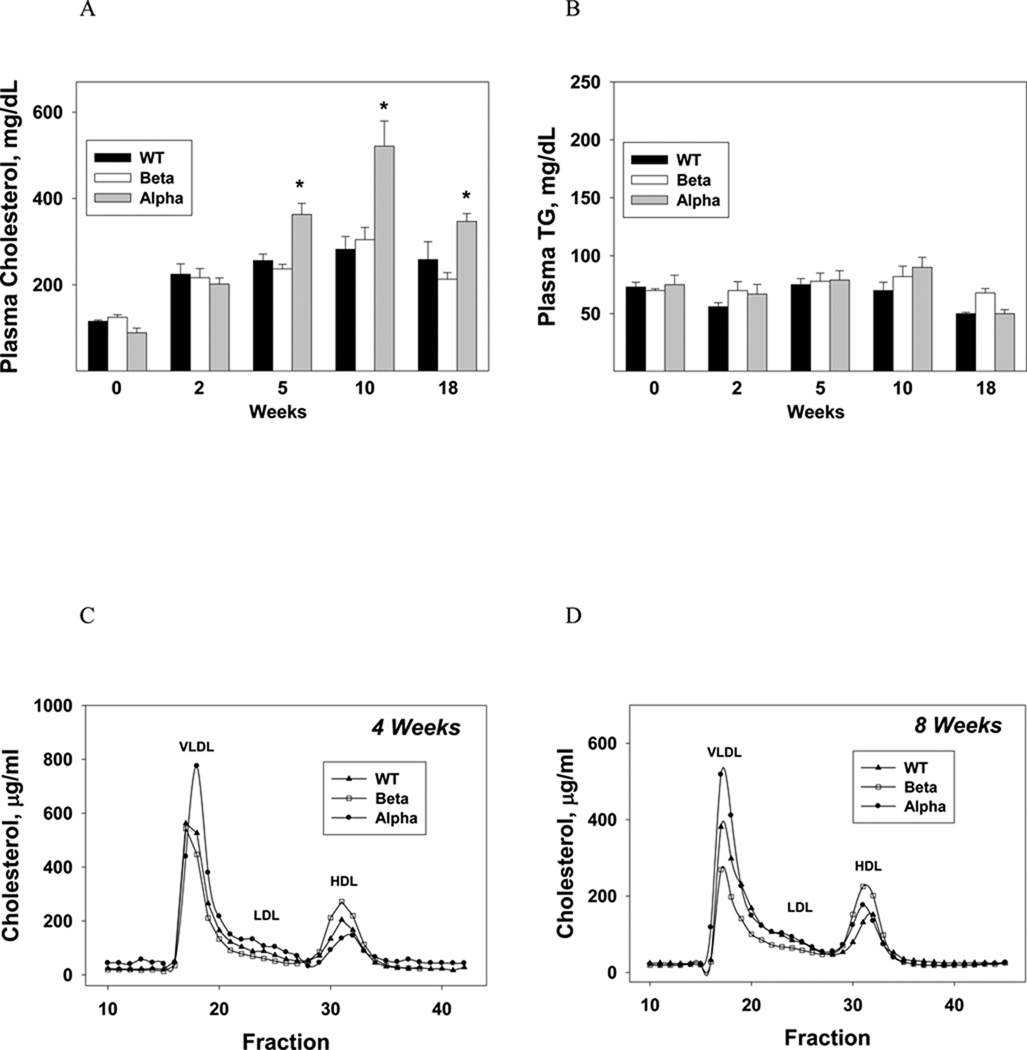
Plasma total cholesterol (A) and TG (B) levels in WT, IL-1β and IL-1α KO mice (n=5 in each group) were measured at baseline and after 2, 5, 10, and 18 weeks of the atherogenic diet. Data represent mean±SE of each group. *p<0.05 (A) compared to WT and IL-1β KO mice. Analysis of the distribution of serum lipoprotein cholesterol in WT, IL-1β and IL-1α KO mice was performed at 4 weeks (C) and after 8 weeks of the atherogenic diet (D). Gel filtration chromatography profiles of plasma lipoproteins from ▲ WT, □ IL-1β and ● IL-1α KO mice (n=5 in each group). Blood was obtained from fasted animals and plasma samples were pooled in each group.
The pro-atherogenic plasma lipid profile in IL-1α-deficient mice was associated with more severe steatosis
WT, IL-1α KO and IL-1β KO chow-fed mice had normal and similar liver weight and serum liver enzymes (Fig. 2), and showed normal morphology by histology (data not shown). Up to ten weeks of the atherogenic diet, there was an increase in liver weight in all three groups (Fig. 2A) with accumulation of liver cholesterol (Fig. 2B). Liver weight and cholesterol content were significantly higher in IL-1α KO compared to IL-1β KO and WT mice (Fig. 2A, 2B). The atherogenic diet reduced hepatic TG levels similarly in all groups (Fig. 2C). Serum bilirubin and albumin were not changed by the atherogenic diet (data not shown). Serum ALT levels increased by about two fold in all groups (Fig. 2D). There was an increase in the number and size of intracellular vacuoles, characteristic of lipid deposits, which were more prominent in IL-1α KO compared to WT and IL-1β KO livers (Fig. 3A, Table 1). Importantly, until 10 weeks of the atherogenic diet, there were only mild signs of liver inflammation with scanty areas of fibrosis in all three groups (Fig. 3A, Table 1).
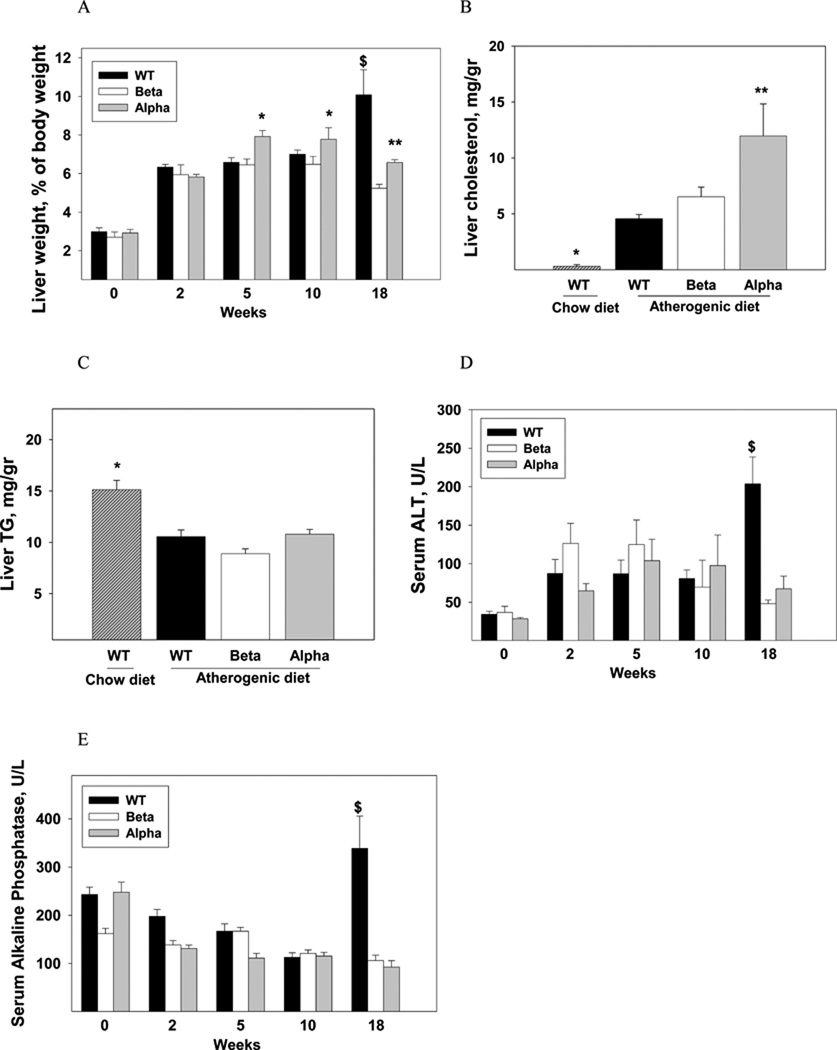
Liver to body weight ratio (A) in WT, IL-1β KO (Beta) and IL-1α KO (Alpha) mice (n=5 in each group) were determined at baseline and after 2, 5, 10, and 18 weeks of the atherogenic diet. Data represent mean±SE of each group. *p<0.05 compared to WT and IL-1β KO mice, **p<0.05 compared to IL-1β KO mice, $p<0.05 compared to IL-1β KO and IL-1α KO mice. Hepatic cholesterol (B) and TG (C) levels were determined in chow-fed WT and WT, IL-1β KO (Beta) and IL-1α KO (Alpha) mice (n=5 in each group) after 10 weeks of the atherogenic diet. Data represent mean±SE of each group. *p<0.05 compared to atherogenic diet-fed WT, IL-1β KO (Beta) and IL-1α KO (Alpha) mice. **p<0.05 compared to atherogenic diet-fed WT and IL-1β KO mice. Serum levels of ALT (D) and alkaline phosphatase (E) were determined in mice described in (A). Data represent mean±SE of each group, $p<0.05 compared to IL-1β and IL-1α KO mice.
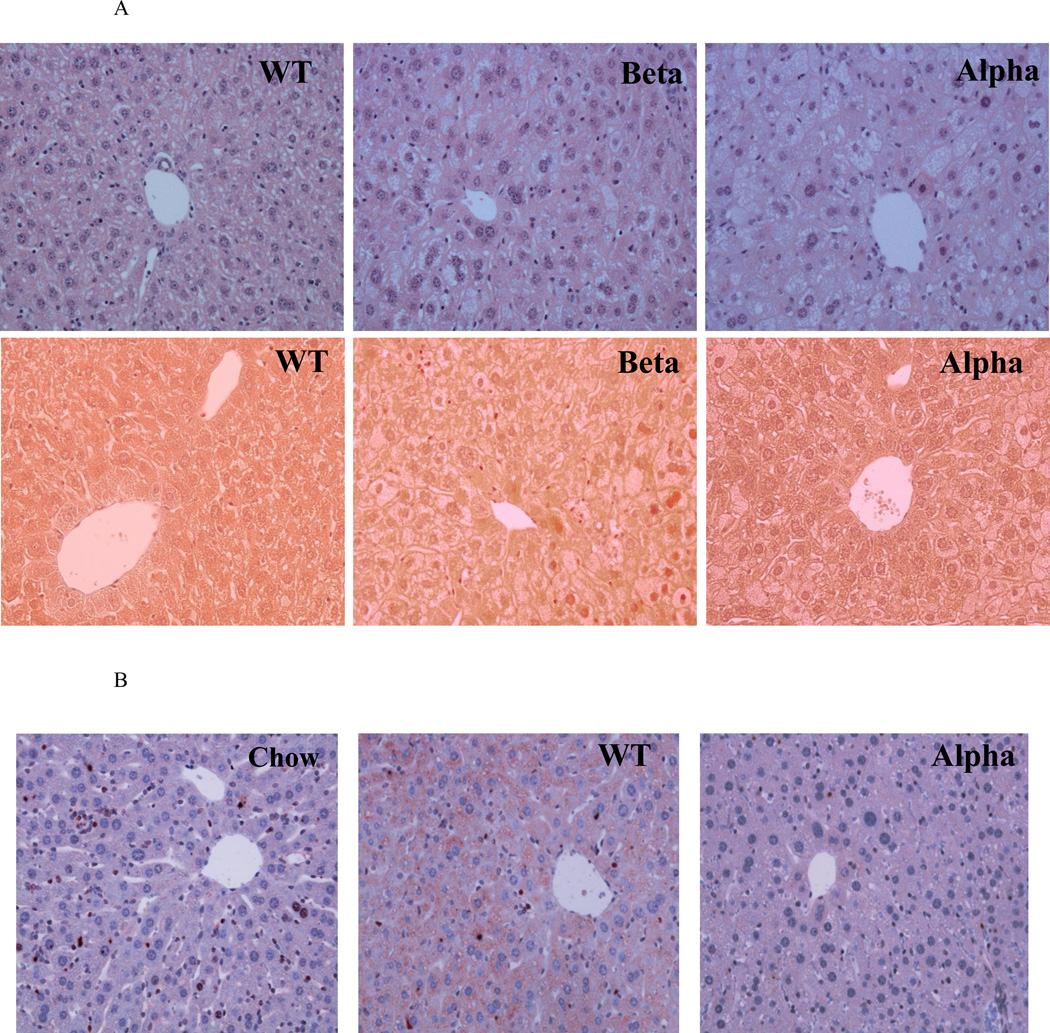
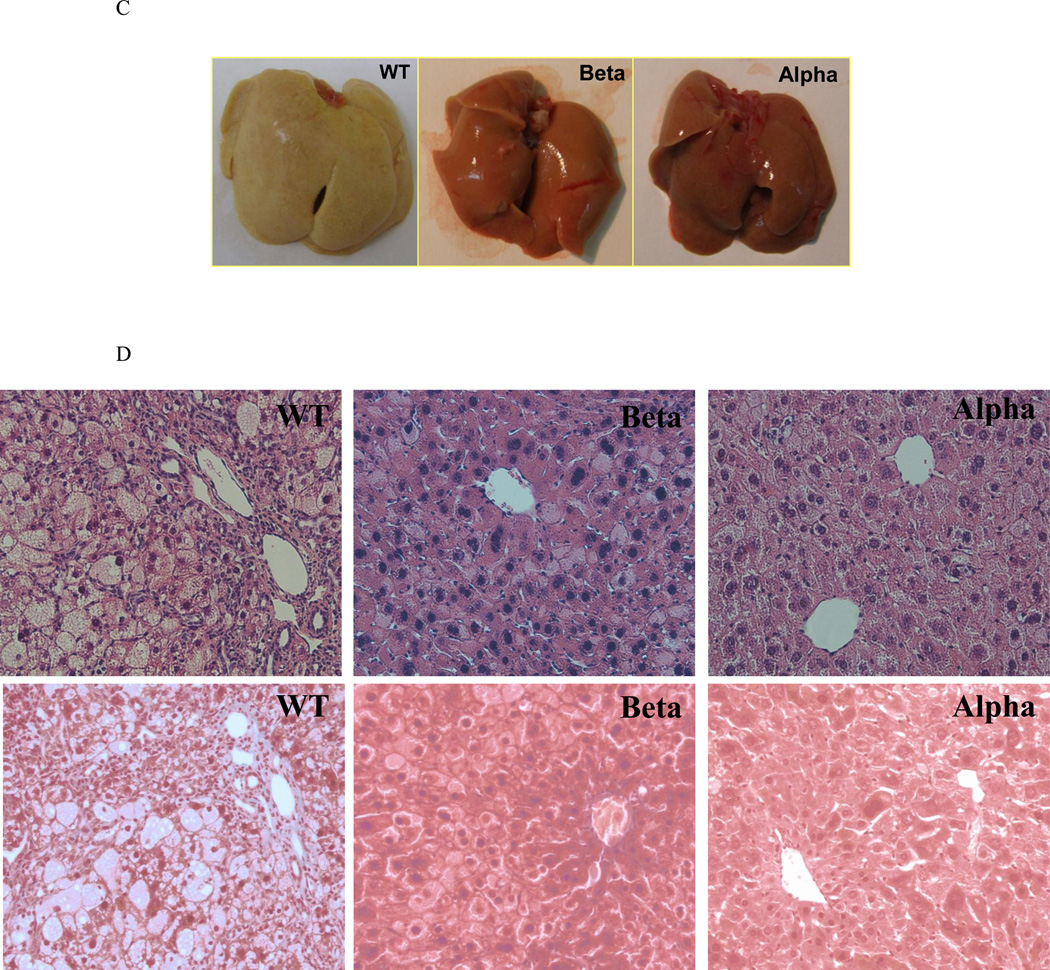
Liver specimens were obtained from the specified experimental groups after 10 and 18 weeks of the atherogenic diet. IL-1α deficiency was associated with more steatosis. (A) Representative images of H&E (upper panel) and Masson (lower panel) staining of livers from WT, IL-1β KO (Beta), and IL-1α KO (Alpha) mice after 10 weeks of atherogenic diet (Magnification ×200). (B) Immunohistochemical staining for IL-1α protein in livers from WT and IL-1α KO (Alpha) mice after 10 weeks of atherogenic diet compared to WT on chow (Chow) diet (Magnification ×200). IL-1α or IL-1β deficiency protected from the development of hepatic inflammation and collagen deposition. (C) Representative macroscopic photographs of livers from WT, IL-1β KO (Beta) and IL-1α KO (Alpha) mice after 18 weeks of atherogenic diet. (D) Representative images of H&E (upper panel) and Masson (lower panel) staining are shown from WT, IL-1β KO (Beta) and IL-1α KO (Alpha) mice after 18 weeks of atherogenic diet.
Table 1
H&E score of livers from WT mice fed chow diet, and WT, IL-1β KO (Beta) and IL-1α KO (Alpha) mice after 10 weeks of the atherogenic diet.
| Chow | WT | Beta | Alpha | Scale | |
|---|---|---|---|---|---|
| Steatosis parenchymal involvement | 0±0 | 1.3±0.3 | 1.4±0.4 | 2.4±0.2* | 0–3 |
| Fibrosis | 0±0 | 0.3±0.2 | 0.2±0.2 | 0.2±0.2 | 0–4 |
| Inflammation Lobular | 0.4±0.2 | 1.2±0.2 | 1.6±0.3 | 1.2±0.2 | 0–3 |
| Portal | 0.1±0.1 | 1.2±0.4 | 0.8±0.4 | 1.2±0.2 | 0–3 |
| Liver cell injury Ballooning | 0.4±0.2 | 1.0±0 | 1.4±0.2 | 2.0±0 | 0–2 |
| Acidophil bodies | 0±0 | 0.8±0.2 | 1.0±0.2 | 1.6±0.2 | 0–1 |
*Key: Steatosis parenchymal involvement (<5%=0, 5–33%=1, 33–66%=2, >66%=3); Fibrosis (None=0, Perisinusoidal/ Periportal=1, Perisinusoidal and Periportal=2, Bridging fibrosis=3, Cirrhosis=4); Portal Inflammation (None/Minimal=0, Mild=1, Moderate=2, Severe=3); Lobular Inflammation (None=0, Mild=1, Moderate=2, and Severe=3); Liver cell injury: Ballooning (None=0, Few=1, Many=2); Acidophil bodies (None/rare=0, Many=1).
Steatosis development was accompanied by increased expression of IL-1α
We assessed the expression of a panel of inflammation and fibrosis related genes (Table 2). Steatosis development was associated with a 3.2 fold increase in mRNA transcripts of IL-1α compared to chow-fed mice (p=0.001), with a 1.5 fold non-significant elevation in IL-1β gene expression (Table 2). Immunohistochemical staining showed increased cytoplasmatic staining of IL-1α protein in hepatocytes (Fig. 3B). Livers from IL-1α KO mice on atherogenic diet did not show staining for IL-1α (negative control).
Table 2
Gene expression in livers of WT mice following 10 (WT 10W) or 18 weeks (WT 18W) of atherogenic diet (relative to chow-fed mice, n=4–5 in each group). Relative mRNA in livers of IL-1α KO (Alpha 18W) compared to WT mice following 18 weeks of atherogenic diet (n=4 in each group). Quantitative real-time PCR analysis used the TaqMan low-density array based on an Applied Biosystems 7900HT Micro Fluidic Card. HPRT-1, TBP, GAPDH and 18S were used as reference genes. Data analysis using the comparative CT (ΔΔCT) method for relative quantization (RQ) of gene expression was performed with DataAssist™ Software v2.0 Software.
| Gene name | WT 10W | p | WT 18W | P | Alpha 18W | P |
|---|---|---|---|---|---|---|
| Pro-inflammatory cytokines | ||||||
| IL-1β | 1.5 | 0.35 | 4.6 | 0.001 | 0.33 | 0.01 |
| CD40 | 2.4 | 0.04 | 5.0 | 0.002 | 0.52 | 0.13 |
| IL-1α | 3.2 | 0.001 | 16 | 0.002 | 0.001 | 0.002 |
| IL-18 | 1.4 | 0.01 | 1.4 | 0.005 | 0.84 | 0.33 |
| CSF-1 | 1.3 | 0.07 | 2.3 | 0.01 | 0.53 | 0.05 |
| CD68 | 2.6 | 0.01 | 5.3 | 0.02 | 0.55 | 0.08 |
| IL-2Rα | 1.7 | 0.19 | 3.6 | 0.02 | 0.50 | 0.06 |
| IL-6 | 4.0 | 0.18 | 6.7 | 0.03 | 0.20 | 0.03 |
| IL-12 | 2.8 | 0.20 | 3.1 | 0.03 | 0.48 | 0.28 |
| IL-5 | 0.9 | 0.40 | 1.5 | 0.04 | 1.16 | 0.50 |
| TNFα | 2.5 | 0.04 | 5.1 | 0.04 | 0.27 | 0.05 |
| IL-33 | 1.5 | 0.01 | 2.4 | 0.06 | 0.43 | 0.06 |
| CSF-3 | 1.1 | 0.79 | 1.3 | 0.15 | 1.16 | 0.50 |
| IL-1R1 | 1.7 | 0.11 | 1.4 | 0.22 | 1.01 | 0.98 |
| CD40 ligand | 1.5 | 0.12 | 1.4 | 0.42 | 0.83 | 0.78 |
| IL-17 | 0.4 | 0.32 | 0.6 | 0.56 | 1.16 | 0.50 |
| IFNγ | 1.2 | 0.73 | 1.0 | 0.95 | 0.97 | 0.95 |
| Anti-inflammatory cytokines | ||||||
| IL-1R antagonist | 18 | 0.06 | 130 | 0.01 | 0.16 | 0.01 |
| IL-10 | 1.5 | 0.39 | 2.3 | 0.12 | 0.35 | 0.10 |
| Chemotaxis | ||||||
| CXCR-3 | 1.9 | 0.02 | 3.7 | 0.0004 | 0.83 | 0.25 |
| CCR-7 | 2.2 | 0.05 | 4.9 | 0.008 | 0.44 | 0.04 |
| CCR-2 | 3.9 | 0.05 | 15 | 0.01 | 0.37 | 0.03 |
| CCL-3 | 1.9 | 0.13 | 7.5 | 0.02 | 0.30 | 0.04 |
| CXCL-1 | 1.0 | 0.99 | 4.1 | 0.04 | 0.16 | 0.03 |
| CCL-2 | 3.3 | 0.15 | 12 | 0.06 | 0.26 | 0.09 |
| CXCL-2 | 2.7 | 0.15 | 12 | 0.11 | 0.14 | 0.12 |
| Cell adhesion | ||||||
| ICAM-1 | 1.8 | 0.002 | 2.8 | 0.002 | 0.72 | 0.34 |
| P-selectin | 1.6 | 0.11 | 3.0 | 0.01 | 0.27 | 0.01 |
| VCAM-1 | 1.5 | 0.23 | 2.7 | 0.04 | 0.78 | 0.35 |
| E-selectin | 4.6 | 0.13 | 18 | 0.05 | 0.44 | 0.18 |
| Fibrosis | ||||||
| TGFβ | 1.8 | 0.001 | 3.1 | 0.0001 | 0.58 | 0.06 |
| PDGFRβ | 1.5 | 0.27 | 3.6 | 0.005 | 0.67 | 0.37 |
| TGFβR1 | 1.1 | 0.30 | 2.2 | 0.01 | 0.69 | 0.12 |
| Collagen | 4.9 | 0.07 | 38 | 0.04 | 0.25 | 0.07 |
| CTGF | 0.8 | 0.45 | 3.4 | 0.04 | 0.44 | 0.09 |
| MMP-9 | 1.5 | 0.21 | 8.4 | 0.05 | 0.18 | 0.05 |
| PDGFβ | 1.5 | 0.08 | 4.2 | 0.05 | 0.44 | 0.11 |
| PDGFRα | 1.2 | 0.43 | 2.3 | 0.06 | 1.00 | 0.99 |
| TIMP-1 | 6.9 | 0.06 | 57 | 0.06 | 0.21 | 0.09 |
| Acta-2 | 1.0 | 0.97 | 2.7 | 0.06 | 0.32 | 0.06 |
| PDGFα | 1.4 | 0.03 | 1.6 | 0.10 | 0.78 | 0.31 |
| TGFβR2 | 1.4 | 0.05 | 1.5 | 0.11 | 0.74 | 0.26 |
| Bambi | 1.6 | 0.33 | 1.3 | 0.72 | 1.02 | 0.97 |
| TGFβR3 | 1.0 | 0.96 | 1.0 | 0.94 | 0.97 | 0.92 |
Deficiency of IL-1α or IL-1β inhibited the progression of steatosis to steatohepatitis
At 18 weeks, livers of WT mice had a gray color with a hard and rubbery consistency compared to the yellowy and soft livers of IL-1α KO and IL-1β KO mice (Fig. 3C). Liver weight in WT mice was 94% and 53% higher than IL-1β KO and IL-1α KO mice, respectively (Fig. 2A). Liver weight of IL-1α KO mice was 27% higher compared with IL-1β KO (p<0.05). Liver cholesterol was significantly lower in IL-1β KO compared to WT (13±1.3 and 20±2.4 mg/gr, respectively, p<0.05), with no difference between IL-1α KO (18±2.2 mg/gr) and WT mice. Serum ALT levels in WT mice were 325% and 200% higher than IL-1β KO and IL-1α KO mice, respectively (Fig. 2D). Serum alkaline phosphatase levels were also higher in WT compared with IL-1β KO and IL-1α KO mice (Fig. 2E). The morphology of WT livers had been dramatically altered with lobular and portal inflammation, and extensive lobular, portal and interstitial fibrosis (Fig. 3D, Table 3). These morphological changes are compatible with severe steatohepatitis. Consistent with the histopathological findings, there was a marked induction of several inflammatory- and fibrosis-related genes in livers from WT mice, including a 16 and 4.6 fold-elevations in mRNA levels of IL-1α and IL-1β, respectively (Table 2). Compared to the severe steatohepatitis in WT mice, IL-1α KO and IL-1β KO mice had preserved liver morphology, with markedly less inflammation and collagen deposition (Fig. 3D, Table 3). IL-1β KO mice had also a lower degree of steatosis compared to WT mice (Fig. 3D, Table 3). The reduction in liver damage in IL-1α KO mice was accompanied by lower mRNA levels of inflammation and fibrosis related genes, including a 3 and 5 fold reduction in the expression of IL-1β and IL-6, respectively and a 50% decrease in TGFβ (Table 2, Table 4). Importantly, liver IL-1α mRNA levels were 2 fold lower in IL-1β KO compared to WT mice (RQ 0.51±0.04 and 1.0±0.08, respectively, p<0.001).
Table 3
H&E score of livers from WT, IL-1β KO (Beta) and IL-1α KO (Alpha) mice after 18 weeks of the atherogenic diet.
| WT | Beta | Alpha | Scale | |
|---|---|---|---|---|
| Steatosis parenchymal involvement | 2.9±0.1 | 1.8±0.2* | 2.5±0.2 | 0–3 |
| Fibrosis | 2.4±0.2 | 0±0* | 0.6±0.4* | 0–4 |
| Inflammation Portal | 2.2±0.1 | 0.8±0.3* | 1.2±0.2* | 0–3 |
| Lobular | 2.8±0.1 | 1.2±0.2* | 1.2±0.2* | 0–3 |
| Liver cell injury Ballooning | 2.0±0 | 1.0±0* | 1.4±0.2 | 0–2 |
| Acidophil bodies | 1.0±0 | 0±0* | 0.4±0.2 | 0–1 |
Table 4
Relative mRNA of individual genes (validation) in livers of IL-1α KO and WT mice following 18 weeks of atherogenic diet (Mean±SEM, n=6–8 in each group).
| Gene name | RQ WT | RQ IL-1α KO | P-Value |
|---|---|---|---|
| TNFα | 1.0±0.20 | 0.20±0.05 | 0.004 |
| TGFβ | 1.0±0.10 | 0.56±0.05 | 0.004 |
| Collagen | 1.0±0.30 | 0.13±0.04 | 0.036 |
| CD68 | 1.0±0.15 | 0.60±0.05 | 0.070 |
Plasma SAA levels were markedly reduced in IL-1α KO mice
The atherogenic diet induced a marked increase in SAA levels compared with chow-fed WT mice (p<0.05, Fig. 4A). IL-1α KO mice had remarkably lower plasma SAA levels compared to WT both at the steatosis and steatohepatitis stages (p<0.05, Fig. 4A, 4B).
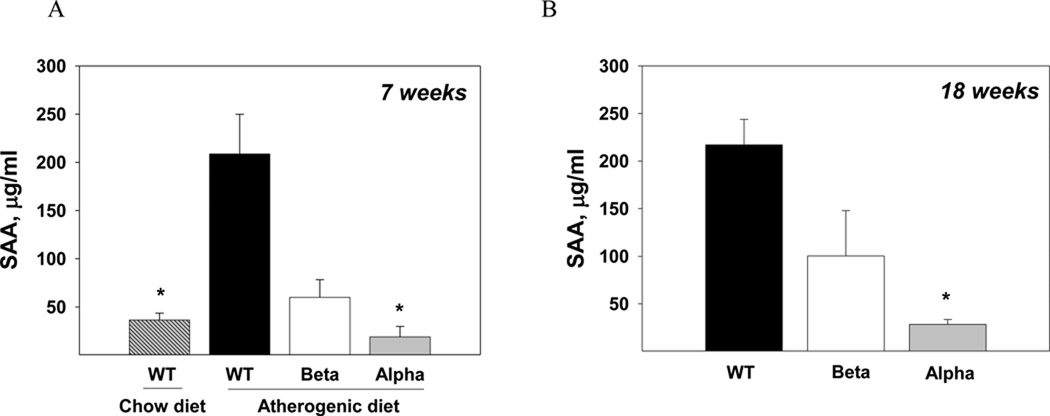
Plasma SAA levels were determined in chow-fed WT and atherogenic diet-fed WT, IL-1β KO and IL-1α KO mice (n=5 in each group) after 7 weeks (A) and in WT, IL-1β KO and IL-1α KO mice (n=5 in each group) after 18 weeks of atherogenic diet (B). Data represent mean±SE of each group. *p<0.05 compared to atherogenic diet-fed WT mice.
Hepatic rather than bone marrow-derived IL-1α or IL-1β deficiency protected against development of steatohepatitis
Induction of steatohepatitis was accelerated in the BM transplantation model and required only 9 weeks of feeding the atherogenic diet. IL-1β KO mice were more sensitive to total body irradiation with a 50% mortality rate (8/16), which resulted in a smaller sample size in Beta to Beta and WT to Beta (n=4 in each group). IL-1α deficiency resulted in reduced liver weight compared to WT to WT (p<0.05; Fig. 5A). Selective expression of IL-1α or IL-1β in bone marrow-derived cells did not increase liver weight in IL-1α or IL-1β deficient mice; WT to Beta and WT to Alpha had reduced liver weight and serum ALT and alkaline phosphatase levels compared to WT to WT (Fig. 5A, 5B, 5C). Furthermore, selective deficiency of IL-1 in BM-derived cells did not attenuate the increase in liver weight (Fig. 5A). Deficiency of IL-1α resulted in a 47% reduction in SAA compared to WT to WT (p<0.05; Fig. 5D). Immunohistochemical staining of livers from WT to WT fed the atherogenic diet showed increased staining of IL-1α protein both in hepatocytes and in inflammatory foci (Fig. 6A). Livers from Alpha to WT showed increased expression of IL-1α in hepatocytes but a substantially lower number of IL-1α positive inflammatory cells compared to WT to WT (Fig. 6A). Deficiency of IL-1β or IL-1α in the liver (IL-1 KO recipients) inhibited steatohepatitis and liver fibrosis development, despite expression of IL-1 in BM-derived cells (Fig. 6B). Collectively, these data show that hepatic and not bone marrow-IL-1 deficiency protects against atherogenic diet induced inflammation and fibrosis.
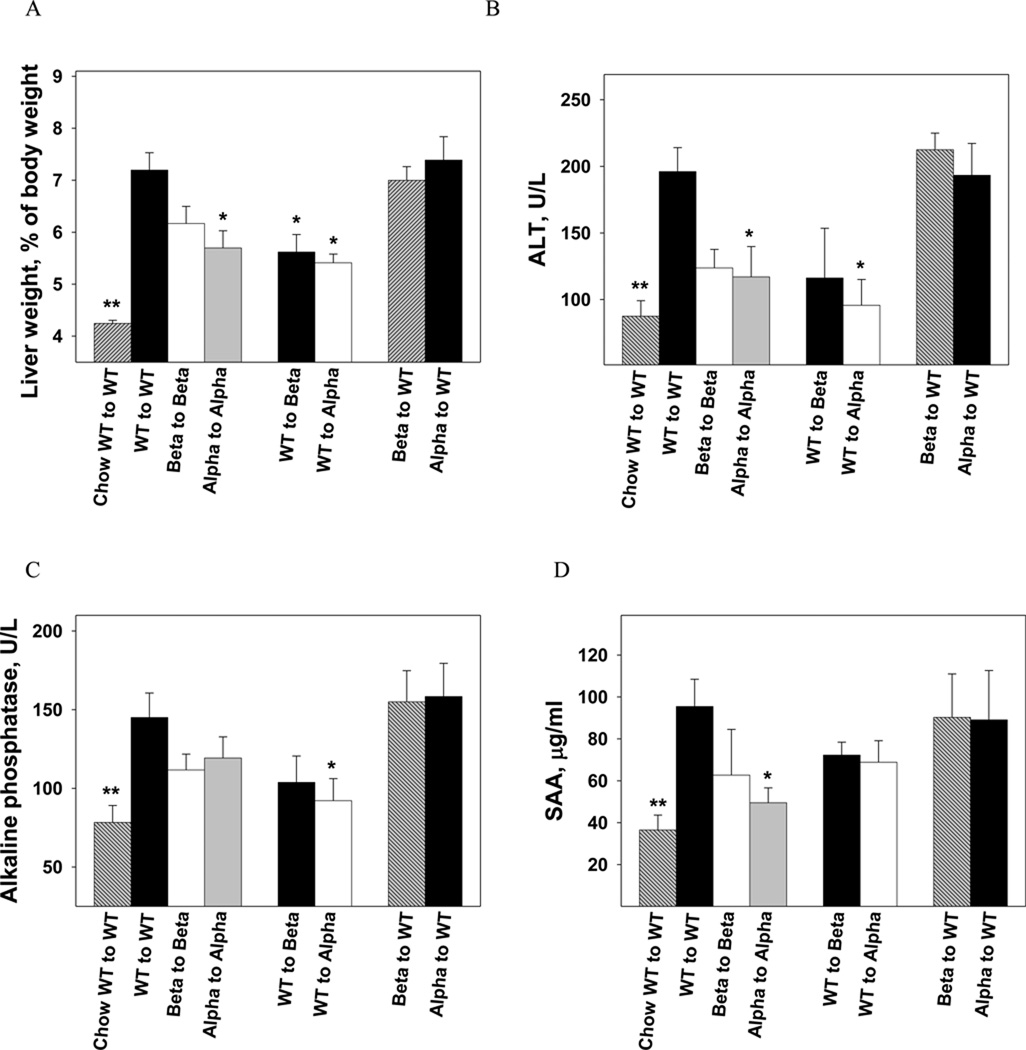
WT mice reconstituted with either WT, IL-1β−/− or IL-1α−/− BM cells (WT to WT; Beta to WT; Alpha to WT), IL-1β KO mice with WT or IL-1β−/− BM cells (WT to Beta; Beta to Beta), and IL-1α KO mice with WT or IL-1α−/− BM cells (WT to Alpha; Alpha to Alpha), were given the atherogenic diet for 9 weeks. A group of WT to WT mice was maintained on chow diet (Chow WT to WT). Liver to body weight ratio (A) in Chow WT to WT (n=6), WT to WT (n=13), Beta to Beta (n=4), Alpha to Alpha (n=7), WT to Beta (n=4), WT to Alpha (n=7), Beta to WT (n=9), and Alpha to WT (n=9) mice, were determined at the end of the study. Data represent mean±SE of each group. **p<0.05 compared to all other groups, *p<0.05 compared to WT to WT. Serum ALT levels (B), alkaline phosphatase (C) and plasma SAA levels (D) were determined in mice described in (A). *p<0.05 compared to WT to WT.
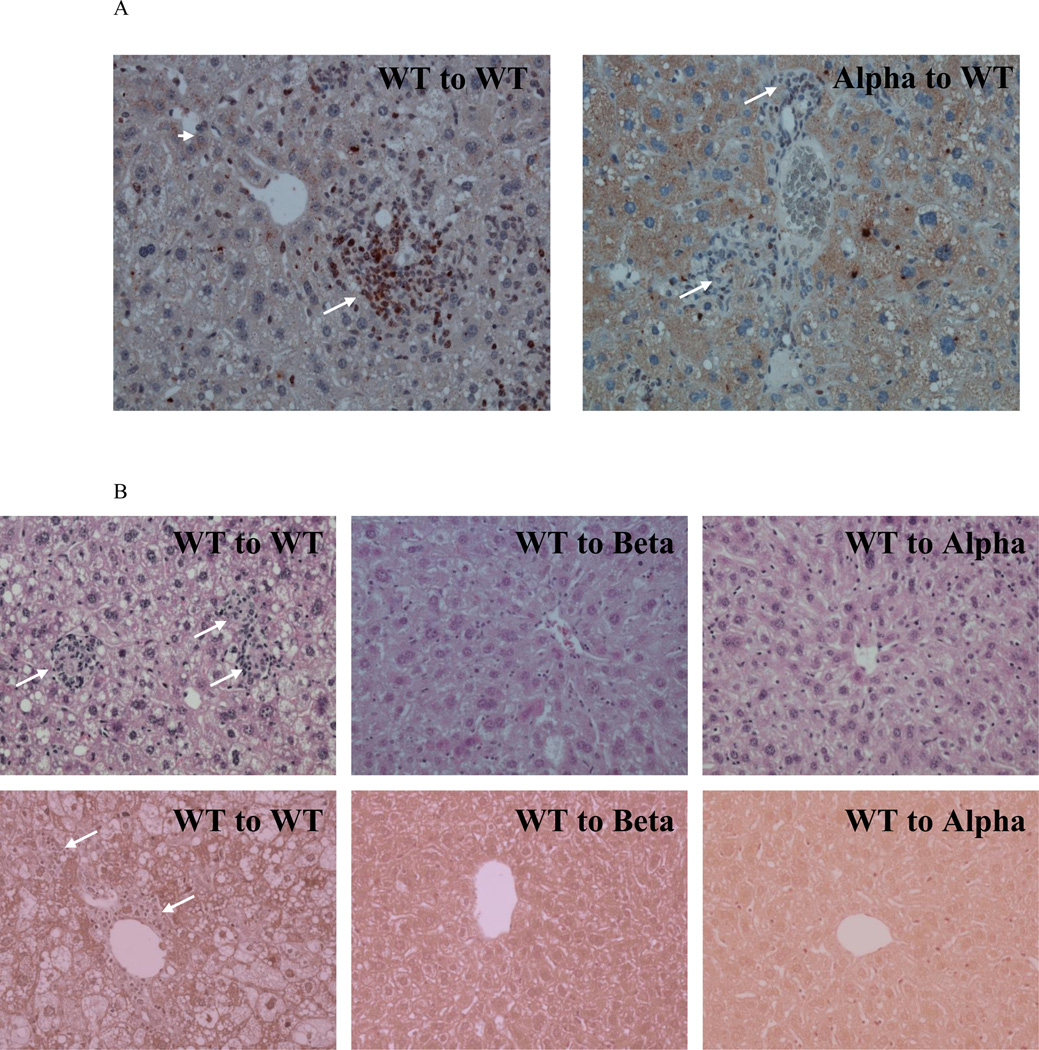
Liver specimens were obtained from the specified experimental groups as described in figure 5. (A) Representative images of immunohistochemistry staining for IL-1α in livers from WT to WT and Alpha to WT mice. WT to WT livers showed increased staining of IL-1α protein both in hepatocytes (Arrow head) and in cells within inflammatory foci (Arrows). Livers from Alpha to WT also showed increased expression in hepatocytes (Arrow head) but showed a substantially lower number of cells with positive IL-1α staining in inflammatory foci (Arrows). (B) Representative images of H&E (upper panel) and Masson trichrome (lower panel) staining of livers from WT to WT, WT to Beta, and WT to Alpha mice are shown. Arrows in H&E staining show inflammatory foci. Arrows in Masson staining show collagen deposition. Magnification ×200.
Discussion
We report that deficiency of IL-1α or IL-1β markedly reduces the progression of steatosis to steatohepatitis and liver fibrosis in a mouse model of atherogenic diet-induced steatohepatitis. The protective effect in IL-1α-deficient mice was particularly unexpected as there was increased hepatic cholesterol deposition, and thus this observation supports the dominant role of inflammation driven by IL-1α in development of steatohepatitis.
The common "two-hit" hypothesis for the pathogenesis of NAFLD implicates inflammation as the link between steatosis and steatohepatitis. Furthermore, recent reports have shown that inflammation through cytokine signaling pathways, including IL-1β signaling, can also aggravate liver accumulation of cholesterol[18, 25] and TGs[7, 26], creating an autoinflammatory circuit of inflammation through cytokine-induced lipid accumulation. Indeed, during the "second hit" phase, IL-1β-deficient mice had less liver inflammation, and also less steatosis compared to WT mice. Unexpectedly, IL-1α-deficient mice developed more pronounced steatosis with higher hepatic cholesterol and had similar liver cholesterol levels at the steatohepatitis stage, despite markedly reduced liver inflammation. This dissociation between cholesterol accumulation and inflammation raises the possibility that IL-1α may be involved in regulation of cholesterol homeostasis during steatosis, and emphasizes that inhibition of liver inflammation was not due to reduced fat accumulation. The concept that the type as opposed to the quantity of lipids accumulating play a central role in NASH development[27, 28] raises the possibility that more pronounced steatosis (without an increase in hepatic TG) in IL-1α-deficient mice, could exert protective, even anti-inflammatory effects mediated by yet unidentified types of lipids beyond TG.
Cholesterol accumulation in hepatocytes plays a pivotal role in lipid-induced liver inflammation.[29–31] Therefore, one would expect that increased hepatic cholesterol in IL-1α-deficient mice would accelerate the development of steatohepatitis. Remarkably, deficiency of IL-1α protected from the progression of steatosis to steatohepatitis and liver fibrosis. IL-1α has been recently characterized as a "danger signal" that is released from necrotic cells, including liver cells, and induces sterile inflammation.[8, 32] We have recently shown that the chromatin bound-IL-1α precursor is released from necrotic, but not apoptotic cells, and induces sterile inflammation as manifested by recruitment of myeloid lineage inflammatory cells.[13] In the current model, hepatic gene expression for IL-1α was induced early during liver fat accumulation, and the development of severe steatohepatitis in WT mice was accompanied by a robust 16 fold increase in the expression of IL-1α. As shown by Sakurai et al in liver carcinogenesis, [32] we propose that the precursor form of IL-1α induced by fat accumulation in liver cells is released from necrotic cells and initiates local inflammation. Indeed, deficiency of IL-1α markedly reduced plasma levels of the acute phase protein SAA, and liver expression of inflammatory genes, including the adhesion molecule P-selectin, chemotactic protein CXCL1, and proinflammatory cytokines IL-6, TNFα and IL-1β. IL-1 is known to induce the expression of adhesion molecules such as P-selectin in endothelial cells, including liver sinusoidal cells.[5, 33] Furthermore, IL-1α released from necrotic cells, including liver cells, plays a critical role in recruitment of inflammatory cells through induction of the chemokine CXCL1.[34] Therefore, the data from our study suggest that IL-1α either directly or through induction of other cytokines such as IL-6, TNFα and IL-1β, promotes development of steatohepatitis by increasing the expression of adhesion molecules and chemotactic factor. Next, recruitment of inflammatory cells to the fat-induced injured liver takes place.
A marked reduction in collagen deposition was detected in livers of IL-1α KO mice. Liver fibrosis results from unresolved inflammation associated with the production of cytokines such as transforming growth factor-β (TGF-β), which exert fibrogenic stimuli to hepatic stellate cells (HSCs).[35] Stimulation of HSCs with IL-1α provokes HSC activation and production of matrix metalloproteinases (MMPs).[36] Furthermore, gene ablation of IL-1 receptor type-1 or MMP-9 attenuated the progression from liver injury to fibrogenesis in vivo.[37] However, little is known about the role of IL-1 in fibrosis development due to NAFLD. The reduced inflammation accompanied by lower expression of TGFβ and MMP-9 in livers of IL-1α KO compared to WT mice may explain the marked reduction in collagen deposition in livers of IL-1α KO mice.
Deficiency of either IL-1α or IL-1β was sufficient to protect against NASH development, which was surprising since both cytokines bind to the same surface receptor and mediate similar downstream signaling effects. The earlier and more pronounced elevation of IL-1α mRNA levels compared to IL-1β, pointed toward a specific role of IL-1α. Furthermore, our finding that IL-1α-deficient mice had lower liver IL-1β mRNA transcripts and IL-1β-deficient mice had lower IL-1α mRNA levels demonstrates for the first time in the liver mutual induction of IL-1α and IL-1β in vivo, similar to previous reports in other cell types.[5, 20] Considering the mutual regulation of IL-1α and IL-1β, it is likely that lower expression of IL-1α in the liver contributed to the protection of IL-1β-deficient mice from steatohepatitis and vice versa.
The contribution of IL-1 from infiltrating bone marrow-derived cells to steatohepatitis development was assayed in bone-marrow transplantation experiments. Both resident liver cells and recruited inflammatory cells express IL-1.[10, 32, 38] Furthermore, stimulated peripheral blood monocytes from NASH patients overproduced IL-1α.[9] Here we have demonstrated that IL-1α or IL-1β from resident liver cells, rather than recruited bone marrow-derived cells, are critical for the development of steatohepatitis. This observation differs from our previous finding showing that deficiency of IL-1 in bone marrow-derived cells attenuated atherosclerosis development.[19] Full reconstitution of Kupffer cells with transplanted cells occurs after a 9 week recovery period.[39] Therefore, the role of Kupffer cell-derived IL-1 in steatohepatitis cannot be determined in these studies since a two week recovery period did not ensure repopulation of all Kupffer cells.
In summary, these findings highlight the critical role of IL-1α and IL-1β in transformation of steatosis to steatohepatitis and liver fibrosis. Therefore, the potential of neutralizing IL-1α and/or IL-1β activity to protect against development of steatohepatitis should be explored.
Acknowledgments
These studies are supported by the Angela and Sami Shamoon Vascular Biology research fund (Y.K), the Talpiot Sheba Medical Leadership Program (Y.K) and NIH Grants AI-15614 (to CAD).
Abbreviations
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| IL-1 | interleukin-1 |
| TNF | tumor necrosis factor |
| IL-1Ra | IL-1 receptor antagonist |
| IL-1RI | IL-1 receptor type I |
| preIL-1β | precursor of IL-1β |
| preIL-1α | precursor of IL-1α |
| WT | Wild type |
| KO | knockout |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| SAA | serum amyloid A |
| TG | triglyceride |
| BM | bone marrow |
| TGF-β | transforming growth factor-β |
| HSCs | hepatic stellate cells |
| MMP | matrix metalloproteinase |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jhep.2011.01.048
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3210940?pdf=render
Citations & impact
Impact metrics
Article citations
Innate Immunity and MASLD.
Biomolecules, 14(4):476, 13 Apr 2024
Cited by: 2 articles | PMID: 38672492 | PMCID: PMC11048298
Review Free full text in Europe PMC
Intrauterine food restriction impairs the lipogenesis process in the mesenteric adipocytes from low-birth-weight rats into adulthood.
Front Endocrinol (Lausanne), 14:1259854, 31 Oct 2023
Cited by: 0 articles | PMID: 38027196 | PMCID: PMC10651082
Multifunctional roles of inflammation and its causative factors in primary liver cancer: A literature review.
World J Hepatol, 15(12):1258-1271, 01 Dec 2023
Cited by: 0 articles | PMID: 38223416 | PMCID: PMC10784815
Review Free full text in Europe PMC
Thrombin-activated interleukin-1α drives atherogenesis, but also promotes vascular smooth muscle cell proliferation and collagen production.
Cardiovasc Res, 119(12):2179-2189, 01 Oct 2023
Cited by: 0 articles | PMID: 37309666 | PMCID: PMC10578913
C-C motif chemokine receptor 2 inhibition reduces liver fibrosis by restoring the immune cell landscape.
Int J Biol Sci, 19(8):2572-2587, 08 May 2023
Cited by: 5 articles | PMID: 37215993 | PMCID: PMC10197881
Go to all (167) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Lack of interleukin-1α in Kupffer cells attenuates liver inflammation and expression of inflammatory cytokines in hypercholesterolaemic mice.
Dig Liver Dis, 46(5):433-439, 26 Feb 2014
Cited by: 20 articles | PMID: 24582082
Interleukin-1 participates in the progression from liver injury to fibrosis.
Am J Physiol Gastrointest Liver Physiol, 296(6):G1324-31, 02 Apr 2009
Cited by: 156 articles | PMID: 19342509 | PMCID: PMC2697947
Interleukin-1α deficiency attenuates endoplasmic reticulum stress-induced liver damage and CHOP expression in mice.
J Hepatol, 63(4):926-933, 27 May 2015
Cited by: 17 articles | PMID: 26022690
IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice.
J Clin Invest, 122(10):3476-3489, 04 Sep 2012
Cited by: 416 articles | PMID: 22945633 | PMCID: PMC3461900
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (4)
Grant ID: AI-15614
Grant ID: R01 AI015614
Grant ID: R56 AI015614
Grant ID: R01 AI015614-30





