Abstract
Objective
Corticotropin-releasing factor (CRF) and somatostatin both play important roles in mediating responses to acute and chronic stress. The purpose of this study was to measure CSF concentrations of CRF and somatostatin in patients with chronic combat-related post-traumatic stress disorder (PTSD) and comparison subjects.Method
Lumbar punctures for collection of CSF were performed in Vietnam combat veterans with PTSD (N = 11) and comparison subjects (N = 17). CSF concentrations of CRF and somatostatin were compared between the two groups.Results
CSF concentrations of CRF were higher in the PTSD patients than in the comparison subjects (mean = 29.0 pg/ml, SD = 7.8, versus mean = 21.9 pg/ml, SD = 6.0). This group difference remained significant after covariance for age. CSF somatostatin concentrations in PTSD patients were higher than those of the comparison subjects (mean = 19.9 pg/ml, SD = 5.4, versus mean = 13.7 pg/ml, SD = 8.0). However, covarying for age reduced the level of significance.Conclusions
Higher CSF CRF concentrations in patients with PTSD may reflect alterations in stress-related neurotransmitter systems. The higher CSF CRF concentrations may play a role in disturbances of arousal in patients with PTSD.Free full text

Elevated CSF Corticotropin-Releasing Factor Concentrations in Posttraumatic Stress Disorder
Abstract
Objective
Corticotropin-releasing factor (CRF) and somatostatin both play important roles in mediating responses to acute and chronic stress. The purpose of this study was to measure CSF concentrations of CRF and somatostatin in patients with chronic combat-related post-traumatic stress disorder (PTSD) and comparison subjects.
Method
Lumbar punctures for collection of CSF were performed in Vietnam combat veterans with PTSD (N=11) and comparison subjects (N=17). CSF concentrations of CRF and somatostatin were compared between the two groups.
Results
CSF concentrations of CRF were higher in the PTSD patients than in the comparison subjects (mean=29.0 pg/ml, SD=7.8, versus mean=21.9 pg/ml, SD=6.0). This group difference remained significant after covariance for age. CSF somatostatin concentrations in PTSD patients were higher than those of the comparison subjects (mean=19.9 pg/ml, SD=5.4, versus mean=13.7 pg/ml, SD=8.0). However, covarying for age reduced the level of significance.
Conclusions
Higher CSF CRF concentrations in patients with PTSD may reflect alterations in stress-related neurotransmitter systems. The higher CSF CRF concentrations may play a role in disturbances of arousal in patients with PTSD.
Corticotropin-releasing factor (CRF) plays an important role in mediating the mammalian stress response (1). CRF released during stress (2) from nerve terminals originating in the paraventricular nucleus of the hypothalamus increases the secretion of adrenocorticotropin hormone (ACTH) from the anterior pituitary, which in turn stimulates release of cortisol from the adrenal (3). Cortisol has a number of effects that are beneficial to short-term survival, including suppression of reproductive and immune function, promotion of analgesia, activation of the peripheral autonomic system, suppression of gastric motility and gastric acid secretion, and increases in total oxygen consumption and glucose and glucagon concentrations in plasma (3). Chronic stress results in sustained increases in plasma glucocorticoid levels (4), with a potentiation of glucocorticoid responsiveness to subsequent stressors (5, 6) and neuronal “hypersecretion” of CRF.
CRF neurons are distributed in a variety of locations outside of the hypothalamus, including the amygdala, bed nucleus of the stria terminalis, central gray area, locus ceruleus, parabrachial area, and dorsal vagus complex (7). Intraventricular administration of CRF to laboratory animals results in behavioral activation, as evidenced by 1) an increase in EEG activity (8); 2) increases in plasma norepinephrine and epinephrine concentrations (9, 10); 3) an increase in firing of the locus ceruleus, site of the majority of noradrenergic cell bodies in the brain, which results in a state of increased attention and arousal (11); 4) increased locomotor activity, grooming, and rearing (12); and 5) behaviors consistent with anxiety and fear, including a decrease in exploratory activity in an open field (13), decreased punished responding in the conflict test (14), and potentiation of acoustic startle (15, 16). These studies support an important role for CRF in the promotion of anxiety and fear-related behaviors.
Findings from clinical studies are consistent with a relationship among CRF, stress, and depression (which has been conceptualized as a chronic stress disorder). Studies in patients with depression consistently reveal an increase in CSF concentrations of CRF and a blunted ACTH response to intravenously administered CRF (17–19). Patients with posttraumatic stress disorder (PTSD) have been found to exhibit alterations in hypothalamic-pituitary-adrenal (HPA) axis function (20, 21), although no studies have directly measured CRF in the CSF in patients with PTSD. Somatostatin is a tetradecapeptide with widespread localization in the CNS that also appears to play a role in the stress response (22–24). Somatostatin and CRF levels tend to covary with one another, so it would be predicted that increased levels of CRF would be associated with increased levels of somatostatin. CSF somatostatin concentrations in patients with affective disorders, however, have been found to be either lower than (25–27) or the same as (28) those of comparison subjects (although elevated levels of somatostatin are seen in mania [29]). The purpose of the present study was to measure CSF CRF and somatostatin concentrations in Vietnam combat veterans with PTSD and matched comparison subjects. We hypothesized that PTSD would be associated with elevated CSF levels of CRF and somatostatin.
METHOD
Patients were Vietnam veterans with combat-related PTSD (N=11) who gave written informed consent for participation in the study and who were admitted to the inpatient unit of the National Center for Posttraumatic Stress Disorder over a 3-year period from 1992 to 1995. Patients were admitted to the unit on an elective basis and received appointment dates for their admission. Only patients who were clinically stable and were not in crisis were admitted to this specialized program. Patients with a history of combat exposure in Vietnam were included if they fulfilled criteria for PTSD on the basis of the Structured Clinical Interview for DSM-III-R (SCID) (30) and the diagnosis (based on DSM-III-R criteria) of a research psychiatrist. Patients were excluded if they had a history of current alcohol or substance abuse or dependence, or schizophrenia, as determined by the SCID; serious medical disorder, as determined by laboratory tests and physical examination; organic mental disorder; neurological disorder; or head trauma. All patients were free of medication, alcohol, and drugs for 4 weeks or more before the study, as determined by frequent (a minimum of twice-a-week), random tests with a device to determine the alcohol content of a breath sample and urine toxicology tests that were performed while the patients were on the inpatient unit.
Patients were evaluated with the SCID for comorbid psychiatric diagnoses. Nine (82%) of 11 patients fulfilled criteria for a lifetime history of major depression, and five (45%) for current major depression. Two patients (18%) fulfilled criteria for lifetime history of dysthymia, and two (18%) for current dysthymia. Two patients (18%) fulfilled criteria for lifetime history of bipolar disorder, and one (9%) for current bipolar disorder. One patient (9%) fulfilled criteria for lifetime history of hypomania, and none for current hypomania. Two (18%) of 11 patients fulfilled criteria for lifetime history of and current panic disorder with agoraphobia, three (27%) for lifetime history of panic disorder without agoraphobia, and two (18%) for current panic disorder without agoraphobia. Two (18%) of 11 fulfilled criteria for lifetime history of and current agoraphobia without panic disorder, five (45%) for lifetime history of social phobia, four (36%) for current social phobia, three (27%) for lifetime history of and current obsessive-compulsive disorder, two (18%) for lifetime history of and current simple phobia, and two (18%) for lifetime history of and current generalized anxiety disorder. No patients fulfilled criteria for a lifetime history of or current schizophrenia, while one patient (9%) fulfilled criteria for a lifetime history of and current psychotic disorder not otherwise specified. No patients fulfilled criteria for a lifetime history of or current anorexia nervosa, bulimia, somatization disorder, somatic pain disorder, undifferentiated somatization disorder, or hypochondriasis.
There was a high rate of comorbid lifetime diagnoses of alcohol and substance abuse disorders in the group of patients with PTSD. All patients fulfilled criteria for a lifetime history of alcohol dependence, four (36%) for sedative/hypnotic/anxiolytic dependence and none for abuse, five (45%) for cannabis dependence and none for cannabis abuse, four (36%) for stimulant dependence and none for abuse, two (18%) for opiate dependence and none for abuse, four (36%) for cocaine dependence and one (9%) for cocaine abuse, two (18%) for hallucinogen/phencyclidine dependence and none for abuse, and none for polydrug dependence or abuse.
Comparison subjects were recruited by newspaper advertisement from the local area. Comparison subjects included were physically healthy and did not have a history of a psychiatric disorder, as determined by a psychiatric interview that employed DSM-III-R criteria. They were between 18 and 65 years of age and provided written informed consent for participation. Comparison subjects were excluded if they had a history of current alcohol or substance abuse, as determined by psychiatric interview and urine toxicology; serious medical disorder, as determined by laboratory tests and physical examination; organic mental disorder; neurological disorder; a history of exposure to psychological trauma, as determined by an interview with a psychiatrist; or a history of head trauma. There was a trend toward patients with PTSD being older than the comparison subjects (mean=45.9 years, SD=1.3, versus mean=40.3, SD=11.9), although this did not reach statistical significance (t=1.9, df=26, p<0.10). Some of the comparison subjects were included in a previous study (31).
Several clinical assessments were administered to the PTSD patients in this study. These included the Mississippi Scale for Combat-Related Posttraumatic Stress Disorder, a self-report measure of current PTSD symptom severity (32), and the Combat Exposure Scale, a self-report instrument for the measurement of level of exposure to combat (33).
Subjects were fasting and had refrained from consumption of caffeine after midnight the night before the procedure. All PTSD patients had been inpatients for 4 or more weeks before the study and slept the previous night in the hospital where the procedure was performed. Comparison subjects either stayed the night before in the hospital (N=4) or arrived the morning of the procedure (N=13). There was no difference between those who stayed in the hospital and those who did not for both CRF measurements (24.5 pg/ml versus 21.2) and somatostatin measurements (16.9 pg/ml versus 12.8). CSF samples of CRF and somatostatin were obtained by lumbar puncture between 8:00 a.m. and 9:00 a.m. on the day of the study. We decided to have most of the comparison subjects arrive the day of the study because this approximated their normal routine. Lumbar punctures were performed under sterile conditions and with local anesthesia, with patients in the lateral decubitus position. There were no complications from the procedure in any subjects. CSF samples were frozen immediately and stored at −68°C, coded, and assayed by a member of the research team (M.J.O.) who was blind to diagnosis and subject identity.
CSF concentrations of CRF were measured in duplicate by modification of a previously described specific radioimmunoassay for CRF (19), by using an antiserum (oC33) raised in rabbits against ovine CRF. Briefly, duplicate 450-μl aliquots of CSF were lyophilized and reconstituted in 200 μl of assay buffer and incubated at 4°C for 18 hours with 100 μl of antiserum at a final dilution of 1:21,875 in assay buffer containing 1% normal rabbit serum. Radiolabeled [125I]-Tyr0-rat/human CRF was added (20,000 counts/minute in 50-μl buffer) after 18 hours to each tube. After incubation for 24 hours at 4°C, 10 μl of goat-antirabbit serum was added to precipitate bound CRF. The standard curve containing 450 μl of artificial CSF and 0.625 pg/tube to 640 pg/tube was prepared by using rat/human CRF and was treated identically as the human CSF samples. The sensitivity of the assay was 1.25 pg/tube with 50% displacement of radiolabeled CRF (IC50) at 30 pg/tube. The inter- and intra-assay coefficients of variation have been measured every 6 months for the past 11 years with a set of identical samples (pooled human CSF) run in two separate assays. The difference between these values has ranged over this period of time between 10% and 13% for interassay and between 2% and 6% for intra-assay measurements.
CSF concentrations of somatostatin were measured in duplicate by modification of a previously described specific radioimmunoassay for somatostatin (34, 35), by using an antiserum (number 486) raised in sheep. Briefly, duplicate 250-μl aliquots of CSF were lyophilized and reconstituted in 200 μl of assay buffer and incubated at 4°C for 24 hours with 100 μl of antiserum at a final dilution of 1:35,000 in assay buffer containing 1% normal sheep serum. Radiolabeled [125I]-Tyr0-somatostatin was added (20,000 counts/minute in 50-μl buffer) after 24 hours to each tube. After incubation for 24 hours at 4°C, 5 μl of rabbit-antisheep serum was added to precipitate bound somatostatin. The standard curve containing 250 μl of artificial CSF and 0.625 pg/tube to 640 pg/tube was prepared by using somatostatin1–14 and was treated identically as the human CSF samples. The sensitivity of the assay was 0.625 pg/tube with 50% displacement of radiolabeled somatostatin (IC50) at 32 pg/tube. The inter- and intra-assay coefficients of variation (which used the methodology described earlier for CRF samples) are 8%–11% and 2%–5%, respectively. All samples from all subjects (including all patients and comparison subjects) were run together in a single CRF or somatostatin assay through use of the same laboratory techniques.
Data were analyzed by using a nonpaired two-tailed t test. In order to control for differences in age between the patients and comparison subjects, an analysis of covariance (ANCOVA) was performed for both CRF and somatostatin.
RESULTS
CSF CRF concentrations were significantly higher in the PTSD patients than in the comparison subjects (mean=29.0 pg/ml, SD=7.8, versus mean=21.9 pg/ml, SD=6.0; p<0.05) (figure 1). The higher level of CSF CRF concentrations in PTSD patients was still significant after adjustment for differences in age between the two groups by using ANCOVA (F=6.71, df=1, 26, p<0.05). CSF somatostatin concentrations were also significantly higher in PTSD patients than in comparison subjects (mean=19.9 pg/ml, SD=5.4, versus mean=13.7 pg/ml, SD=8.0; p<0.05) (figure 2). This difference was a statistical trend for a higher concentration in PTSD patients after adjustment for differences in age by using ANCOVA (F=3.40, df=1, 25, p=0.08).
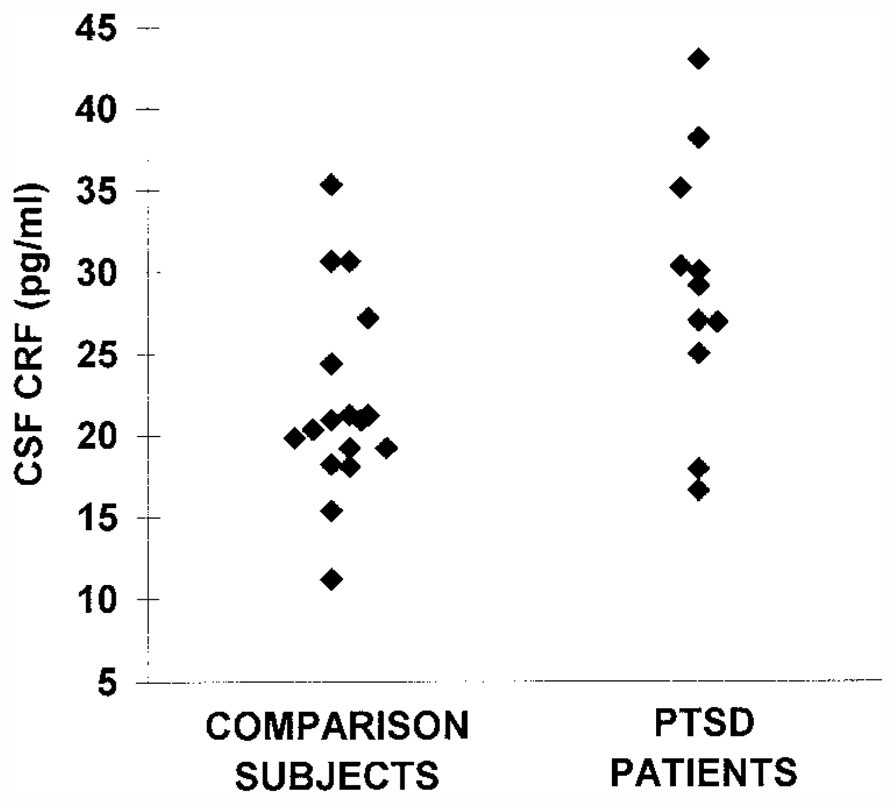
aCSF CRF levels were significantly higher in PTSD patients than in comparison subjects (t=2.7, df=26, p<0.05).
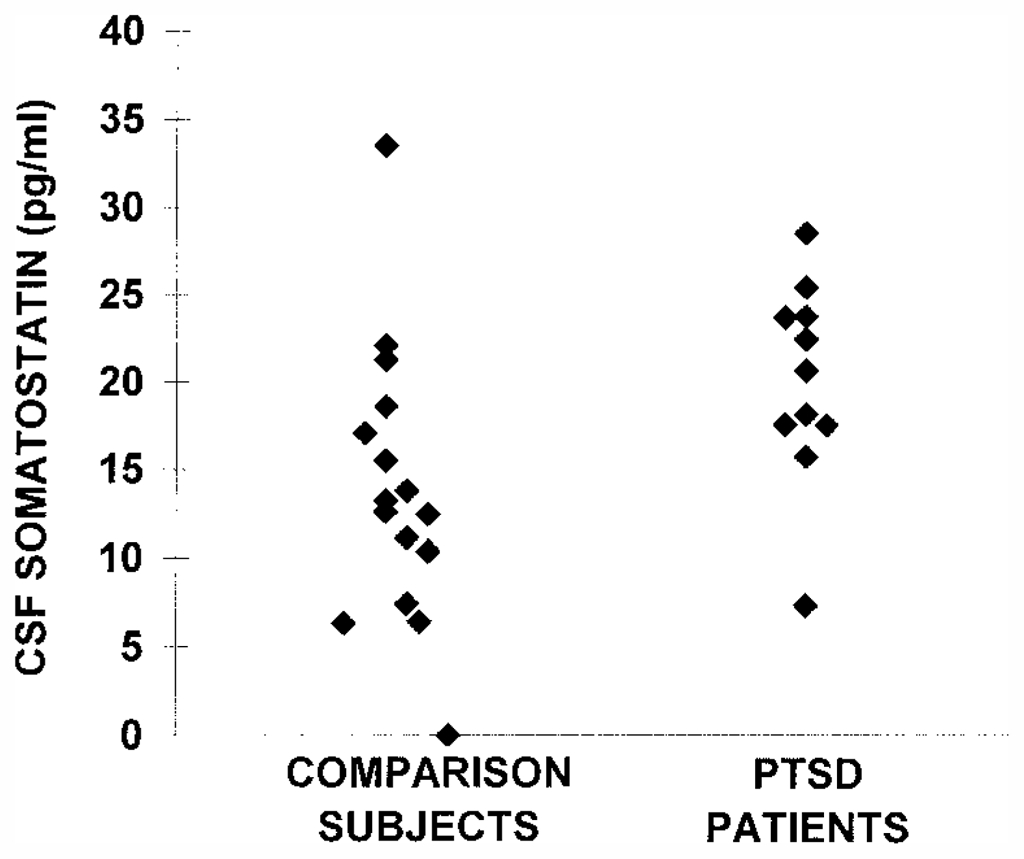
aCSF somatostatin levels were significantly higher in patients with PTSD than in comparison subjects (t=2.3, df=25, p<0.05). This effect was less significant after control for differences in age with ANCOVA.
There was a highly significant correlation between CSF CRF and somatostatin in the PTSD patients when considered alone (p=0.006). This was predicted on the basis of the hypothesis that increases in CRF would be associated with increases in somatostatin. There was no significant correlation between CSF CRF and somatostatin in the comparison subjects when considered alone (figure 3). We used multivariate analyses to examine the hypothesis of a significant relationship between CRF and somatostatin in the patients but not in the comparison subjects. When the relationship between CRF and somatostatin levels was examined through use of multiple linear regression, with CRF as the dependent variable and somatostatin and diagnosis as independent variables, there was a highly significant interaction between somatostatin and diagnosis relative to the dependent variable, CRF (F=12.89, df=1, 25, p=0.002).
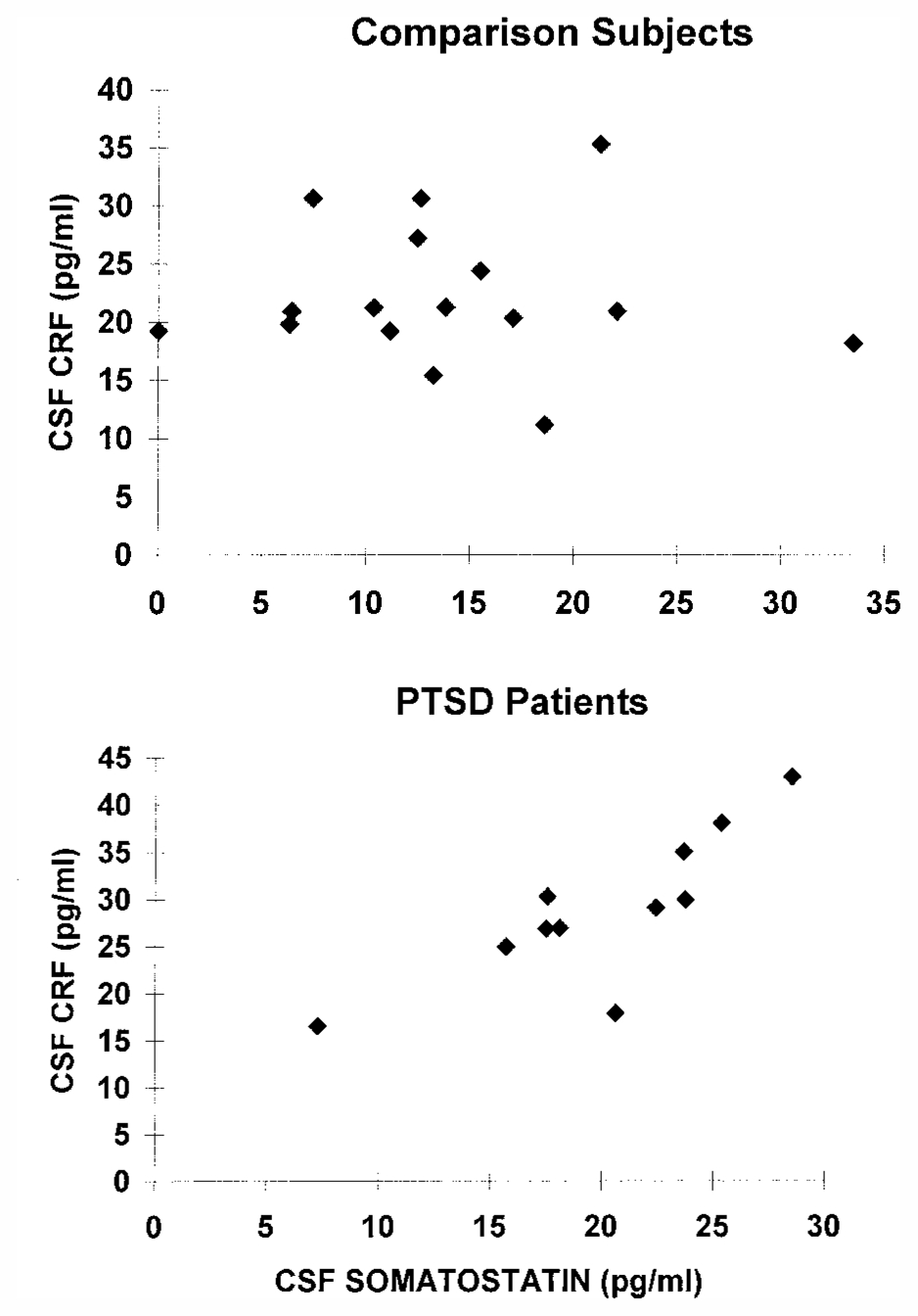
aThere was a highly significant correlation between CSF somatostatin and CSF CRF within the PTSD patient group (r=0.77, df=9, p=0.006) but not within the comparison group.
There was no difference in CSF concentrations of CRF between PTSD patients with and without comorbid major depression (mean=30.9 pg/ml, SD=4.9, versus mean=27.5 pg/ml, SD=7.0). In addition, there was no difference in CSF concentrations of somatostatin between PTSD patients with and without comorbid major depression (mean=20.5 pg/ml, SD=4.0, versus mean=19.8 pg/ml, SD=4.9).
There was no correlation between CSF CRF or somatostatin and measures of PTSD symptoms (Mississippi scale score) or level of exposure to combat stress as measured by the Combat Exposure Scale within the PTSD patients alone. There was no correlation between CSF CRF or somatostatin and age in either patients or comparison subjects.
DISCUSSION
In this study CSF concentrations of CRF were higher in patients with PTSD than in comparison subjects. CSF concentrations of somatostatin were also elevated in PTSD patients. However, the intergroup difference in somatostatin levels was not as great as that for CRF, and the level of significance was reduced for somatostatin levels after control for differences in age between the two groups through use of ANCOVA. CSF CRF and somatostatin concentrations were highly correlated in the PTSD patients but not in the comparison subjects.
An increase in CSF CRF is consistent with the hypothesis of a hypersecretion of neuronal CRF in PTSD. On the basis of findings related to the sites of origin of CSF levels of CRF, this increase is probably derived from brain regions outside of the median eminence (36), such as hippocampus, cerebral cortex, amygdala, bed nucleus of the stria terminalis, and cortical areas (7). Several studies have found evidence for altered HPA/CRF axis function in PTSD (37–42), including a blunted ACTH response to CRF (37), which could be explained by a down-regulation of CRF receptors at the level of the anterior pituitary secondary to chronic increases in CRF. Consistent with this, preclinical studies showed a chronic increase in neuronal CRF release following exposure to stress (5). Preclinical studies showed that glucocorticoids are associated with damage to the hippocampus (43, 44), a brain region involved in memory that has been suggested to have an inhibitory effect on glucocorticoid release from the hypothalamus. Stress-induced damage was associated with an increase in levels of CRF mRNA in the para-ventricular nuclei of the hypothalamus (45), as well as a decrease in the sensitivity of rats to dexamethasone suppression of HPA function (46, 47). Consistent with this, we found smaller right hippocampal volume in Vietnam combat veterans with PTSD than in matched comparison subjects (48). Alterations in hippocampal function may be associated with neuronal hypersecretion of CRF in PTSD.
We carefully considered the possible effects of comorbid diagnoses on our results. Patients with affective disorders have been found to have increased CSF CRF (18, 19) (but see reference 49). We did not find a difference in CSF CRF between PTSD patients with and without major depression in the current study. Hypercortisolemia and an increase in rates of nonsuppression with the standard dexamethasone suppression test have been associated with depression, while hypocortisolemia and a supersuppression with low doses of dexamethasone have been associated with PTSD. This suggests that these disorders have different neurobiological bases, especially with regard to the CRF/HPA axis. It is of interest that nonhuman primates with variable foraging mothers (a model for early-life stress) had elevated CSF CRF and decreased CSF cortisol levels in adulthood, a picture that is closer to PTSD than depression (50). Furthermore, no difference has been found in rates of supersuppression with low-dose dexamethasone between PTSD patients with and without comorbid major depression (41); this finding has been interpreted as the neurobiology of PTSD “overriding” the neurobiology of depression in those patients who have comorbid depression and PTSD. Patients with other psychiatric disorders, including mania (28), generalized anxiety disorder (28, 51, 52), panic disorder (28, 51, 52), somatization disorder (28), dementia without depression (28), and schizophrenia (19, 53), have not been found to have a difference in CRF in comparison to control subjects. No studies have specifically examined CSF CRF in substance abuse, and the one study in alcoholics (which did find increased concentrations of CSF CRF) was conducted in individuals who were undergoing alcohol withdrawal (54). This finding was not applicable to our current group, since none of the patients was in alcohol withdrawal. In summary, there is not evidence at this time to support the idea that findings related to CRF/HPA axis function in patients with PTSD are related to comorbidity with other psychiatric disorders.
There are some limitations of our study that are worthy of mention. Although all of the PTSD patients were voluntarily admitted as psychiatric inpatients at the time of the procedure, not all of the comparison subjects stayed overnight the night before the test. There may be differences based on hospitalization status that could represent confounding factors in our study results. High levels of comorbidity with other psychiatric diagnoses in the PTSD patients may represent another confounding factor in the study results.
This study demonstrated higher CSF somatostatin concentrations in PTSD patients than in comparison subjects. As reviewed earlier, an increase in somatostatin release has been associated with exposure to stress. We found a correlation between somatostatin and CRF in the PTSD patients but not in the normal subjects. Somatostatin blocks CRF-induced anorexia (55) and inhibition of growth hormone secretion (56), a phenomenon observed during stress. CRF levels correlate positively with somatostatin levels (57), while increased levels of peripheral glucocorticoids are associated with low levels of somatostatin (57, 58). Patients with chronic PTSD may have two separate factors that lead to increased somatostatin, low levels of peripheral glucocorticoids, and elevated levels of CSF CRF. Elevated levels of peripheral glucocorticoids may explain why somatostatin levels have been found to be low in depression (25–27), a disorder associated with elevated CRF (although elevated levels of somatostatin are seen in mania [29]). Although a previous study found a significant correlation between CRF and somatostatin in healthy subjects (unlike in the current study), this correlation was modest (r=0.37) and much less than that in a population of patients with Cushing’s disease who had altered CRF levels and a strong correlation between CRF and somatostatin (r=0.64) (57).
Acknowledgments
Supported by a grant from the National Center for Posttraumatic Stress Disorder, a VA Career Development Award to Dr. Bremner, NIMH grant MH-42088 and grant MH-51761 to Drs. Nemeroff and Owens, and an Alma Foster Davis Investigator Award from the National Alliance for Research on Schizophrenia and Depression to Dr. Licinio.
The authors thank Sandi Capelli, R.N., Valinda Ouelette, R.N., Angie Genovese, R.N., and Pat Barry, R.N., for assistance in conducting these studies.
Footnotes
Antiserums oC33 and 486 were provided by Dr. Wylie Vale, Salk Institute, La Jolla, Calif.
References
Full text links
Read article at publisher's site: https://doi.org/10.1176/ajp.154.5.624
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3233756?pdf=render
Free after 12 months at ajp.psychiatryonline.org
http://ajp.psychiatryonline.org/cgi/reprint/154/5/624.pdf
Free to read at ajp.psychiatryonline.org
http://ajp.psychiatryonline.org/cgi/content/abstract/154/5/624
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1176/ajp.154.5.624
Article citations
Sex differences in contextual fear conditioning and extinction after acute and chronic nicotine treatment.
Biol Sex Differ, 15(1):88, 31 Oct 2024
Cited by: 0 articles | PMID: 39482781 | PMCID: PMC11529327
Post traumatic stress disorder associated hypothalamic-pituitary-adrenal axis dysregulation and physical illness.
Brain Behav Immun Health, 41:100849, 20 Aug 2024
Cited by: 0 articles | PMID: 39280087 | PMCID: PMC11401111
Review Free full text in Europe PMC
Advances in the role of the GADD45 family in neurodevelopmental, neurodegenerative, and neuropsychiatric disorders.
Front Neurosci, 18:1349409, 25 Jan 2024
Cited by: 0 articles | PMID: 38332860 | PMCID: PMC10850240
Review Free full text in Europe PMC
Sex Differences in Stress Response: Classical Mechanisms and Beyond.
Curr Neuropharmacol, 22(3):475-494, 01 Jan 2024
Cited by: 7 articles | PMID: 37855285 | PMCID: PMC10845083
Review Free full text in Europe PMC
Treatment of Posttraumatic Stress Disorder: A State-of-the-art Review.
Curr Neuropharmacol, 22(4):557-635, 01 Jan 2024
Cited by: 15 articles | PMID: 37132142 | PMCID: PMC10845104
Review Free full text in Europe PMC
Go to all (358) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects.
Biol Psychiatry, 54(12):1382-1388, 01 Dec 2003
Cited by: 87 articles | PMID: 14675802
Corticotropin-releasing factor, interleukin-6, brain-derived neurotrophic factor, insulin-like growth factor-1, and substance P in the cerebrospinal fluid of civilians with posttraumatic stress disorder before and after treatment with paroxetine.
J Clin Psychiatry, 72(8):1124-1128, 28 Dec 2010
Cited by: 44 articles | PMID: 21208596
Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder.
Am J Psychiatry, 162(5):992-994, 01 May 2005
Cited by: 75 articles | PMID: 15863803
Funding
Funders who supported this work.
NCRR NIH HHS (1)
Grant ID: M01 RR000125
NIMH NIH HHS (5)
Grant ID: MH-42088
Grant ID: R01 MH056120
Grant ID: R01 MH056120-12
Grant ID: R01 MH042088
Grant ID: MH-51761





