Abstract
Background
BRCA1 and BRCA2 germline mutations are associated with an elevated risk for pancreas adenocarcinoma (PAC). Other BRCA-associated cancers have been shown to have greater sensitivity to platinum and poly(ADP-ribose) polymerase (PARP) inhibitors with better clinical outcomes than in sporadic cases; however, outcomes in BRCA-associated PAC have not been reported.Methods
Patients with a known BRCA1 or BRCA2 mutation and a diagnosis of PAC were identified from the Gastrointestinal Oncology Service, Familial Pancreas Cancer Registry, and Clinical Genetics Service at Memorial Sloan-Kettering Cancer Center.Results
Fifteen patients, five male, with a BRCA1 (n = 4) or BRCA2 (n = 11) mutation and PAC and one patient with a BRCA1 mutation and acinar cell carcinoma of the pancreas were identified. Seven female patients (70%) had a prior history of breast cancer. Four patients received a PARP inhibitor alone or in combination with chemotherapy; three demonstrated an initial radiographic partial response by Response Evaluation Criteria in Solid Tumors whereas one patient had stable disease for 6 months. Six patients received platinum-based chemotherapy first line for metastatic disease; five of those patients had a radiographic partial response.Conclusion
BRCA mutation-associated PAC represents an underidentified, but clinically important, subgroup of patients. This is of particular relevance given the ongoing development of therapeutic agents targeting DNA repair, which may potentially offer a significant benefit to a genetically selected population. We anticipate that further study and understanding of the clinical and biologic features of BRCA-mutant PAC will aid in the identification of tissue biomarkers indicating defective tumor DNA repair pathways in sporadic PAC.Free full text

An Emerging Entity: Pancreatic Adenocarcinoma Associated with a Known BRCA Mutation: Clinical Descriptors, Treatment Implications, and Future Directions
Learning Objectives
After completing this course, the reader will be able to:
Describe the genetic syndromes associated with pancreas adenocarcinoma.
Explain the potential role of platinum-based therapy and PARP inhibitors in BRCA-mutated pancreas adenocarcinoma.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
BRCA1 and BRCA2 germline mutations are associated with an elevated risk for pancreas adenocarcinoma (PAC). Other BRCA-associated cancers have been shown to have greater sensitivity to platinum and poly(ADP-ribose) polymerase (PARP) inhibitors with better clinical outcomes than in sporadic cases; however, outcomes in BRCA-associated PAC have not been reported.
Methods.
Patients with a known BRCA1 or BRCA2 mutation and a diagnosis of PAC were identified from the Gastrointestinal Oncology Service, Familial Pancreas Cancer Registry, and Clinical Genetics Service at Memorial Sloan-Kettering Cancer Center.
Results.
Fifteen patients, five male, with a BRCA1 (n = 4) or BRCA2 (n = 11) mutation and PAC and one patient with a BRCA1 mutation and acinar cell carcinoma of the pancreas were identified. Seven female patients (70%) had a prior history of breast cancer. Four patients received a PARP inhibitor alone or in combination with chemotherapy; three demonstrated an initial radiographic partial response by Response Evaluation Criteria in Solid Tumors whereas one patient had stable disease for 6 months. Six patients received platinum-based chemotherapy first line for metastatic disease; five of those patients had a radiographic partial response.
Conclusion.
BRCA mutation–associated PAC represents an underidentified, but clinically important, subgroup of patients. This is of particular relevance given the ongoing development of therapeutic agents targeting DNA repair, which may potentially offer a significant benefit to a genetically selected population. We anticipate that further study and understanding of the clinical and biologic features of BRCA-mutant PAC will aid in the identification of tissue biomarkers indicating defective tumor DNA repair pathways in sporadic PAC.
Introduction
Despite advances in the development of cytotoxic and targeted therapies, pancreatic adenocarcinoma (PAC) remains a significant cause of cancer mortality worldwide [1]. Although the majority of cases of PAC are sporadic, up to one in 10 cases occurs in the setting of a hereditary cancer predisposition syndrome, the most common of which is a germline BRCA1 or BRCA2 mutation [2]. The lifetime risk for PAC in a BRCA2 mutation carrier is estimated at 3.5–10 times that of the general population [2, 3]. Recently, BRCA1 was also implicated as carrying an elevated risk for the development of PAC, although this risk appears to be lower than that seen in BRCA2 mutation carriers; the frequency of BRCA1 mutations compared with BRCA2 mutations in familial pancreatic cancer kindreds is also less [4]. There is currently no consensus regarding standard indications for BRCA1 and BRCA2 testing in patients with PAC.
Study of the clinical and pathologic features of sporadic serous ovarian and triple-negative breast cancers has shown significant overlap with that of BRCA1 mutation–associated cases with the respective malignancy. Moreover, the presence of a germline BRCA1 or BRCA2 mutation has been identified as an independent predictive factor for overall survival in unselected advanced ovarian cancer patients [5]. In contrast, the literature to date regarding the clinical features of BRCA mutation–associated PAC is limited. The largest series to date of eight patients with a BRCA1 or BRCA2 mutation and PAC was reported from Memorial Sloan-Kettering Cancer Center (MSKCC) in 2009 and was limited to patients of Ashkenazi Jewish heritage with resected PAC; data on response to systemic therapy and outcomes were not reported [6]. Defining and targeting tumors deficient in DNA repair have become increasingly important over recent years because of the ongoing development of novel therapeutic strategies with the ability to exploit tumor DNA repair abnormalities [7]. BRCA mutation–associated PAC represents a small, but clinically significant, group of patients who may potentially benefit from therapies synthetically lethal with a deficiency in homologous recombination; it is also possible that clinical outcomes may differ in this genetically selected population from outcomes in sporadic PAC.
To further explore these concepts, we identified 15 patients with a known BRCA1 or BRCA2 mutation and PAC seen at our institution over a 7-year period, representing the largest reported series of BRCA mutation–associated PAC to date.
Patients and Methods
Patients with a known BRCA1 or BRCA2 mutation and PAC were identified from the prospectively collected MSKCC Pancreatic Cancer Family Registry and retrospectively from electronic records of the Clinical Genetics Service. Prior approval for the project was obtained from the institutional review/privacy board. None of the patients identified herein were included in the prior series of eight BRCA mutation–associated PAC cases published from our institution [6]. Clinical and pathological data were collected from chart review, including age at diagnosis, sex, epidemiologic information (where available), personal and family history of malignancy, presenting symptoms, tumor site, pathology/cytology results, date and type of operation, systemic therapy and/or radiation therapy, and status at last follow-up. Records were reviewed to determine treatment regimens and radiology results were reviewed to determine response to therapy. Overall survival was calculated from the date of cytological or pathological confirmation of diagnosis to the date of death or last follow-up, and was calculated using the Kaplan–Meier method.
Results
Fifteen patients with a BRCA germline mutation and PAC were identified. Four patients (27%) had a BRCA1 mutation identified and 11 (73%) had a BRCA2 mutation. Eight (53%) patients had Jewish heritage, nine (60%) had a prior history of malignancy, and 12 (80%) had a first-degree relative with breast, ovarian, prostate, or pancreatic cancer. Figure 1 shows a family pedigree from a 36-year-old Asian male BRCA1 mutation carrier with PAC. Seven female patients (70%) had a prior history of breast cancer; other prior malignant diagnoses included squamous cell carcinoma of the tonsil, papillary thyroid carcinoma, and melanoma. The clinicopathologic features of patients with a BRCA mutation and PAC are summarized in Table 1. One additional patient with an acinar cell pancreas malignancy and a BRCA1 mutation was also identified.
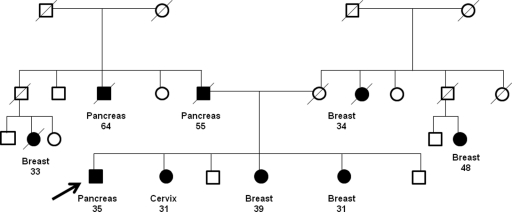
Family pedigree of a patient with a BRCA1 mutation and pancreatic adenocarcinoma.
Note: Pedigree was altered to protect the confidentiality and privacy of family members without compromising relevant content.
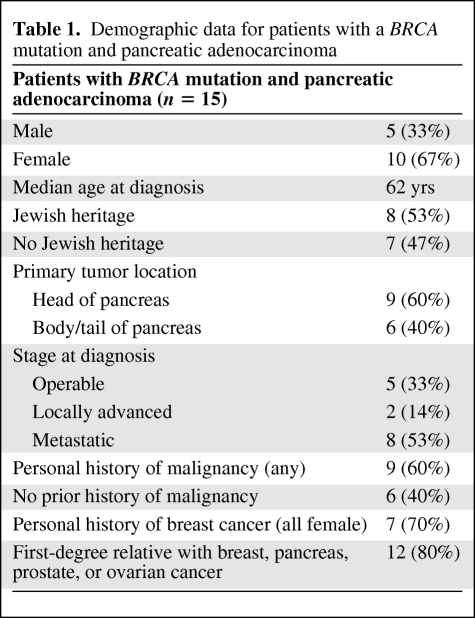
Table 1.
Demographic data for patients with a BRCA mutation and pancreatic adenocarcinoma
Five patients (33%) had localized, operable disease at presentation; the remainder had locally advanced (n = 2, 14%) or metastatic (n = 8, 53%) disease. All patients had recurred at the time of last follow-up; nine (60%) were alive with disease and six (40%) had died as a result of progressive PAC. The median overall survival time for all 15 patients was 27.6 months. Three patients received a poly(ADP-ribose) polymerase (PARP) inhibitor in combination with chemotherapy; two demonstrated an initial radiographic partial response by the Response Evaluation Criteria in Solid Tumors (RECIST) with subsequent progression of disease at 5 months and 6 months, whereas the third patient had stable disease as the best response. Figure 2 demonstrates a radiographic partial response to gemcitabine in combination with a PARP inhibitor. One patient received a PARP inhibitor as single-agent therapy for metastatic disease and demonstrated a partial radiographic response to therapy. Six patients received platinum-based chemotherapy as first-line therapy for metastatic disease; five of those patients had a radiographic partial response by the RECIST, including one patient with a complete response to infusional 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan. Treatment history, including the radiographic best response, and survival outcomes are summarized in Table 2.
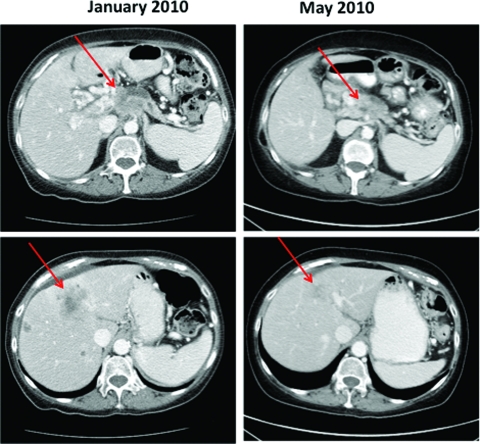
Patient with stage IV pancreas adenocarcinoma and a BRCA1 mutation: Response to gemcitabine plus a poly(ADP-ribose) polymerase inhibitor.
Table 2.
BRCA mutation carriers with pancreas adenocarcinoma
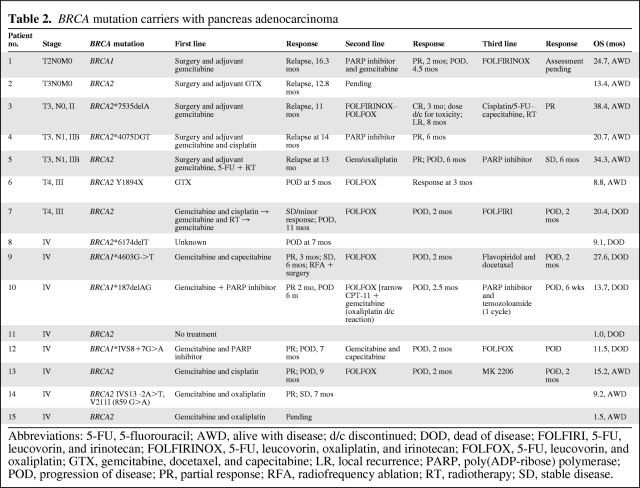
Abbreviations: 5-FU, 5-fluorouracil; AWD, alive with disease; d/c discontinued; DOD, dead of disease; FOLFIRI, 5-FU, leucovorin, and irinotecan; FOLFIRINOX, 5-FU, leucovorin, oxaliplatin, and irinotecan; FOLFOX, 5-FU, leucovorin, and oxaliplatin; GTX, gemcitabine, docetaxel, and capecitabine; LR, local recurrence; PARP, poly(ADP-ribose) polymerase; POD, progression of disease; PR, partial response; RFA, radiofrequency ablation; RT, radiotherapy; SD, stable disease.
Discussion
Germline mutations in BRCA1 and BRCA2 are estimated to occur in up to 7% of patients with PAC, whereas in patients with familial PAC, the frequency of BRCA1 or BRCA2 mutation carriers is estimated at 11%–17% [2]. All but one of the 15 patients we identified as carrying a BRCA1 or BRCA2 germline mutation had either a personal history of a prior malignancy or a documented case of BRCA-associated malignancy in a first-degree relative, which may reflect the biased sample assessed in that such observations prompted referral for genetic testing. However, it is worth noting that seven of the 10 female carriers identified had a prior history of breast cancer. The median age at diagnosis of PAC was 62 years, 10 years below the average age at diagnosis in an unselected population reported from the Surveillance, Epidemiology, and End Results (SEER) data.
One patient with acinar cell carcinoma of the pancreas, a rare histological subtype of pancreatic malignancy, and a BRCA1 mutation was identified; an association between these two conditions has not been described previously. That patient had a complex past medical history with prior diagnoses including acromegaly, colonic adenocarcinoma, and papillary renal cell carcinoma and reported a significant family history of early-onset breast cancer in multiple first- and second-degree relatives. Colon cancer, thyroid cancer, and renal cell cancer have not been demonstrated to occur at significantly higher rates in BRCA1 or BRCA2 mutation carriers [8].
Over 6,000 patients were evaluated at MSKCC with a diagnosis of PAC in 2000–2010; the identification of 15 BRCA1 or BRCA2 mutation carriers and PAC in 2003–2010 is likely to be a substantial underestimation of the true frequency of mutation carriers in this population because the vast majority of patients do not undergo genetic testing. In a prior study at our institution of 38 breast cancer survivors who subsequently developed PAC, just two of the 38 women in that group had undergone BRCA1 or BRCA2 testing [9]. In contrast to breast and ovarian cancers, for which referral for genetic counseling and testing is routine, the majority of patients with PAC and a personal or family history suggestive of BRCA mutation do not undergo genetic testing. Several barriers to genetic testing in this population exist, including a lack of awareness of the association between BRCA mutation and PAC among treating professionals and patients, difficulty in obtaining reimbursement for the cost of genetic testing, and rapid disease progression precluding attendance for genetic counseling and testing. With greater recognition, better access, and a more rapid turnaround time for BRCA genetic testing, we anticipate that in the near future at least some of these current barriers to genetic counseling and testing for PAC patients can be overcome.
In this series of BRCA mutation–associated PAC patients, clinical and partial radiologic responses were seen in two of the three patients treated with the combination of a PARP inhibitor and gemcitabine, and one additional patient had a partial radiographic response to single-agent PARP inhibitor treatment. Five of six patients (83%) treated with platinum-based first-line chemotherapy for metastatic disease demonstrated a radiographic complete or partial response by the RECIST. This observation lends support to the hypothesis that PARP inhibitors are synthetically lethal in BRCA-deficient PAC. PARP-1 and PARP-2 are key components of the cellular DNA repair mechanism for single-strand breaks and nucleoside base damage; inhibition of PARP in tumor cells leads to transformation of background single-strand breaks into double-strand breaks (DSBs) [10]. These lesions are cytotoxic in cells without functional BRCA1 or BRCA2 because they are unable to effectively repair DSBs by homologous recombination and instead rely on the error-prone nonhomologous end-joining repair mechanism. Platinum chemotherapy drugs exert their cytotoxic effect by binding directly to DNA, causing crosslinking of DNA strands and thereby inducing DNA DSBs, which also are ineffectively repaired in cells lacking functioning BRCA1 or BRCA2. Pancreatic cancer cell lines deficient in BRCA2 or another component of the Fanconi anemia pathway have been shown to have marked sensitivity to DNA crosslinking agents [11]. Previous anecdotal reports also indicate a substantial response to alkylating agents in patients with a known BRCA mutation and PAC [12]. Notably, all patients treated with a PARP inhibitor experienced progression of disease after several months of therapy, despite an initial clinical and radiographic response. The mechanism of acquired resistance in vivo to PARP inhibition is unknown, but it may potentially occur as a result of a secondary mutation resulting in restored competency of homologous repair in BRCA-mutant cells [13].
Interpretation of survival outcomes in this series is limited by the small number of patients and varying stages of disease at presentation. The possibility of survival bias must also be considered because patients who live longer are more likely to present for genetic testing. In this cohort of 15 patients, only five of whom underwent surgical resection, the overall median survival duration for the cohort was 27.6 months. Although this appears better than the reported SEER survival data in unselected PAC cases [1], the prognostic significance of BRCA mutation remains unclear.
We believe that there is a strong therapeutic rationale for the development of PARP inhibitors for BRCA1 or BRCA2 mutation–associated PAC. In collaboration with other investigators and in partnership with the Cancer Therapeutic and Evaluation Program and the Lustgarten Foundation, we plan a randomized phase II trial evaluating the addition of PARP inhibition to platinum-based therapy in a genetically selected population of BRCA1, BRCA2, or PALB2 mutation carriers with PAC. Selected other PARP inhibitor trials in pancreas cancer are noted in Table 3.
Table 3.
Trials of PARP inhibitors including pancreas adenocarcinoma patients
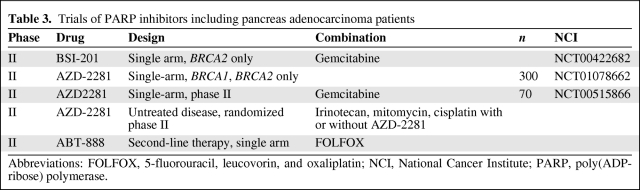
Abbreviations: FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; NCI, National Cancer Institute; PARP, poly(ADP-ribose) polymerase.
In conclusion, BRCA mutation–associated PAC represents an underidentified, but clinically important, subgroup of patients. Improved awareness of the association between BRCA mutation and PAC among patients and physicians is important to identify potential mutation carriers on the basis of a family or personal history of malignancy or a predisposing genetic background. This is of particular relevance given the ongoing development of therapeutic agents targeting DNA repair, which may potentially offer a significant benefit to a genetically selected population and provide a first “proof of principle” of a targeted therapy approach in PAC patients. We anticipate that further study and understanding of the clinical and biologic features of BRCA-mutant PAC will aid in the identification and refinement of tissue biomarkers indicating defective tumor DNA repair pathways in sporadic PAC cases.
Author Contributions
Conception/Design: Eileen M. O'Reilly, Maeve A. Lowery, David Kelsen, Kenneth Yu, Zsofia Stadler, Emmy Ludwig, Erin Salo-Mullen, Mark Robson, Peter J. Allen, Robert C. Kurtz, David R. D'Adamo
Provision of study material or patients: Eileen M. O'Reilly, Maeve A. Lowery, David Kelsen, Kenneth Yu, Zsofia Stadler, Yelena Y. Janjigian, Emmy Ludwig, Erin Salo-Mullen, Mark Robson, Peter J. Allen, Robert C. Kurtz, David R. D'Adamo
Collection and/or assembly of data: Eileen M. O'Reilly, Maeve A. Lowery
Data analysis and interpretation: Eileen M. O'Reilly, Maeve A. Lowery
Manuscript writing: Maeve A. Lowery
Final approval of manuscript: Eileen M. O'Reilly, David Kelsen, Kenneth Yu, Zsofia Stadler, Yelena Y. Janjigian, Emmy Ludwig, Erin Salo-Mullen, Mark Robson, Peter J. Allen, Robert C. Kurtz, David R. D'Adamo
References
Articles from The Oncologist are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1634/theoncologist.2011-0185
Read article for free, from open access legal sources, via Unpaywall:
http://theoncologist.alphamedpress.org/content/16/10/1397.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/170532277
Article citations
Clinical utility of BRCA and ATM mutation status in circulating tumour DNA for treatment selection in advanced pancreatic cancer.
Br J Cancer, 131(7):1237-1245, 28 Aug 2024
Cited by: 0 articles | PMID: 39198618
The role of germline BRCA1 & BRCA2 mutations in familial pancreatic cancer: A systematic review and meta-analysis.
PLoS One, 19(5):e0299276, 29 May 2024
Cited by: 0 articles | PMID: 38809921 | PMCID: PMC11135687
Review Free full text in Europe PMC
Understanding the Genetic Landscape of Pancreatic Ductal Adenocarcinoma to Support Personalized Medicine: A Systematic Review.
Cancers (Basel), 16(1):56, 21 Dec 2023
Cited by: 2 articles | PMID: 38201484 | PMCID: PMC10778202
Review Free full text in Europe PMC
Targeting BRCA and PALB2 in Pancreatic Cancer.
Curr Treat Options Oncol, 25(3):346-363, 04 Jan 2024
Cited by: 0 articles | PMID: 38311708
Review
Therapeutic developments in pancreatic cancer.
Nat Rev Gastroenterol Hepatol, 21(1):7-24, 05 Oct 2023
Cited by: 28 articles | PMID: 37798442
Review
Go to all (141) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Identification of germline genetic mutations in patients with pancreatic cancer.
Cancer, 121(24):4382-4388, 06 Oct 2015
Cited by: 109 articles | PMID: 26440929 | PMCID: PMC5193099
BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma.
J Clin Oncol, 27(3):433-438, 08 Dec 2008
Cited by: 125 articles | PMID: 19064968 | PMCID: PMC3657622
Clinical outcomes in pancreatic adenocarcinoma associated with BRCA-2 mutation.
Anticancer Drugs, 26(2):224-226, 01 Feb 2015
Cited by: 21 articles | PMID: 25304989
Emerging strategies in BRCA-positive pancreatic cancer.
J Cancer Res Clin Oncol, 144(8):1503-1507, 18 May 2018
Cited by: 24 articles | PMID: 29777302 | PMCID: PMC6061050
Review Free full text in Europe PMC
 a
a



