Abstract
Objective
The incidence of type 1 diabetes is increasing. Delivery by cesarean section is also more prevalent, and it is suggested that cesarean section is associated with type 1 diabetes risk. We examine associations between cesarean delivery, islet autoimmunity and type 1 diabetes, and genes involved in type 1 diabetes susceptibility.Research design and methods
Cesarean section was examined as a risk factor in 1,650 children born to a parent with type 1 diabetes and followed from birth for the development of islet autoantibodies and type 1 diabetes.Results
Children delivered by cesarean section (n = 495) had more than twofold higher risk for type 1 diabetes than children born by vaginal delivery (hazard ratio [HR] 2.5; 95% CI 1.4-4.3; P = 0.001). Cesarean section did not increase the risk for islet autoantibodies (P = 0.6) but was associated with a faster progression to diabetes after the appearance of autoimmunity (P = 0.015). Cesarean section-associated risk was independent of potential confounder variables (adjusted HR 2.7;1.5-5.0; P = 0.001) and observed in children with and without high-risk HLA genotypes. Interestingly, cesarean section appeared to interact with immune response genes, including CD25 and in particular the interferon-induced helicase 1 gene, where increased risk for type 1 diabetes was only seen in children who were delivered by cesarean section and had type 1 diabetes-susceptible IFIH1 genotypes (12-year risk, 9.1 vs. <3% for all other combinations; P < 0.0001).Conclusions
These findings suggest that type 1 diabetes risk modification by cesarean section may be linked to viral responses in the preclinical autoantibody-positive disease phase.Free full text

Cesarean Section and Interferon-Induced Helicase Gene Polymorphisms Combine to Increase Childhood Type 1 Diabetes Risk
Abstract
OBJECTIVE
The incidence of type 1 diabetes is increasing. Delivery by cesarean section is also more prevalent, and it is suggested that cesarean section is associated with type 1 diabetes risk. We examine associations between cesarean delivery, islet autoimmunity and type 1 diabetes, and genes involved in type 1 diabetes susceptibility.
RESEARCH DESIGN AND METHODS
Cesarean section was examined as a risk factor in 1,650 children born to a parent with type 1 diabetes and followed from birth for the development of islet autoantibodies and type 1 diabetes.
RESULTS
Children delivered by cesarean section (n = 495) had more than twofold higher risk for type 1 diabetes than children born by vaginal delivery (hazard ratio [HR] 2.5; 95% CI 1.4–4.3; P = 0.001). Cesarean section did not increase the risk for islet autoantibodies (P = 0.6) but was associated with a faster progression to diabetes after the appearance of autoimmunity (P = 0.015). Cesarean section–associated risk was independent of potential confounder variables (adjusted HR 2.7;1.5–5.0; P = 0.001) and observed in children with and without high-risk HLA genotypes. Interestingly, cesarean section appeared to interact with immune response genes, including CD25 and in particular the interferon-induced helicase 1 gene, where increased risk for type 1 diabetes was only seen in children who were delivered by cesarean section and had type 1 diabetes–susceptible IFIH1 genotypes (12-year risk, 9.1 vs. <3% for all other combinations; P < 0.0001).
CONCLUSIONS
These findings suggest that type 1 diabetes risk modification by cesarean section may be linked to viral responses in the preclinical autoantibody-positive disease phase.
Type 1 diabetes is characterized by autoimmunity and a genetic susceptibility encoded in multiple genes (1). The last decades have seen a marked increase in the incidence of type 1 diabetes among children (2), strongly suggesting that environmental factors contribute to overall type 1 diabetes risk. Among the myriad of changes in the environment over recent decades, rapid increases in the rate of cesarean section have occurred in parallel to the rate of diabetes (3). A meta-analysis of 20 retrospective studies revealed a 20% increase in the risk of childhood-onset type 1 diabetes in children delivered by cesarean section (4). Children delivered by cesarean section have been shown to have altered gut microbiotic composition and immune response (5), both of which are relevant to the development of diabetes (6). Potentially related to these effects of cesarean section is that genetic susceptibility for type 1 diabetes is determined by genes that influence host immune response, including response to microbial environment (7).
We recently demonstrated that the onset of autoimmunity and the subsequent progression to diabetes are distinct phases (8) that can be affected differently by genes that are associated with type 1 diabetes risk (9). Interactions between genes and environment to modify risk either specifically at autoimmunity or specifically at progression would provide evidence that the gene and environmental factors act through a common pathogenetic mechanism. Here, we use cesarean section as an environmental factor and search for association with islet autoimmunity and/or progression to diabetes and interactions with type 1 diabetes susceptibility genes. We examined these associations in the BABYDIAB cohort, which prospectively follows children born to a parent with type 1 diabetes, allowing us to define both autoimmunity and progression. The findings provide evidence for interaction between cesarean section and immune response genes in influencing progression to type 1 diabetes after seroconversion.
RESEARCH DESIGN AND METHODS
Study cohort, participants, and samples.
The study was performed in children from the BABYDIAB study, a longitudinal study examining the natural history of islet autoimmunity and type 1 diabetes in 1,650 children born to a mother or father with type 1 diabetes (10). Recruitment began in 1989 and ended in 2000. All children were recruited from Germany. The cohort is not population based, and 97% of families are German Caucasian. Venous blood samples were obtained from children at study visits scheduled at 9 months and at 2, 5, 8, 11, 14, 17, and 20 years of age. Autoantibodies against insulin (IAAs), GAD (GADAs), insulinoma antigen 2 (IA-2As), and zinc transporter 8 (ZnT8As) were measured in samples taken at all scheduled visits and every 6 months in children with islet autoantibodies. The median follow-up time from birth to last sample was 11.0 years (interquartile range 8.0–12.5) and from birth to last contact was 13.9 years (11.9–15.6). The BABYDIAB study was approved by the ethical committee of Bavaria, Germany (Bayerische Landesärztekammer number 95357). All families gave written informed consent to participate in the study. Investigations were carried out in accordance with the principles of the Declaration of Helsinki, as revised in 2000.
Islet autoantibody measurements.
IAAs, GADAs, IA-2As, and ZnT8As were determined centrally by the Institute of Diabetes Research Munich using radiobinding assays as previously described (10,11). Briefly, IAAs were measured by protein A/G radiobinding assays using [125I]-recombinant human insulin labeled at tyrosine amino acid 14, and GADAs, IA-2As, and ZnT8As were measured separately by protein A radiobinding assays using [35S]-methionine–labeled in vitro transcribed/translated recombinant human GAD65, intracellular portion of IA-2, and the COOH-terminal portion of ZnT8 for the two major variants at amino acid 325, respectively. The upper limit of normal for each assay was determined using Q–Q plots and corresponded to the 99th percentile of control children. Offspring were considered positive for islet autoantibodies when two consecutive samples collected after birth were positive. Islet autoantibody assays were evaluated by the Diabetes Autoantibody Standardization Program (laboratory 121) (12,13).
Subject characteristics and environmental exposure.
Perinatal and anthropometric data were collected from each child’s pediatric record. Relevant to the current study, this included maternal age at delivery, gestational age, mode of delivery, sex of the child, and singleton birth status. Where no pediatric record was provided, the mode of delivery was recorded as missing. Parity status and smoking behavior during pregnancy were self-reported in a questionnaire completed by the mothers before or at delivery.
Genotyping.
HLA-DRB1, HLA-DQA1, and HLA-DQB1 alleles were typed using PCR-amplified DNA and nonradioactive sequence-specific oligonucleotide probes as described previously (14). Classification into high-risk HLA genotypes was based on The Environmental Determinants of Diabetes in the Young (TEDDY) study inclusion genotypes for first-degree relatives (15): DR4-DQA1*030X-DQB1*0302@/DR3-DQA1*0501-DQB1*0201; DR4-DQA1*030X-DQB1*0302@/DR4-DQA1*030X-DQB1*0302@; DR4-DQA1*030X-DQB1*0302@/DR8- DQA1*0401-DQB1*0402, DR3-DQA1*0501-DQB1*0201/DR3-DQA1*0501-DQB1*0201; DR4-DQA1*030X-DQB1*0302@/DR4-DQA1*030X-DQB1*020X; DR4-DQA1*030X-DQB1*0302@/DR1-DQA1*0101-DQB1*0501; DR4-DQA1*030X-DQB1*0302@/DR13-DQA1*0102-DQB1*0604, DR4-DQA1*030X-DQB1*0302/DR4-DQA1*030X-DQB1*0304, DR4-DQA1*030×-DQB1*0302@/DR9-DQA1*030X-DQB1*0303; DR3-DQA1*0501-DQB1*0201/DR9-DQA1*030X-DQB1*0303; where @ includes DQB1*0302 and DQB1*0304.
Interferon-induced with helicase C domain (IFIH1), CD25, and PTPN22 genotypes were determined in 1,289, 1,283, and 1,295 children, respectively. The remaining children were not typed because of unsuitable or missing DNA samples or unsuccessful typing. Single-nucleotide polymorphism (SNP) genotyping was performed with the MassARRAY system using iPLEX chemistry (Sequenom, San Diego, CA), as previously described (16). SNP CD25 rs11594656 and the proxy SNPs IFIH1 rs2111485 and PTPN22 rs6679677 were typed. Reproducibility was assessed by duplicate genotyping in 16.3% of samples (discordance rate, <0.5%). For each of the SNP typings, deviation from Hardy-Weinberg equilibrium was previously tested in a case-control set of samples.
Statistical analysis.
Time to event analyses (Kaplan-Meier and Cox proportional hazards model) were used for diabetes and islet autoantibody outcomes. Kaplan-Meier analysis was used to calculate risk and to compare probabilities of type 1 diabetes and islet autoantibodies in children stratified for delivery mode and stratified for delivery mode and genotype. The Cox proportional hazards model was used to determine hazard ratios (HRs) for multiple covariates. To identify potential confounders associated with cesarean section (Table 1), the χ2 test was used. Variables that were associated with cesarean section were subsequently included as covariates in the Cox proportional hazards model. For the Cox model, these covariates were categorized as yes/no (maternal diabetes, singleton birth, firstborn child, premature birth, breastfeeding <3 months, and HLA TEDDY risk genotype) or above and below median (birth weight). For IFIH1, CD25, and PTPN22 SNPs, children were categorized as either homozygous for the common allele or other, corresponding to a recessive susceptible model for IFIH1 and CD25 and a dominant/codominant model for PTPN22. For analyses with type 1 diabetes as the outcome, the age at diagnosis of diabetes or the age at last follow-up was used as the event time. For analyses with islet autoantibody status as the outcome, the age at the first sample positive for one or more islet autoantibodies was used as the event time. Analyses were censored for loss to follow-up and for antibody-negative status at the subject’s last autoantibody-negative sample according to age. The log-rank test was used to compare categories in the Kaplan-Meier analysis. As a test for homogeneity for findings with respect to cesarean section, a stratified analysis was performed after dividing the cohort into children born in the months January, March, May, July, September, or November (odd months) and children born in the months February, April, June, August, October, or December (even months). A permutation analysis (n = 100) was performed for the Cox proportional hazards model that included cesarean section and the type 1 diabetes susceptibility genes.
TABLE 1
Association of cesarean section with pregnancy and birth factors
| n | Cesarean section [n (%)] | P* | |
|---|---|---|---|
| Maternal type 1 diabetes | 10−23 | ||
 Yes Yes | 917 | 433 (47.2) | |
 No No | 588 | 127 (21.6) | |
| Singleton birth | 0.016 | ||
 Yes Yes | 1,468 | 537 (36.6) | |
 No No | 37 | 23 (62.2) | |
| Premature (<37 weeks’ gestation) | 10−15 | ||
 Yes Yes | 191 | 123 (64.4) | |
 No No | 1,314 | 437 (33.3) | |
| Firstborn child | 5 × 10−5 | ||
 Yes Yes | 857 | 364 (42.5) | |
 No No | 624 | 187 (30.0) | |
| Maternal smoking during pregnancy | 0.16 | ||
 Yes Yes | 163 | 73 (44.8) | |
 No No | 1,342 | 487 (36.3) | |
| Maternal age (years) | 1.0 | ||
 <28.1 <28.1 | 471 | 172 (36.5) | |
 28.2–31.6 28.2–31.6 | 511 | 189 (37.0) | |
 >31.6 >31.6 | 506 | 193 (38.1) | |
| Birth wt (g) | 0.048 | ||
 <3,450 <3,450 | 700 | 281 (40.0) | |
 ≥3,450 ≥3,450 | 727 | 241 (33.2) | |
| Breast feeding (months) | 4 × 10−10 | ||
 ≤3 ≤3 | 720 | 330 (45.8) | |
 >3 >3 | 737 | 215 (29.2) |
*Bonferroni-corrected values.
Evidence for interaction between cesarean section and type 1 diabetes susceptibility genes associated with type 1 diabetes in the cohort was investigated by 1) adding the term cesarean section×genotype as a covariate in the Cox proportional hazards model; 2) Cox proportional hazards model testing of cesarean section association with diabetes conditioned by a gene SNP genotype categorized as either homozygous for the common allele or other; and 3) Kaplan-Meier analysis after stratification for genotype as either homozygous for the common allele or other.
Where indicated, P values have been corrected using Bonferroni correction, which was by a factor of 8 for covariates shown in Table 1 and by a factor of 5 for Kaplan-Meier analyses with gene stratification in Fig. 4. For all analyses, two-tailed P values of 0.05 were considered significant. All statistical analyses were performed using the Statistical Package for Social Science (SPSS 18.0; Chicago, IL).
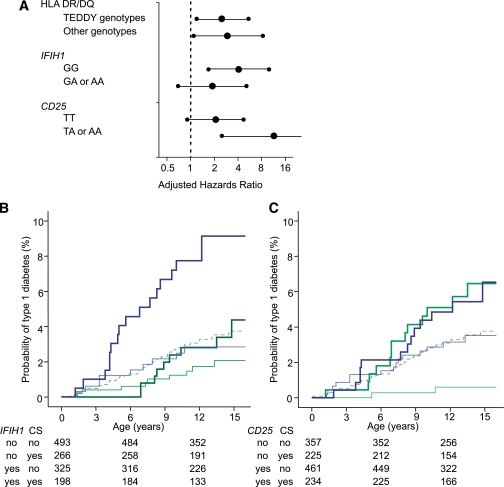
Interactions between cesarean section and type 1 diabetes susceptibility genes. A: Adjusted HRs (large dots) for cesarean section versus vaginal delivery as a risk for type 1 diabetes are shown after stratification for genotype at HLA, IFIH1, and CD25 genes. For the IFIH1 and CD25 SNPs, the risk genotype used was the homozygous common allele, which is consistent with a recessive susceptibility model. Error bars represent 95% CIs. B and C: Kaplan-Meier survival curves for type 1 diabetes risk according to delivery mode stratified for IFIH1 (B) and CD25 (C) genotype. Type 1 diabetes development curves for cesarean section and vaginal delivery are shown as thick and thin lines, respectively, and the color codes represent children with the type 1 diabetes susceptible (blue) and nonsusceptible (green) genotypes, as predicted by recessive susceptibility models. Numbers under the abscissa are the number of children still followed at each time point. P values for comparisons are provided in the text.
RESULTS
Cesarean section is associated with increased type 1 diabetes risk.
Of the 1,505 children with delivery mode data, 560 were born by cesarean section and 945 by vaginal delivery (Fig. 1). Delivery by cesarean section was more frequent in children who were born to mothers with type 1 diabetes (47%) than in children of nondiabetic mothers (22%; Pcorrected <0.0001), in nonsingleton births (62%; Pcorrected = 0.016), in births at <37 weeks of gestation (64%; Pcorrected <0.0001), in firstborn children (42%; Pcorrected <0.0001), and in children with birth weight less than the cohort median (40%, Pcorrected = 0.048), but was not associated with maternal age at delivery or maternal smoking during pregnancy (Table 1). Children who were delivered by cesarean section were breastfed less than children from vaginal delivery (Pcorrected <0.001).
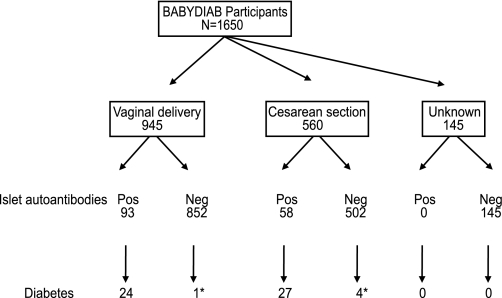
Study participants and outcome. Schematic diagram shows study participants, mode of delivery, and outcome with respect to islet autoantibodies and type 1 diabetes. *The five cases of diabetes that occurred in children without islet autoantibodies were not tested for islet autoantibodies for 9, 7.7, 6.8, 4, and 1.2 years before diabetes onset, respectively.
A total of 51 children developed diabetes during follow-up. The risk for developing type 1 diabetes by age 12 years was higher in children who were delivered by cesarean section (4.8%; 95% CI 3.0–6.6%) than children born by vaginal delivery (2.2%; 1.2–3.2; P = 0.002; Fig. 2A). The increased risk for diabetes among children delivered by cesarean section remained after adjusting for maternal diabetes, nonsingleton birth, premature delivery, firstborn children, and smoking during pregnancy (adjusted HR 2.8;1.5–5.1; P = 0.001; Table 2). This increased risk was also observed when the analysis was limited to children of fathers with type 1 diabetes and nondiabetic mothers (adjusted HR 4.7; 1.8–12.1; P = 0.001; n = 630) or when the cohort was stratified according to month of birth as a check for data homogeneity (adjusted HR 2.5, P = 0.048 for odd months; adjusted HR 3.4, P = 0.003 for even months).
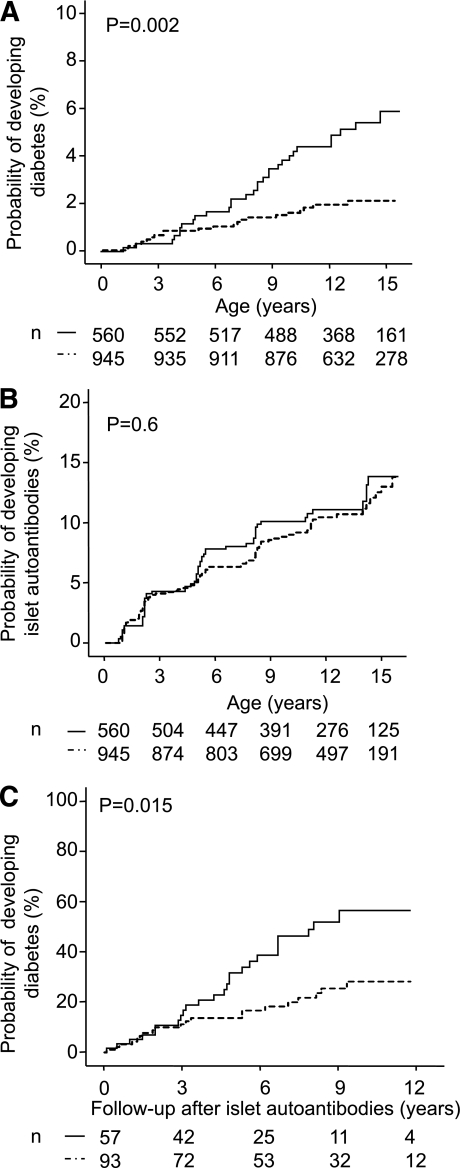
Cesarean section and risk for type 1 diabetes and islet autoantibodies. Kaplan-Meier analysis for the probability of developing type 1 diabetes (A) and islet autoantibodies (B) and for the progression to overt diabetes from first appearance of islet antibodies (C) in children delivered by cesarean section (solid line) or vaginally (dashed line). Numbers under the abscissa are the number of children still followed at each time point. P values are derived from log-rank tests comparing children delivered by cesarean section with children born by vaginal delivery.
TABLE 2
Multivariate Cox proportional hazards model for developing type 1 diabetes
| Univariate HR | P | Multivariate HR | P | |
|---|---|---|---|---|
| Cesarean section | 2.5 (1.4–4.3) | 0.001* | 2.8 (1.5–5.0) | 0.001 |
| Maternal type 1 diabetes | 0.9 (0.5–1.6) | 0.8 | 0.8 (0.4–1.4) | 0.5 |
| Nonsingleton birth | NA† | NA* | ||
| Premature birth | 1.2 (0.6–2.5) | 0.7 | 1.2 (0.5–2.6) | 0.7 |
| Firstborn child | 1.0 (0.6–1.7) | 0.9 | 1.2 (0.6–2.1) | 0.6 |
| Breast feeding <3 months | 0.9 (0.5–1.6) | 0.7 | 0.8 (0.4–1.5) | 0.5 |
| Birth wt <3,450 g | 1.4 (0.8–2.5) | 0.2 | 1.5 (0.8–2.6) | 0.2 |
*Bonferroni-corrected P = 0.007.
†Not applicable, since there were no diabetes cases in the 37 nonsingleton birth children.
Cesarean section increases progression from autoimmunity to type 1 diabetes.
Islet autoantibodies precede the development of type 1 diabetes and were identified in multiple samples from 147 of the BABYDIAB children. However, there was no increase in the risk of islet autoantibodies in children who were delivered by cesarean section (13.8%; 95% CI 9.9–17.7%) compared with children with vaginal delivery (13%; 10–16%; P = 0.6; Fig. 2B). No differences between cesarean section and vaginal delivery were also observed for the probability of developing autoantibodies associated with thyroid autoimmunity and celiac disease (Supplementary Fig. 1). Instead, as shown in Fig. 2C, the rate of progression from the development of islet autoantibodies to overt type 1 diabetes in autoantibody-positive children was higher in children delivered by cesarean section (57% within 10 years; 41–73%) versus children born by vaginal delivery (28%; 17–39%; P = 0.015). This remained significant after adjustment for potential confounding variables associated with cesarean section (adjusted HR 2.4; 1.2–4.4; P = 0.008).
Cesarean section interacts with immune response genes in modifying type 1 diabetes risk.
Cesarean delivery is reported to be accompanied by a change in the infant’s microbial and viral environment and immune response (5). In addition to HLA class II genes (17), the viral immune response–associated gene IFIH1 is associated with type 1 diabetes development in our cohort (9), and the CD25 gene and PTPN22 gene affect both immune response and type 1 diabetes risk (7). We therefore examined genotypes of these four gene regions for association with type 1 diabetes and for possible interactions with cesarean section in the association with type 1 diabetes risk. With respect to association, a multivariate Cox proportional hazards model showed that HLA DR/DQ genotype (adjusted HR 7.3; 95% CI 4.0–13.4; P < 0.0001), IFIH1 genotype (2.4; 1.3–4.5; P = 0.005), and cesarean section (2.7; 1.5–4.9; P = 0.001) contributed to the probability of developing type 1 diabetes in the BABYDIAB cohort (Fig. 3). Permutation analysis in the multivariate Cox model confirmed that HLA DR/DQ genotype (P < 0.01), IFIH1 genotype (P < 0.01), and cesarean section (P < 0.01) were associated with type 1 diabetes risk and further suggested an association with CD25 genotype (P = 0.04), but not PTPN22 genotype (P = 0.49), in the cohort.
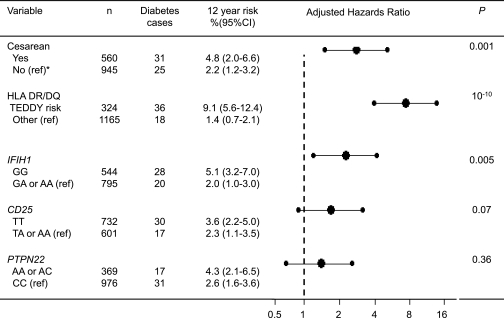
Type 1 diabetes outcome and risk according to delivery mode and type 1 diabetes susceptibility genes. Type 1 diabetes outcome stratified for delivery mode or the type 1 diabetes susceptibility genes is shown in the left-hand column. Delivery mode is categorized as cesarean section or vaginal delivery, HLA is categorized as TEDDY-defined risk genotypes or other, and SNP genotype is categorized as homogeneous for the common allele versus other. *The reference group used to calculate adjusted HRs in the Cox proportional hazards model is indicated for each covariate (ref). The adjusted HRs (large dots) and 95% CIs (error bars) for type 1 diabetes in the multivariate Cox proportional hazards model are shown in the diagram. The model also included potential confounder variables associated with cesarean section in the BABYDIAB study (Table 1). The P values are obtained from the multivariate Cox proportional hazards model.
To search for potential interaction between cesarean section and susceptibility genes in influencing type 1 diabetes risk, we first added the interaction item cesarean section×genotype for each of the genes in the Cox proportional hazards model. HRs for the interaction item were suggestive of interaction for IFIH1 (adjusted HR 2.3; P = 0.18) and for CD25 (0.2; P = 0.04), but not for HLA (0.96; P = 0.9), prompting further analysis. We then examined type 1 diabetes risk associated with cesarean section conditioned by the genotypes of each of these three gene regions (Fig. 4). Adjusted HRs for cesarean section were similar for both HLA risk and nonrisk genotypes, again suggesting that there was no interaction between cesarean section and HLA genotype. In contrast, there were pronounced differences in adjusted HRs of cesarean section between children with risk and nonrisk genotypes at the IFIH1 and CD25 genes, again suggesting interaction.
Finally, we performed Kaplan-Meier analyses after stratification for IFIH1 and CD25 genotypes. For the IFIH1 genotypes examined, the increased risk associated with cesarean section was only evident in children who had both the diabetes-susceptible IFIH1 GG genotype and cesarean section. The probability of type 1 diabetes by age 12 years in children with the IFIH1 GG genotype was 9.1% (95% CI 4.9–13.3) in individuals delivered by cesarean section versus 2.8% (0.9–4.7) in individuals delivered vaginally (Pcorrected = 0.014). In comparison, the probability for developing type 1 diabetes in children with the GA or AA IFIH1 genotypes was 2.8% (0.8–4.8) if delivered by cesarean section and 1.7% (0.5–2.9; Pcorrected = 0.9) if delivered vaginally. For CD25, cesarean section was associated with increased risk for type 1 diabetes, regardless of CD25 genotype. However, there was a markedly reduced 12-year risk for type 1 diabetes in children who had nonsusceptible genotypes and vaginal delivery (0.6%; 0.1–1.4) compared with all other groups: nonsusceptible genotypes and cesarean section, 5.7% (2.5–8.9; Pcorrected = 0.0005); susceptible genotypes and vaginal delivery, 3.2% (1.6–4.8; Pcorrected = 0.04); and susceptible genotypes and cesarean section, 5.9% (3.1–8.7%; Pcorrected = 0.0007).
DISCUSSION
Delivery by cesarean section has become more prevalent over recent decades. In this prospective study of children who are genetically at risk for type 1 diabetes and who have been followed from birth, we found that delivery by cesarean section is associated with a more than twofold increase in the risk of developing type 1 diabetes. Notably, this increase was not accompanied by higher numbers of children developing islet autoimmunity, but by a faster progression from the onset of islet autoimmunity to overt diabetes. Moreover, the data were indicative of an interaction between cesarean section and the type 1 diabetes susceptible immune response genes IFIH1 and CD25, suggesting that cesarean section could affect the risk for type 1 diabetes by modifying the host’s environment and immune response.
This is the first prospective longitudinal study of children followed from birth in which cesarean section was examined as a risk factor. The prospective nature of this study, which includes the assessment of islet autoantibodies and onset of overt diabetes, allowed us to distinguish between the risk for autoimmunity and the risk for progression to diabetes. This design also offers some insight into the potential mechanisms underlying these associations. Potential limitations of the study are that it is not population-based and that it is limited to children who have a parent, most commonly mothers, with type 1 diabetes. Nevertheless, the association between cesarean section and type 1 diabetes was strong in children of nondiabetic mothers, suggesting that the findings may be representative of the broader population. It should also be considered that the associations observed here could be secondary to confounding factors, particularly those that are associated with high rates of cesarean section. However, none of the factors associated with an increased prevalence of cesarean section in the BABYDIAB cohort were associated with an increased risk for type 1 diabetes. Furthermore, cesarean section remained an important risk factor after adjustment for these factors. Additional potential confounding factors are social status and maternal care, which could not be formally analyzed in the BABYDIAB cohort. Smoking during pregnancy is associated with lower social economic status, but was not significantly associated with the risk for type 1 diabetes in the BABYDIAB cohort (data not shown).
Previous studies have suggested that cesarean section is a risk factor for type 1 diabetes (18–20). Thus, our findings in a prospective cohort are consistent with these reports. Our findings that cesarean section is more prevalent in women with type 1 diabetes, in premature births, and in multiparous births are also consistent with previous reports (21). Inconsistent with previous reports is the magnitude of the increased risk for type 1 diabetes associated with cesarean section in our cohort. Our study finds that cesarean section more than doubles risk, whereas the meta-analysis of previous case-control studies estimated a 20–30% increase in risk (4). Our study differs from previous analysis in that it is performed in children with an a priori type 1 diabetes family history. It is possible that the influence of cesarean section on type 1 diabetes risk is enhanced in cohorts rich in type 1 diabetes susceptibility genotypes, such as in the BABYDIAB cohort. The relationships seen here with some of the genetic susceptibility genotypes would support this.
The observation is intriguing that cesarean section increases risk for type 1 diabetes and that the prevalence of cesarean section in children born to mothers with type 1 diabetes is high. This result appears at odds with the fact that children born to mothers with type 1 diabetes have a lower risk for diabetes than children born to fathers with type 1 diabetes and nondiabetic mothers (22). However, it is important to note that within the BABYDIAB cohort, the reduced risk for type 1 diabetes seen in children of mothers with type 1 diabetes is associated with protection against the development of islet autoimmunity (23), whereas cesarean section only affected the rate of progression from islet autoimmunity to overt diabetes. Thus, it appears that the type 1 diabetes protection associated with maternal diabetes is strong and potentially hierarchical over increased risk provided by cesarean section. This is consistent with the fact that autoimmunity is almost always observed before the development of type 1 diabetes, implying that it is an important prerequisite for disease.
In terms of how cesarean section increases the risk for type 1 diabetes, our findings suggest that the overall risk for autoimmunity, including autoimmunity seen in thyroid and celiac disease, was not affected and suggest that the period after the initiation of autoimmunity is influenced in some way. To consolidate this notion, we examined relationships between cesarean section and genetic susceptibility. We reasoned that factors influencing progression to diabetes should have little or no interaction with genes that mainly affected the risk for developing islet autoantibodies, but that there could be interaction with genes that also affected progression. HLA strongly affects the risk for autoantibodies and only minimally, if at all, progression to diabetes (17,24,25). Notably, and consistent with our reasoning, cesarean section provided a similar increase in the risk for type 1 diabetes in children with and children without high-risk HLA genotypes. In contrast to HLA, the IFIH1 gene influences progression from autoimmunity to type 1 diabetes in our cohort (9). Unlike what we observed for the HLA genotype, stratification for the IFIH1 genotype suggested interaction between cesarean section and the IFIH1 gene. Cesarean section had an HR for type 1 diabetes in children with a susceptible IFIH1 genotype that was twice that observed in children with nonsusceptible IFIH1 genotypes. Moreover, the probability of developing type 1 diabetes was increased only when children were delivered by cesarean section and had the high-risk IFIH1 genotype.
Relevant to our findings, both the IFIH1 gene and cesarean section affect innate and adaptive immunity. IFIH1 triggers the secretion of immune mediators, such as interferons, in response to viral infection (26). These mediators inhibit viral replication and enhance the expression of surface major histocompatibility complex-1 molecules, which are relevant to the destruction of β-cells by CD8+ T lymphocytes (27). Cesarean section is associated with stronger nonspecific humoral immune responses and with a marked variation in the microbiome of children, with fewer bifidobacteria at an early age (5). The gut microbiome has been suggested to be skewed in a similar manner in children who progress to diabetes (28). Consistent with this, the hygiene hypothesis suggests that children with reduced or delayed exposure to infection may have an increased risk for type 1 diabetes (29). Of interest, a second immune response gene associated with type 1 diabetes, CD25, also showed evidence of interaction with cesarean section in modifying the risk for type 1 diabetes in our cohort, whereas the PTPN22 gene showed little effect on type 1 diabetes risk in our cohort. Whereas we are eager to suggest mechanisms involving the immune response effects of cesarean section, it is also possible that cesarean section modifies parental behavior and that this is the primary reason for the observed associations with type 1 diabetes.
In conclusion, the data reinforce the association between delivery mode and type 1 diabetes. They further suggest that cesarean section increases the risk for the progression to type 1 diabetes after the initiation of islet autoimmunity, and this increased risk appears to be influenced by environment response genes. These data support the notion that environmental factors can act on disease pathogenesis after autoimmunity has initiated, which is consistent with recent reports of an association of enterovirus infection with type 1 diabetes progression in the Diabetes Autoimmunity Study in the Young (DAISY) study (30) and with findings in animal models of autoimmune diabetes (31). Second, they expose novel possibilities to interfere with rate of progression to type 1 diabetes in children who have already mounted an immune response to pancreatic islet β-cell antigens, which include treatments that affect the host’s immune response to environmental factors.
ACKNOWLEDGMENTS
The work was supported by grants from the Kompetenznetz Diabetes Mellitus (Competence Network for Diabetes Mellitus) funded by the Federal Ministry of Education and Research (FKZ 01GI0805-07), the European Union (EP7-HEALTH-2007, DIAPREPP N202013), and the Juvenile Diabetes Research Foundation (JDRF 1-2006-665). E.B. was supported by the Deutsche Forschungsgemeinschaft (DFG) Research Center and Cluster of Excellence, Center for Regenerative Therapies Dresden (FZ 111).
No potential conflicts of interest relevant to this article were reported.
E.B. provided major input to analysis and interpretation of data and revised the article critically. K.W. and C.W. contributed to acquisition, analysis, and interpretation of data and drafted the article. M.W. contributed to acquisition of data. A.-G.Z. designed the study, is principal investigator of the BABYDIAB study, contributed to writing the article, and revised the article critically.
The authors thank Annette Knopff, Ulrike Mollenhauer, Claudia Lauber (Forschergruppe Diabetes e.V. at Helmholtz Center Munich, Neuherberg, Germany), and Marina Zwilling (Forschergruppe Diabetes, Klinikum Rechts der Isar, University of Technology, Munich) for data collection and expert technical assistance; Thomas Illig and Harald Grallert (Institute of Epidemiology, Helmholtz Center Munich, Neuherberg, Germany) for IFIH1 SNP genotyping; and Kerstin Adler (Forschergruppe Diabetes, Klinikum Rechts der Isar, University of Technology, Munich) for laboratory management. The authors also thank the pediatricians and family doctors in Germany for participating in the BABYDIAB study.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/10.2337/db11-0729/-/DC1.
REFERENCES
Articles from Diabetes are provided here courtesy of American Diabetes Association
Full text links
Read article at publisher's site: https://doi.org/10.2337/db11-0729
Read article for free, from open access legal sources, via Unpaywall:
https://diabetes.diabetesjournals.org/content/diabetes/60/12/3300.full.pdf
Citations & impact
Impact metrics
Article citations
Caesarean section and risk of type 1 diabetes.
Diabetologia, 67(8):1582-1587, 31 May 2024
Cited by: 0 articles | PMID: 38819466 | PMCID: PMC11343945
Probiotics in Allergy and Immunological Diseases: A Comprehensive Review.
Cureus, 16(3):e55817, 08 Mar 2024
Cited by: 1 article | PMID: 38590477 | PMCID: PMC10999892
Review Free full text in Europe PMC
Pathogenesis of autoimmune disease.
Nat Rev Nephrol, 19(8):509-524, 10 May 2023
Cited by: 85 articles | PMID: 37165096 | PMCID: PMC10171171
Review Free full text in Europe PMC
Mechanistic Insights into Immune-Microbiota Interactions and Preventive Role of Probiotics Against Autoimmune Diabetes Mellitus.
Probiotics Antimicrob Proteins, 15(4):983-1000, 12 May 2023
Cited by: 1 article | PMID: 37171690
Review
Can type 1 diabetes be an unexpected complication of obesity?
Front Endocrinol (Lausanne), 14:1121303, 31 Mar 2023
Cited by: 5 articles | PMID: 37065759 | PMCID: PMC10102381
Review Free full text in Europe PMC
Go to all (56) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
SNPs (3)
- (1 citation) dbSNP - rs11594656
- (1 citation) dbSNP - rs6679677
- (1 citation) dbSNP - rs2111485
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Characteristics of rapid vs slow progression to type 1 diabetes in multiple islet autoantibody-positive children.
Diabetologia, 56(7):1615-1622, 29 Mar 2013
Cited by: 43 articles | PMID: 23539116
A strategy to find gene combinations that identify children who progress rapidly to type 1 diabetes after islet autoantibody seroconversion.
Acta Diabetol, 51(3):403-411, 19 Nov 2013
Cited by: 18 articles | PMID: 24249616
An interferon-induced helicase (IFIH1) gene polymorphism associates with different rates of progression from autoimmunity to type 1 diabetes.
Diabetes, 60(2):685-690, 01 Feb 2011
Cited by: 46 articles | PMID: 21270278 | PMCID: PMC3028371
Genetic determinants of diabetes are similarly associated with other immune-mediated diseases.
Curr Opin Allergy Clin Immunol, 7(6):468-474, 01 Dec 2007
Cited by: 11 articles | PMID: 17989522
Review





