Abstract
Free full text

Gelsolin Associates with the N Terminus of Syntaxin 4 to Regulate Insulin Granule Exocytosis
Abstract
The plasma membrane soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) protein syntaxin (Syn)4 is required for biphasic insulin secretion, although how it regulates each phase remains unclear. In a screen to identify new Syn4-interacting factors, the calcium-activated F-actin-severing protein gelsolin was revealed. Gelsolin has been previously implicated as a positive effector of insulin secretion, although a molecular mechanism to underlie this function is lacking. Toward this, our in vitro binding studies showed the Syn4-gelsolin interaction to be direct and mediated by the N-terminal Ha domain (amino acid residues 39–70) of Syn4. Syn4-gelsolin complexes formed under basal conditions and dissociated upon acute glucose or KCl stimulation; nifedipine blocked dissociation. The dissociating action of secretagogues could be mimicked by expression of the N-terminal Ha domain of Syn4 fused to green fluorescent protein (GFP) (GFP-39–70). Furthermore, GFP-39–70 expression in isolated mouse islet and clonal MIN6 β-cells initiated insulin release in the absence of appropriate stimuli. Consistent with this, the inhibitory GFP-39–70 peptide also initiated Syn4 activation in the absence of stimuli. Moreover, although MIN6 β-cells expressing the GFP-39–70 peptide maintained normal calcium influx in response to KCl, KCl-stimulated insulin secretion and the triggering pathway of insulin secretion were significantly impaired. Taken together, these data support a mechanistic model for gelsolin's role in insulin exocytosis: gelsolin clamps unsolicited soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE)-regulated exocytosis through direct association with Syn4 in the absence of appropriate stimuli, which is relieved upon stimulus-induced calcium influx to activate gelsolin and induce its dissociation from Syn4 to facilitate insulin exocytosis.
In response to a sharp increase in glucose concentration, such as that induced by intake of a meal, pancreatic islet β-cells secrete insulin, doing so in a highly regulated and biphasic manner (1). The first phase is rapid and robust, occurring within 10 min of stimulation, and thought to be accounted for by a readily releasable pool of granules already present at the plasma membrane (PM). The second phase follows at a sustained, lower rate of secretion that can last for hours (2) and is elicited only in response to fuel-type secretagogues, such as glucose. In order for insulin to be secreted, insulin granules must fuse with the PM in a stimulus-dependent and highly regulated manner; this process is carried out by soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins (3–6). Two known functional target membrane-SNARE (t-SNARE) isoforms from the syntaxin (Syn) protein family, Syn1A and Syn4, facilitate selective insulin granule fusion in β-cells via functioning as docking sites at the PM; Syn1A is required only for first phase secretion, whereas Syn4 is important for both first and second phases (7, 8). Both Syn isoforms pair with a second t-SNARE, SNAP-25 (synaptosomal-associated protein-25), under both unstimulated/basal and stimulated conditions (9, 10). Insulin granules are equipped with the vesicle-SNARE protein vesicule-associated membrane protein-2 (VAMP2)/synaptobrevin (11) and, upon arrival at the PM, form heterotrimeric SNARE core complexes by docking with t-SNARE to facilitate membrane fusion (reviewed in Refs. 12, 13). Importantly, insulin release must be clamped under basal conditions, to provide the steep gradient necessary for a regulated stimulus response to appropriate secretagogues. Defects in clamping unsolicited insulin exocytosis are associated with elevated basal insulin levels and are disruptive to maintenance of glucose homeostasis in vivo (14).
To sustain insulin release, mature insulin granules in intracellular storage pools must be mobilized toward the PM. This process appears to coincide with glucose-induced remodeling of the actin cytoskeleton (9, 15, 16). Filamentous actin (F-actin) functions as a barrier to restrain insulin granule accumulation at the PM (17, 18), such that its depolymerization results in more morphologically docked granules and may confer release competence (10, 19). Evidence of positive effects of the cytoskeleton in stimulus-induced insulin secretion exists as well (20–23). In fact, insulin granules are known to interact with F-actin filaments and require microtubule tracks, upon which to traffic to the F-actin network toward the PM (24–26). Beyond impact upon trafficking, disruption of F-actin remodeling with agents that induce either F-actin disassembly or polymerization reveals that both actions jointly impact stimulated exocytosis outcomes (27). F-actin reorganization is important for stimulated exocytosis in many cell types other than β-cells, including neuroendocrine cells, adrenal chromaffin cells, platelets, and endothelial cells (28–30). In addition to controlling stimulated exocytosis, the actin cytoskeleton affects control of basal exocytosis through focal adhesion kinase, EphA-Ephrin-A signaling, and cell contact (23, 31, 32). However, molecular mechanisms to describe how the cytoskeleton might participate in clamping of exocytotic machinery to ensure low levels of insulin secretion in the absence of stimuli remain unresolved.
Multiple SNARE proteins are linked with the F-actin cytoskeleton (10, 28, 33), although only Syn4 can directly interact with F-actin in vitro. Granule docking at Syn4 sites may be an important limiting factor in insulin exocytosis, given that increasing the number of Syn4 docking sites in vivo augments biphasic insulin secretion while maintaining normal low basal levels (7). Syn4 binds to F-actin via a spectrin-like region within the first two coiled-coil domains of the N terminus of Syn4 (10). Use of this region as a competitive peptide inhibitor reduces endogenous F-actin-Syn4 complex formation, concomitant with enhanced glucose-stimulated insulin secretion from MIN6 β-cells. Although these data argue that F-actin anchors at Syn4 sites at the PM, the potential roles of multiple actin binding proteins that are implicated in, or shown to be necessary for, proper glucose-stimulated insulin secretion have yet to be tested for integration into this particular mechanism (20, 34–36). One particular protein of interest is gelsolin, an F-actin-severing/capping protein that plays a positive role in insulin secretion and has been proposed to be important for glucose-induced F-actin remodeling, which occurs in β-cells through an undefined mechanism (36, 37).
In this study, we provide the first evidence for formation of a novel and direct interaction between Syn4 and gelsolin, mediated via the N-terminal Ha domain of Syn4. In β-cells, this complex is dissociated in response to acute glucose or KCl stimulation. Introduction of an Ha-domain peptide (amino acid residues 39–70) into MIN6 β-cells mimicked the action of these secretagogues by inducing dissociation of endogenous Syn4-gelsolin complexes. Functionally, these binding alterations were accompanied by elevated basal insulin release in mouse islet and MIN6 β-cells, coordinate with inappropriate activation of Syn4. The peptide-induced dissociation of Syn4-gelsolin complexes in MIN6 β-cells also attenuated acute glucose- and KCl-stimulated insulin exocytosis, coinciding with attenuation of the ATP-sensitive potassium channel (KATP)-channel-dependent triggering pathway in the diazoxide paradigm. These data support a new model for a clamping mechanism underlying regulated SNARE protein-mediated exocytosis. Given that 1) gelsolin and Syn4 are ubiquitously expressed proteins, 2) F-actin remodeling occurs in non-β-cell types, and 3) the effects upon insulin exocytosis are not secretagogue-specific, we propose that this mechanism may be applicable to regulated exocytosis events on a broader cell biological basis.
Results
Syn4 directly interacts with gelsolin
We first identified gelsolin as a potential Syn4-binding partner in an unbiased yeast two-hybrid screen (Thurmond, D. C., and J. E. Pessin, unpublished results) and subsequently validated gelsolin protein to bind directly to recombinant soluble [lacking the C-terminal transmembrane domain (TM)] glutathione S-transferase (GST)-Syn4 in vitro (Fig. 1A). Because both Syn4 and gelsolin are required for glucose-stimulated insulin exocytosis (7, 36), the presence of full-length endogenous Syn4-gelsolin complexes was subsequently investigated and revealed to exist in MIN6 β-cell lysates (Fig. 1B). Notably, these complexes were found to rapidly and transiently dissociate in response to acute 5-min glucose stimulation. After a 30-min glucose stimulation, Syn4-gelsolin complex abundance resumed to approximately 50% that detected under basal conditions. Changes in complex formation were independent of changes in Syn4 or gelsolin protein abundances in cell lysates.
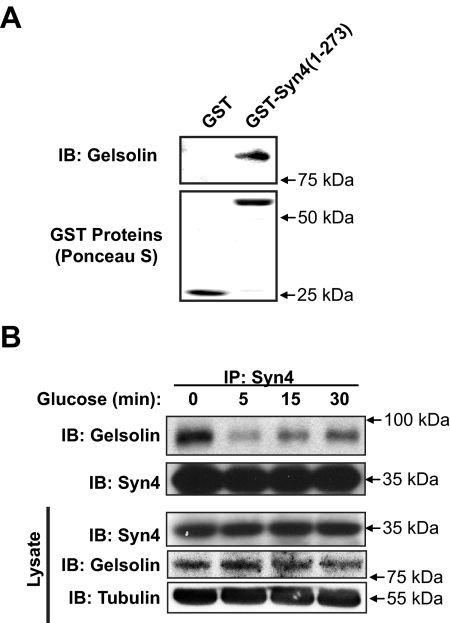
Syn4 and gelsolin directly interact and form complexes in MIN6 β-cells that are sensitive to glucose stimulation. A, Recombinant gelsolin was combined with either GST or GST-Syn4 (1–273) linked to sepharose beads for a 2-h incubation at 4 C. Beads were pelleted and extensively washed, with eluates subjected to 10% SDS-PAGE for immunoblot detection (IB) of gelsolin. Ponceau staining served as control for GST protein loading. Data are representative of at least three independent experiments using two different batches of protein. B, MIN6 cells were preincubated in MKRBB for 2 h and stimulated with 20 mm glucose for 5, 15, or 30 min. Cleared detergent cell lysates were prepared and used in Syn4 immunoprecipitation (IP) reactions. Immunoprecipitates were resolved on 10% SDS-PAGE, and proteins were transferred to PVDF for immunoblot detection of Syn4 and gelsolin. Protein abundances in starting input lysates (100 μg) were confirmed on a separate gel. Data are representative of at least three independent coimmunoprecipitation studies.
Given the novelty of this interaction, we next sought to identify the minimal region of Syn4 required to confer direct binding to gelsolin. Syn4 truncations fused C terminally to GST were analyzed using in vitro binding studies with recombinant gelsolin. As depicted in the schematic representation of Fig. 2A, Syn4 protein is composed of three N-terminal α-helical domains, which, based upon comparison with the x-ray crystal structure of family member Syn1A, pack together to comprise a coiled-coil bundle and are linked to the C-terminal α-helical SNARE domain known to be operational in binding SNARE proteins (38). Elimination of all but the N-terminal most Ha (residues 1–70) domain failed to ablate binding to gelsolin, including the Hb (residues 71–112) region previously shown to be required to confer Syn4 binding to F-actin (Fig. 2B) (10). To evaluate this binding event in a mammalian cell expression system, CHO-K1 cells were electroporated to express Flag-tagged gelsolin with truncated green fluorescent protein (GFP)-Syn4 fusion proteins (GFP-39–112 and GFP-1–70), and detergent solubilized cell lysates were prepared for coimmunoprecipitation analyses. Indeed, anti-Flag (gelsolin) immunoprecipitation coprecipitated with both truncated forms of Syn4 (Fig. 2C). After this, the far N-terminal residues 1–38 were removed to isolate the Ha domain, GFP-39–70, which was sufficient to confer binding to Flag-tagged gelsolin (Fig. 2D). Despite efforts to test for necessity of the Ha domain, deletion of the Ha from either GST- or GFP-fusion protein rendered proteins too unstable to study. Regardless, the ability of this Ha domain to confer binding to gelsolin distinguishes its interaction with Syn4 from that of F-actin, because Ha alone failed to confer Syn4 binding to F-actin (10). Importantly, this is the first demonstration of the Ha domain conferring stable binding with any of the known Syn4 binding partners.
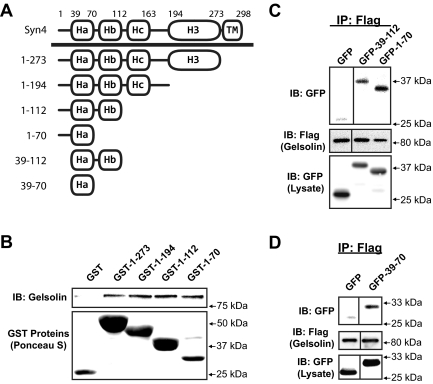
Residues 39–70 of Syn4 are sufficient to confer Syn4-gelsolin binding. A, Depiction of the multiple helical domains of the Syn4 protein. The N-terminal helical domains are denoted as Ha, Hb, and Hc; the C-terminal H3 helix is the SNARE domain, followed by the TM. B, In vitro binding reactions containing recombinant gelsolin protein plus either GST or GST-Syn4 truncation proteins linked to beads were performed as described in Fig. 1, and proteins resolved by 10% SDS-PAGE for immunoblotting (IB). Ponceau S staining shows the presence of the GST proteins in each reaction. Data are representative of two independent experiments. CHO-K1 cells were coelectroporated with pEGFP, pEGFP-Syn4(39–112) or pEGFP-Syn4 (1–70) DNA (C), or pEGFP-Syn4(39–70) DNA along with pIRES-gelsolin-Flag DNA (D), and 48 h later, detergent lysates were prepared for immunoprecipitation (IP) with anti-Flag antibody. Immunoprecipitates were subjected to 10% SDS-PAGE for immunoblot detection of GFP and Flag (gelsolin). Input lysates (50 μg) demonstrate expression and migration of each protein. Vertical lines denote splicing of lanes from within the same gel. Data are representative of three independent immunoprecipitation experiments.
Competitive inhibition of endogenous Syn4-gelsolin complexes
Because there are reported effects of apoptosis and compensatory cytoskeletal effects associated with gelsolin knockdown/depletion approaches (39, 40), we opted to use the Ha-domain peptide (GFP-39–70) as a competitive inhibitor in effort to selectively target the Syn4-gelsolin complex to evaluate its function in insulin exocytosis. In addition to the unusual ability of the Syn4 Ha domain to confer binding to gelsolin, as opposed to how Syn4 binds other partners, the Ha domain (residues 39–70) carries the least identity of all its helical domains (<35%) to Syn1A. Using lysates prepared from MIN6 β-cells expressing the GFP-39–70 protein, anti-Syn4 immunoprecipitation reactions resulted in attenuated coprecipitation of gelsolin under basal (unstimulated) conditions, compared with reactions from control GFP; a scrambled peptide fused to GFP (GFP-Scr), designed to retain helical structure (http://npsa-pbil.ibcp.fr), was without effect (Fig. 3A). Having established specificity of the GFP-39–70 peptide upon Syn4-gelsolin complexes, we next evaluated effects after an acute 5-min glucose stimulation. GFP-expressing cells showed reduced interaction of endogenous Syn4-gelsolin complexes in GFP-expressing cells, similar to that of complexes shown in Fig. 1B. However, glucose stimulation of GFP-39–70-expressing cells failed to further dissociate the endogenous complex (Fig. 3Bi). As we have previously shown that F-actin, but not G-actin, interacts with Syn4 in β-cells in a glucose-sensitive manner, and can directly interact with Syn4 in vitro (10), we questioned whether gelsolin was coupled to this interaction. Coordinate with this, F-actin association with Syn4 in the presence of the GFP-39–70 peptide was diminished in unstimulated cell lysates, relative to GFP alone (Fig. 3Bii). Moreover, pharmacologically induced actin depolymerization using latrunculin B (LAT) caused dissociation of both actin and gelsolin from Syn4 (Fig. 3C).

GFP-39–70 disrupts endogenous Syn4-gelsolin complexes. A, MIN6 cells transfected to express GFP, GFP-39–70, or GFP-Scr (scrambled peptide) were preincubated in MKRBB for 2 h and resultant lysates used for anti-Syn4 immunoprecipitation (IP). Coprecipitated proteins were resolved on 10% SDS-PAGE for immunoblot detection (IB). Data represent at least three independent coimmunoprecipitation experiments. B, MIN6 cells transfected to express either GFP or GFP-39–70 proteins were preincubated and stimulated with 20 mm glucose for 5 min; lysates were used in IP reactions as detailed in A above. Band intensities of actin and gelsolin association with Syn4 were quantitated and expressed as the ratio of gelsolin (Gsn) (i) and actin (ii) to Syn4 (normalized, basal ratio = 1 for each experiment). Bars represent the mean ± se of the three independent experiments (*, P < 0.05, vs. unstimulated GFP). C, MIN6 cells were treated with vehicle [dimethylsulfoxide (DMSO)] or 10 μm LAT for 2 h in MKRBB and resultant cell lysates used for IP as described in A and B above in three independent experiments. Protein abundances in starting input lysates (100 μg) were confirmed on a separate gel. Vertical lines denote splicing of lanes from within the same gel.
Because gelsolin knockdown in MIN6B1 cells exerted global effects upon F-actin remodeling, we investigated this as a potential explanation for the GFP-39–70-induced alterations to Syn4 binding. We first determined that selective attenuation of Syn4-gelsolin binding under basal conditions was unattributable to differential GFP-39–70 subcellular localization, because its principally cytosolic localization was similar to that of GFP, as visualized by confocal microscopy (Fig. 4, panels 1–4). However, unstimulated GFP-39–70-expressing cells contained contiguous cortical F-actin (red, rhodamine-phalloidin staining) similar to that of neighboring untransfected cells and of GFP control cells (Fig. 4, panels 5 and 6, and 9 and 10). Moreover, GFP and GFP-39–70-expressing cells all showed the expected acute glucose-induced actin remodeling, as determined by disruption of the contiguous cortical F-actin rim encircling each cell (Fig. 4, panels 7 and 8, and 11 and 12). These data indicated that the GFP-39–70 peptide sufficed as a competitive inhibitor of endogenous Syn4-gelsolin complexes present in unstimulated MIN6 β-cells and did so without inducing global changes in cortical F-actin structure.
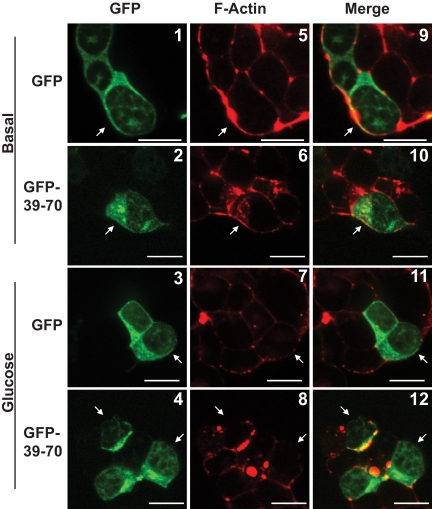
GFP-39–70 does not disrupt normal glucose-induced actin remodeling. MIN6 cells were plated on glass coverslips and transfected to express GFP or GFP-39–70 proteins (green) were preincubated in MKRBB for 2 h and left unstimulated (top panels 1 and 2, 5 and 6, and 9 and 10) or stimulated with 20 mm glucose for 5 min (bottom panels 3 and 4, 7 and 8, and 11 and 12). Cells were fixed, permeabilized, and cortical F-actin staining detected by rhodamine-phalloidin staining (red). Cells in clusters containing both nontransfected and GFP-transfected cells were imaged at the midplane of the cluster by single channel scanning confocal microscopy to determine subcellular distribution of the GFP proteins (panels 1–4) and F-actin remodeling (panels 5–8). Merged GFP and F-actin images are shown in panels 9–12 to permit comparisons between nontransfected and transfected cells. Scale bar, 10 μm. Data shown are representative of three independent sets of experiments.
Syn4-gelsolin complexes are required to clamp unsolicited insulin exocytosis events
The competitive peptide was subsequently used to determine the functional ramifications of disrupting Syn4-gelsolin complexes upon insulin secretion from isolated islets and MIN6 cells. To ensure thorough and efficient expression of the peptide across cell populations for these studies, an adenovirus encoding GFP-39–70 (GFP-39–70-Ad) was generated and validated to exert dissociating actions upon endogenous Syn4-gelsolin complexes akin to that of the plasmid-based delivery system (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Subsequently, islets isolated from wild-type C57BL/6J mice were transduced with GFP-39–70 or GFP adenoviruses and protein expression confirmed (Fig. 5A), albeit GFP-39–70 expression was consistently lower than that of GFP. Islet perifusion experiments were initiated to examine effects of GFP-39–70 expression upon basal and phasic insulin release. GFP-39–70-expressing islets released approximately 4-fold more insulin under basal (2.8 mm glucose) conditions (Fig. 5B), and first phase secretion appeared to be slightly impaired in GFP-39–70-expressing islets (GFP-39–70 peaked 3-fold above basal, GFP peaked 10-fold above basal). However, defective basal secretion impeded reliability of area under the curve analysis to quantify phasic differences. We next employed a short-term static incubation approach as a means to quantify acute/first phase secretion using glucose or KCl secretagogues. Again, basal elevation in GFP-39–70-expressing cells was fully recapitulated in the static culture system, yet short term-stimulated secretions were highly erratic and thwarted efforts to discern significant first phase differences (Fig. 5C). However, this rise in basal secretion in GFP-39–70 islets nearly abolished the overall stimulatory responses compared with those of GFP-expressing islets (stimulation index = glucose-stimulated/basal secretion) (Fig. 5D). Differences in secretion were not due to alterations in insulin content (Fig. 5E). No significant differences in long-term glucose-stimulated insulin secretion (1 h) or associated insulin content were noted between GFP and GFP-39–70-expressing islets (Supplemental Fig. 1, B and C). These data suggested that dissociation of endogenous Syn4-gelsolin complexes under basal conditions permitted or otherwise prompted inappropriate insulin release.

Disruption of Syn4-gelsolin complexes elevates basal insulin secretion from isolated mouse islets. Freshly isolated mouse islets were immediately transduced with GFP or GFP-39–70 adenovirus, and after a 48-h incubation, GFP-positive islets were handpicked under a fluorescence microscope for immunoblot analysis (A), for perifusion analysis (B), and for static culture studies (C). Perifusion entailed use of 50 GFP positive islets per column with GFP and GFP-39–70-expressing islet columns run in parallel; islets were perifused with low glucose (2.8 mm) followed by high glucose (16.7 mm) to capture first phase release. Static secretion reactions contained 10 GFP positive islets/tube and were incubated in low glucose (2.8 mm) KRBH for 2 h and buffer collected for quantitation of basal insulin secretion. Buffers containing stimulatory levels of glucose (16.7 mm) or KCl (35 mm) were added to the islets for 10 min, followed by collection for insulin quantitation. Insulin content in the remaining islets was also determined and used to normalize insulin secretion data; *, P < 0.001 vs. GFP. D, Stimulation index was calculated using the values from C (stimulated insulin release/basal insulin release). E, Insulin content was similar between transduced islet groups. Data represent the mean ± se of three independent islet isolation experiments; *, P < 0.05 vs. GFP. IB, Immunoblotting.
Loss of clamping of basal insulin release could result from a direct effect upon Syn4 activation, or possibly an indirect effect of aberrant Cdc42 signaling, as has been found to occur in other cases of dysregulated basal insulin secretion (41, 42). To test for alterations in Syn4 activation, lysates prepared from MIN6 β-cells transduced to express GFP or GFP-39–70 protein were combined with recombinant GST-VAMP2 (soluble, TM domain deleted) protein, linked to sepharose beads, as a means to selectively precipitate open conformation Syn4 as described previously (10, 41). It is expected that opening increases accessibility of Syn4 to enhance granule docking to promote granule fusion and insulin release. Although GFP-expressing cells exhibited a low level of basal activation and responded to acute glucose stimulation (5 min) with the traditional approximately 2-fold increase in Syn4 activation, GFP-39–70-expressing cells showed significantly elevated basal Syn4 activation, with no further responsiveness to glucose (Fig. 6A). These differences were not a consequence of differential Syn4 protein expression, or between GFP and GFP-39–70 in the starting lysates (Fig. 6B). Further implicating Syn4 activation as a mechanism to explain elevated basal secretion, Cdc42 signaling was unaffected by the GFP-39–70 peptide, as gauged by normal activation (phosphorylation) levels of the immediate downstream Cdc42 effector protein [p21-activated kinase-1 (PAK1)] under basal conditions (Fig. 6C).
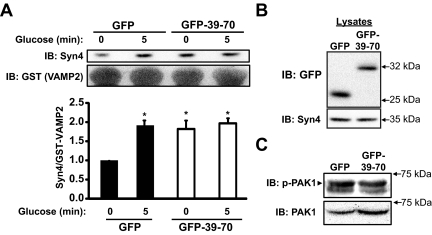
Disruption of Syn4-gelsolin complexes triggers Syn4 activation in the absence of secretagogue stimulation. A, MIN6 cells transduced to express either GFP or GFP-39–70 proteins were preincubated in MKRBB for 2 h and left unstimulated or stimulated with 20 mm glucose for 5 min to generate cleared detergent cell lysates for use in GST-VAMP2 interaction assays. Lysate protein (2–3 mg) was combined with GST-VAMP2 (soluble, TM domain deleted) bound to glutathione sepharose beads, incubated for 2 h and precipitated proteins resolved on 10% SDS-PAGE for immunoblot detection (IB) of Syn4 and GST. Band intensities of Syn4 association with GST-VAMP2 were quantitated and the ratio of Syn4 to GST-VAMP2 determined (normalized to unstimulated GFP-Ad = 1 in each experiment); *, P < 0.05 vs. unstimulated GFP. B, Immunoblot to demonstrate the relative expression of GFP and GFP-39–70 proteins in the starting lysates used in GST-VAMP2 interaction assays. C, Basal Pak1 phosphorylation was evaluated by immunoblotting lysates prepared from transduced and unstimulated MIN6 cells. The marker indicates the upper phospho-Pak1 band; lower band is nonspecific. Blots were subsequently stripped and reprobed for total Pak1 protein. Images are representative of three independent sets of transduced cell lysates.
Syn4-gelsolin complexes and the KATP channel-dependent/triggering pathway in MIN6 β-cells
Islets are composed of multiple cell types. To ascertain whether erratic short-term insulin secretion outcomes from islet studies were due to possible effects of noninsulin-secreting cell types within islets, secretion studies were performed in the clonal MIN6 β-cell system. Mouse clonal MIN6 cells are considered to have similar insulin content and glucose-stimulatory response similar to that of normal islets, where normal responses occur at 16–20 mm glucose (43). As observed in mouse islets, expression of the GFP-39–70 but not the GFP-Scr peptide in MIN6 cells induced elevation of basal secretion in the absence of secretagogue (Fig. 7A and Supplemental Fig. 2), although to a lesser extent than observed in whole islets. Similar to islets, prolonged glucose stimulation failed to unveil significant differences in insulin release between GFP and GFP-39–70-expressing cells (data not shown). By contrast, acute stimulation (10 min) with either glucose or KCl in GFP-39–70-expressing cells elicited small but significant approximately 20% attenuations of acute (10 min) stimulus-induced secretion (Supplemental Fig. 3): 20 mm glucose (205 ± 22 and 169 ± 20 ng/mg protein for GFP vs. GFP-39–70, respectively) and with 35 mm KCl (499 ± 48 and 410 ± 24 ng/mg protein for GFP vs. GFP-39–70, respectively). The combined impairments in basal and secretagogue-stimulated secretion culminated in significant losses in stimulation index (Fig. 7B) and were not due to GFP-39–70-induced β-cell apoptosis, as evaluated by caspase-3 cleavage (Fig. 7C).
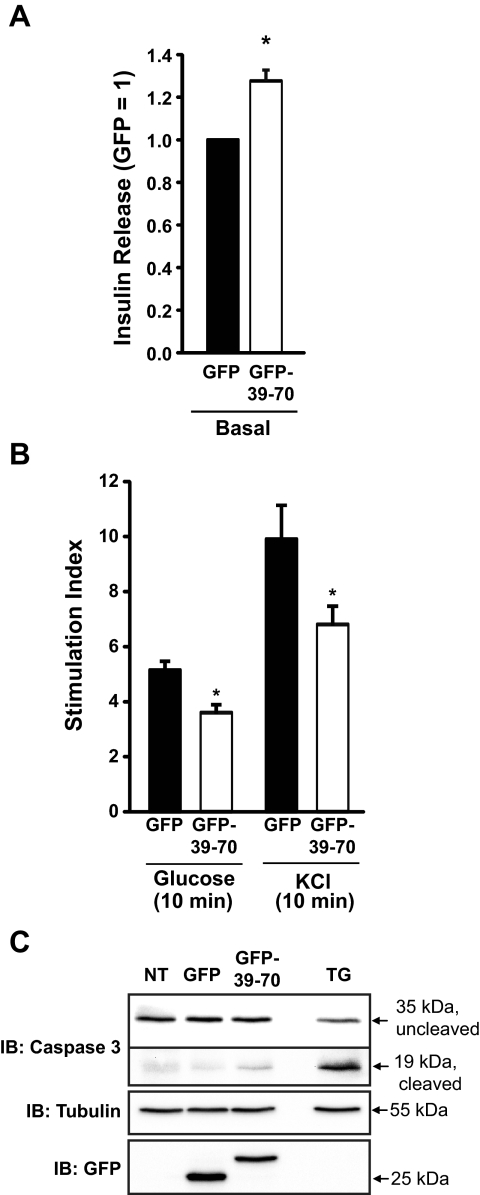
Disruption of Syn4-gelsolin complexes impairs insulin secretion from MIN6 β-cells. MIN6 cells transduced to express either GFP or GFP-39–70 proteins were preincubated in MKRBB for 2 h and left unstimulated (A) or were stimulated with either 20 mm glucose or 35 mm KCl for 10 min (B) to determine the insulin secretion stimulation index (stimulated insulin release/basal insulin release). Data represent the mean ± se of at least three independent islet batches; *, P < 0.05 vs. GFP. C, Caspase cleavage was assessed in MIN6 cells transduced to express GFP or GFP-39–70 by immunoblotting (IB) for caspase-3, which recognizes the 35-kDa inactive protein and the 19-kDa cleaved activated form. Treatment for 16 h with 0.5 μm thapsigargin (TG) to induce apoptosis served as a positive control. The GFP immunoblot shows expression of GFP and GFP-39–70 (NT, Not transduced) and tubulin immunoblot used as a loading control. Data are representative of two independent experiments.
Another method used to assess the phases of glucose stimulation is by the diazoxide paradigm. This pharmacological paradigm is based upon the premise that the first phase release is due to KATP-dependent triggering, whereas the second phase is regulated by KATP channel-independent amplifying effects (44–47). By subjecting MIN6 β-cells to depolarizing KCl concentrations in the presence of the KATP channel opener diazoxide, low glucose simulates triggering, and high glucose simulates amplification (34). Using this paradigm, GFP-39–70-expressing cells showed approximately 30% decrease in the KCl-stimulated triggering of insulin secretion compared with GFP-expressing controls (Fig. 8A). Diazoxide action was verified by abolishment of secretion from cells treated with diazoxide alone or in combination with glucose in the absence of depolarizing KCl concentrations (data not shown). The GFP-expressing cells exhibited the amplifying response, with approximately 3-fold more insulin release upon stimulation with glucose, validating responsiveness of the cells (34, 48). However, the stimulation index of the amplification effect between GFP- and GFP-39–70-expressing cells was similar (Fig. 8A, inset graph). This suggested that artificial disruption of the Syn4-gelsolin complex by the GFP-39–70 peptide may have compromised triggering. However, efforts to use this paradigm in adenovirally transduced islets failed, because a combination of these islets and diazoxide treatment dramatically reduced insulin content (data not shown).
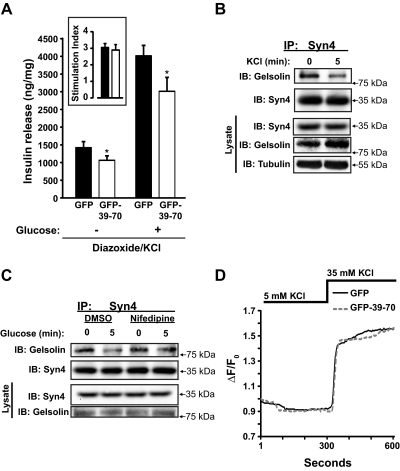
Effects of GFP-39–70 upon the triggering pathway. A, Effects of GFP-39–70 were evaluated by the diazoxide paradigm. MIN6 cells transduced to express GFP or GFP-39–70 proteins were incubated with 250 μm diazoxide and 35 mm KCl, followed by addition of 20 mm glucose for 30 min and upon insulin secretion quantified. Insulin released was normalized for corresponding cellular protein content (nanograms of insulin/milligrams of protein). Inset, Stimulation index = insulin release under diazoxide + KCl + glucose treatment/insulin release under diazoxide + KCl treatment alone (black bar, GFP; open bar, GFP-39–70). Data represent the mean ± se of at least three independent islet batches; *, P < 0.05 vs. GFP. B, MIN6 cells preincubated in MKRBB were stimulated 5 min with KCl (50 mm) and resultant cell lysates used for Syn4 immunoprecipitation (IP) as described in Fig. 1B. Data are representative of five independent experiments. C, MIN6 cells treated with vehicle [dimethylsulfoxide (DMSO)] or nifedipine for 1 h during preincubation in MKRBB were acutely stimulated with glucose (20 mm) for 5 min, in the continued presence of nifedipine/DMSO, and resultant cell lysates used in anti-Syn4 immunoprecipitation reactions. Protein abundances in starting input lysates (100 μg) were confirmed on a separate gel. Data are representative of three independent experiments. D, MIN6 cells on glass coverslips transduced to express GFP or GFP-39–70 proteins were preincubated in MKRBB supplemented with 2 mm glucose for 2 h followed by loading with fura 2-AM. Cells were subjected to perfusion for 300 sec under low KCl (5 mm) conditions and switched to buffer containing stimulatory KCl (35 mm) for an additional 300 sec to evaluate calcium influx. Each 340/380 ratio trace was normalized to the initial basal value (F0) of the respective trace to yield ΔF/F0. IB, Immunoblotting.
We next determined whether triggering steps impacted the Syn4-gelsolin complex dissociation. The triggering pathway is defined as that involving closure of KATP channels, membrane depolarization, opening of voltage-dependent calcium channels, calcium influx and rise in the cytoplasmic-free calcium concentration ([Ca2+]c), and activation of the exocytotic machinery (49). Testing these steps, acute KCl stimulation dissociated Syn4-gelsolin complexes (Fig. 8B). Consistent with this, treatment with nifedipine, which blocks calcium influx through the β-cell's voltage-dependent calcium channels, blocked glucose-induced dissociation of endogenous Syn4-gelsolin complexes (Fig. 8C). However, fura 2 calcium imaging experiments revealed no decrease in KCl-stimulated increases in [Ca2+]c in GFP-39–70-Ad-expressing cells vs. GFP cells (Fig. 8D), suggesting that the GFP-39–70 peptide does not disrupt Syn4-gelsolin complexes and acute insulin secretion via disrupting calcium influx/elevation of [Ca2+]c per se. Taken together with the abnormal activation of SNARE machinery (Syn4) induced by the GFP-39–70 peptide data of Fig. 6, these data support the conclusion that a stimulatory defect induced by the GFP-39–70 peptide was downstream of calcium influx/elevated [Ca2+]c, perhaps at steps of gelsolin activation and/or SNARE-mediated granule docking/fusion. Indeed, the calcium sensitivity of gelsolin activation is approximately 300-1000 nm, consistent with that of insulin exocytosis (50, 51).
Discussion
In this report, we demonstrate the existence of a novel interaction between the SNARE protein Syn4 and the F-actin-severing/capping protein gelsolin and that this interaction was susceptible to disruption upon glucose and KCl stimulation in β-cells. Introduction of a competitive inhibitory peptide, GFP-39–70, into pancreatic β-cells disrupted endogenous Syn4-gelsolin complexes under basal conditions, mimicking the action of secretagogue stimulation, providing strong evidence for a crucial functional role for this complex in clamping unsolicited basal insulin secretion. Mechanistically, disruption of Syn4-gelsolin complexes spurred inappropriate activation of Syn4 under basal conditions, consistent with the concept of gelsolin binding to Syn4 to mitigate unsolicited Syn4-mediated granule docking/fusion events. In addition to a role in the clamping mechanism, data gained from MIN6 cells suggest that Syn4-gelsolin complexes may also participate in the calcium- and KATP channel-dependent/triggering mechanism of insulin release, although further studies will be required to substantiate this possibility in primary cells. Such a dual action of Syn4-gelsolin complexes would be consistent with very recent work showing that actin filaments both prevent and augment the exocytosis of a single regulated secretory granule (52).
Gelsolin is a known calcium-activated F-actin severing/capping protein consisting of six homologous domains, each of which contains calcium binding sites that contribute to calcium-induced alterations to gelsolin's conformation and activity (50, 53–55). Because gelsolin is activated by calcium at levels observed in KCl- and glucose-stimulated β-cells (50, 51), it could be speculated that gelsolin participates within calcium microdomains for F-actin clearance at active sites of exocytosis. If so, one explanation for the effect of the GFP-39–70 peptide is that it displaced Syn4 from a calcium microdomain. However, Syn4 remains at the PM in cells treated with LAT, and moreover, that Syn4 is activated (10) and presumed to be functional given the potentiating effect of LAT upon calcium-stimulated insulin release (9). Regarding gelsolin's role at calcium microdomains for actin clearance, this remains controversial: gelsolin knockdown in the MIN6B1 cell line ablated glucose-induced F-actin remodeling, but the recent analyses of islets from gelsolin knockout mice do not show effects upon F-actin remodeling (56), and now, we also show that the GFP-39–70 peptide fails to impact actin remodeling. The lack of effect of the GFP-39–70 peptide upon actin remodeling under basal conditions could be explained by the need for calcium activation of gelsolin and that its mere dissociation from Syn4 was insufficient to induce actin remodeling. Moreover, the absence of a refractory response from the cytoskeleton to the competitive peptide could suggest that gelsolin's severing activity was unaffected by the peptide. As such, the GFP-39–70 peptide would likely not induce the many and diverse defects associated with gelsolin knockout or knockdown, such as compensatory increases in Rac1 protein, reduced fibroblast motility, and neurological and immune system defects (40, 57, 58), and did not cause apoptosis as was observed in gelsolin-depleted MIN6B1 cells (39). Because the degree of change in stimulated insulin secretion was similar in cells depleted of gelsolin (36%) and those expressing the GFP-39–70 peptide (~30%), the implication is that gelsolin's mechanism of action in glucose-stimulated insulin secretion might be through its interaction with Syn4. This may be further impacted by phosphoinositide binding and regulation of gelsolin (59–61). The lack of a larger deficit in insulin secretion could be due to compensation by a closely related family member, scinderin, which shows expression only in adrenal chromaffin cells, kidney and intestinal cells (62), and pancreatic β-cells (data not shown). Scinderin and gelsolin share more than 60% sequence identity, and a scinderin-derived actin-binding peptide inhibited Ca2+-dependent exocytosis without affecting the whole-cell Ca2+ current, by 61% in mouse pancreatic β-cells (63). Thus, the potential remains that gelsolin and scinderin work in an additive manner, perhaps via Syn4; due to scinderin's more limited expression profile, Syn4-scinderin complexes in the β-cell could present a means to selectively manipulate insulin release.
Poorly regulated/constitutive and nonsecretagogue-specific insulin release from an islet β-cell is characteristic of an aberration of the regulated exocytosis (SNARE protein) machinery; F-actin, which is shown to function both in preventing unsolicited basal exocytosis and in promoting secretagogue-induced exocytosis in endothelial cells (52), may also be defective. Syn4 activation appears to be linked to its association with F-actin, which vacillates in the presence of secretagogue (10). As such, one possible reason that the GFP-39–70 peptide disrupted both basal and stimulated secretion in the MIN6 cells is that it may have interfered with normal activation/deactivation cycling of Syn4: after fusion, SNARE complexes undergo disassembly with t-SNARE proteins deactivated and recycled for subsequent rounds of docking/fusion. Furthermore, residues within the t-SNARE and vesicle-SNARE proteins that become surface exposed upon conformational changes induced by assembly into the heterotrimeric SNARE core complex have been linked directly to calcium triggering of exocytosis from chromaffin cells (64). Because Syn1A can associate directly with the operative L-type voltage-dependent calcium channels in β-cells (65, 66), placing SNARE core complex formation at the site of calcium entry into the cell, future studies of Syn4 binding to channels will be required to determine whether this could also account for GFP-39–70 peptide-induced defects. Alternatively, constitutive Syn4 activation induced by the peptide could have disrupted binding and function of SNARE accessory proteins that bind calcium, such as synaptotagmin 7 and double C2 domain protein-b, both of which function in insulin secretion (67–70). Nevertheless, despite our use of multiple and diverse approaches, we were unable to reproducibly detect defects in first phase/triggering in GFP-39–70-expressing islets. One possibility is that the peptide exerted effects in non-β-cells of the islet, particularly because Syn4 and gelsolin are considered ubiquitously expressed proteins, and this generated sufficient “noise” in our systems to negate detection of the small expected changes in secretion. Deregulation of basal secretion in the islets was also far more substantial (4-fold) than in MIN6 β-cells, effectively blunting any further secretagogue-induced increase in stimulation index. Notably, however, actin filaments prevent hypersecretion as well as promote stimulated secretion (52). Given that changes in actin tethering to Syn4 occur in tandem with gelsolin, it remains possible that GFP-39–70 has impacted this process. Future studies use real-time imaging of in-cell Syn biosensors that distinguish open from closed conformations will be required to determine its cyclic activation patterns in primary islet β-cells.
The Ha domain of Syn4, in isolation, has not previously been shown to be sufficient to confer its binding to any other of Syn4's binding partners other than gelsolin. Syn4 directly interacts with both gelsolin and F-actin, and gelsolin directly binds to F-actin; however, it remains unknown whether the three form a heterotrimeric complex. The simultaneous dissociation of Syn4 from F-actin and from gelsolin in response to acute glucose stimulation in β-cells might support this concept, although efforts to reciprocally coimmunoprecipitate the complex from cell lysates, or efforts to capture the complex in vitro, were unsuccessful due to technical limitations with available reagents. As such, the inappropriate Syn4 activation could potentially be caused by the dissociation of F-actin that happens alongside disruption of the Syn4-gelsolin complex. Indeed, this is seen using a peptide containing the minimal region of Syn4 that binds F-actin (GFP-39–112), or through global F-actin depolymerization using the actin monomer binding agent LAT (10). Oddly, treatment with LAT failed to also elevate basal insulin secretion, suggesting that there is distinction between nonspecifically disrupting the entire F-actin cytoskeleton and disrupting select protein-protein interactions, as in the case of using GFP-39–70 to disrupt Syn4-gelsolin complexes. The idea that basal insulin secretion is influenced by the cytoskeleton is supported by studies of EphA-Ephrin-A signaling, cell-cell contact, and focal adhesion kinase (23, 31, 32).
It is interesting that cells expressing GFP-39–70 and further subjected to acute glucose stimulation did not elicit an additive effect upon disruption of Syn4-gelsolin complexes, such that a contingent of complexes persisted. This phenomenon was also observed with peptide disruption of F-actin-Syn4 binding (10). One possibility is that the peptide is only capable of mimicking the action of secretagogue and only disrupts a subset of complexes; some may be refractory or inaccessible. Furthermore, it remains unknown as to whether Syn4 associates, transiently or otherwise, with gelsolin and/or F-actin during the dynamic process of SNARE complex assembly/disassembly; dissociation events may be so transient that it is not possible to detect all dissociated in sync. Although it also remains possible that the peptide is a relatively poor competitor, despite our lysate evidence showing it to be abundantly expressed, future studies of Syn4-gelsolin complex kinetics are required to address issues related to dissociation susceptibility.
In conclusion, we describe the first mechanistic evidence for gelsolin in regulating the exocytosis machinery, via its direct association with the t-SNARE protein Syn4. Through association with Syn4, gelsolin plays a critical role as a clamp on unsolicited Syn4 activation and aberrant insulin release in the absence of the appropriate secretagogue signal. Calcium may act as the key that unlocks the gelsolin clamp, because it is known to initiate gelsolin conformational changes. Once released from gelsolin, Syn4 activates and joins other SNARE proteins to facilitate granule docking and fusion. Importantly, elevated basal insulin release observed in islets with peptide-induced disruption of Syn4-gelsolin complexes models the constitutive insulin release observed in prediabetic and type 2 diabetic patients, before onset of β-cell apoptosis; this may provide new insight into the dysregulation of insulin exocytosis in diabetes. From a broader perspective, gelsolin and Syn4 are ubiquitously expressed proteins, such that their interaction may represent a more generalized clamping mechanism required for maintaining the “regulated” aspect of SNARE-mediated exocytosis.
Materials and Methods
Materials
Two rabbit polyclonal anti-Syn4 antibodies were obtained for use in coimmunoprecipitation (Chemicon, Temecula, CA) and for immunoblotting (in-house, described in Ref. 41). Rabbit anti-GFP and anti-glyceraldehyde-3-phosphate dehydrogenase antibodies were obtained from Abcam (Cambridge, MA). Monoclonal mouse anti-GFP, mouse anti-Cdc42, and mouse antigelsolin antibodies were purchased from CLONTECH/BD Biosciences (Mountain View, CA). Rabbit anti-actin antibody, Flag-M2 antibody, 4′,6-diamidino-2-phenylindole (DAPI), nifedipine, and diazoxide were purchased from Sigma (St. Louis, MO). LAT was obtained from Calbiochem (San Diego, CA). Rabbit anti-GST was obtained from Affinity Bioreagents (Golden, CO). Rabbit antiphospho-αPak1 (Thr423) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit IgG and rabbit anti-Pak1 were purchased from Cell Signaling (Danvers, MA). Fura 2-AM and rhodamine-phalloidin were purchased from Invitrogen (Carlsbad, CA). The MIN6 cells were a gift from John Hutton (University of Colorado Health Sciences Center, Denver, CO). Goat antimouse horseradish peroxidase secondary antibody was obtained from Thermo Fisher Scientific (Rockford, IL). Goat antirabbit horseradish peroxidase secondary antibody and TransFectin lipid reagent were acquired from Bio-Rad (Hercules, CA). enhanced chemiluminescence reagent and Supersignal Femto were purchased from GE Healthcare (Piscataway, NJ) and Pierce (Rockford, IL), respectively. The rat insulin RIA kits were obtained from Millipore (Billerica, MA).
Plasmids
The pGEX4T-1 plasmids containing rat Syn4 residues 1–273, 1–194, 1–112, and 1–70 and pEGFP-C2 plasmids containing Syn4 residues 1–273, 1–194, and 39–112 were previously described (10). A PCR-generated DNA insert comprising the 39–70 residue region of Syn4 was subcloned into the 5′ EcoRI and 3′ XhoI sites of the pEGFP-C2 vector (CLONTECH) to generate pEGFP-Syn4–39-70; GFP-Scr (scrambled helical peptide) was inserted similarly using annealed primer set sequences: 5′sense strand, attcatgaagttcttcgacaccaagaaggtggagctggagctgacccagatcgtgcagatggagcagatcgagcagctgagcaggaagcagaccagggccgtgtagg-3′. GFP-Scr helicity was predicted to be 87.5%, that closest in helicity to GFP-39–70 that also failed to match with any known mouse peptides in BLAST. The pGFP-39–70-Ad adenoviral expression plasmid was generated by subcloning the GFP-39–70 DNA into the 5′ BamHI and 3′ NotI sites of the pAdCMVK vector (Viraquest, Inc., North Liberty, IA), after which adenoviral production was carried out by Viraquest, Inc. The pIRES-GFP-gelsolin-Flag plasmid was generated by subcloning DNA comprising only the cytoplasmic isoform (missing residues 1–50 of full-length protein) from the full-length mouse cDNA purchased from Open Biosystems (Huntsville, AL) into the 5′ NheI and 3′ XhoI sites of the pIRES-GFP-3XFlag vector; the modified vector was a gift of Raj Khanna (Indiana University School of Medicine). All constructs were verified by DNA sequencing.
Recombinant proteins and interaction assays
All GST fusion proteins were expressed in Escherichia coli and purified by glutathione-sepharose affinity chromatography as described previously (10). GST-Syn4 truncation GST proteins (20 μg) immobilized on sepharose beads were incubated with 20 μg of recombinant his-tagged human gelsolin protein (Cytoskeleton, Inc., Denver, CO) in a 0.25% Nonidet P-40 (NP-40) containing lysis buffer [25 mm HEPES (pH 7.4), 0.25% NP-40, 10% glycerol, 50 mm sodium fluoride, 10 mm sodium pyrophosphate, 137 mm NaCl, 1 mm sodium vanadate, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml pepstatin, and 5 μg/ml leupeptin] for 2 h at 4 C. After three washes with PBS, bound proteins were eluted from the sepharose beads and proteins resolved on 10% SDS-PAGE followed by transfer to polyvinylidene fluoride (PVDF) membrane for immunoblotting.
Cell culture, transient transfection, adenoviral transduction, and secretion assays
MIN6 β-cells were cultured in DMEM (25 mm glucose) supplemented with 15% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 292 μg/ml l-glutamine, and 50 μm β-mercaptoethanol as described previously (69). MIN6 β-cells at approximately 60–70% confluence were transfected using cesium chloride-purified plasmid DNA with TransFectin (Bio-Rad) to obtain approximately 30–50% transfection efficiency. Electroporation of MIN6 cells was carried out as previously described (71). After 48 h of incubation, cells were washed twice with and incubated for 2 h in freshly prepared modified Krebs-Ringer bicarbonate buffer (MKRBB) [5 mm KCl, 120 mm NaCl, 15 mm HEPES (pH 7.4), 24 mm NaHCO3, 1 mm MgCl2, 2 mm CaCl2, and 1 mg/ml RIA-grade BSA]. Cells were stimulated with 20 mm glucose for the times indicated in the figures, after which the buffer was collected and centrifuged for 10 min at 4 C to pellet cell debris and insulin secreted into the buffer was quantitated using a rat insulin RIA kit (Millipore). Cells were harvested in 1% NP-40 lysis buffer (same as 0.25% NP-40 with exception of 1% NP-40), lysed for 10 min at 4 C, and were cleared of insoluble material by centrifugation for 10 min at 4 C for subsequent use in coimmunoprecipitation experiments. For adenoviral overexpression, MIN6 cells at 50–60% confluence were transduced at an multiplicity of infection of 100 for 2 h, washed twice with PBS, and incubated in complete media for 48 h. Cells were subsequently preincubated in MKRBB, subjected to stimulation, and harvested for generation of detergent cell lysates as described above.
Coimmunoprecipitation and immunoblotting
For each immunoprecipitation, 2–3 mg of cleared detergent lysate protein were combined with 1 μg of antibody per milligram of protein and the reaction rotated for 2 h at 4 C. Protein G Plus agarose beads (Santa Cruz Biotechnology, Inc.) were added and reactions rotated at 4 C for an additional 2 h. Beads were pelleted and washed three times with lysis buffer, and resulting immunoprecipitates were resolved on 10–12% SDS-PAGE and transfer to PVDF membranes for immunoblotting. Immunoreactive bands were visualized with enhanced chemiluminescence or Supersignal Femto reagents and imaged using a Chemi-Doc gel documentation system (Bio-Rad).
Calcium imaging
MIN6 cells transduced as described above were preincubated in MKRBB for 2 h, with fura 2-AM (5 μm) added to the cells for an additional 25 min as reported previously (72, 73). Cells were washed with MKRBB to remove excess fura 2-AM and placed in fresh MKRBB containing low (2 mm) glucose and KCl (5 mm). Cells were imaged under constant perfusion (1 ml/min) for 300 sec, followed by stimulation with 35 mm KCl to elicit calcium influx for 300 sec. Fura 2-AM was excited at 340 and 380 nm and emission captured at 510 nm on a Zeiss Axio Observer Apochromat 100X/1.46 objective equipped with a Hamamastu Orca-ER digital camera and analyzed using AxioVision 4.7 software (Carl Zeiss, Oberkochen, Germany). Fura 2 has been shown to exhibit similar fluorescence from cells untransfected or transfected with GFP (74).
Immunofluorescence and confocal microscopy
MIN6 cells plated onto glass coverslips at 30% confluence were transiently transfected with 4 μg of plasmid DNA/35-mm well. After a 48-h incubation, cells were placed in MKRBB for 2 h, followed by stimulation with 20 mm glucose for 5 min, and then immediately fixed and permeabilized in 4% paraformaldehyde and 0.1% Triton X-100 for 10 min at 4 C. Fixed and permeabilized cells were blocked in 1% BSA plus 5% donkey serum for 1 h at room temperature, followed by incubation with 0.17 μm rhodamine-phalloidin for 1 h, per manufacturer instructions. MIN6 cells were then washed three times with PBS (pH 7.4). During the final wash, 4′,6-diamidino-2-phenylindole was added to stain nuclei. All cells were washed again with PBS and mounted (using Vectashield) for confocal fluorescence microscopy. GFP and rhodamine fluorescing cells were imaged using single-channel scanning with a ×60 objective under a ×2 zoom using an Olympus FV1000-MPE confocal microscope (Olympus, Center Valley, PA).
Mouse islet isolation, transduction, perifusion, and static culture
All studies involving mice followed the Guidelines for the Use and Care of Laboratory Animals at Indiana University School of Medicine. Wild-type male C57BL/6J mice were killed for pancreatic islet isolation as previously described (7). All viruses were obtained from Viraquest, Inc. Freshly isolated islets were immediately transduced with 107 plaque-forming unit/islet with either GFP-Ad or GFP-39–70-Ad CsCl-purified particles for 1 h at 37 C in RPMI 1640 medium, washed twice with PBS, and incubated 48 h at 37 C/5% CO2. GFP fluorescence was visualized in greater than 60–70% of islet cells with even penetration to the islet core. GFP positive islets were handpicked into batches of 10/tube for static secretion studies or 50 islets/column for perifusion. Perifusion studies were carried out as previously described (75). Islet batches were incubated 2 h in Kreb's ringer bicarbonate hepes buffer [10 mm HEPES (pH 7.4), 134 mm NaCl, 5 mm NaHCO3, 4.8 mm KCl, 1 mm CaCl2, 1.2 mm MgSO4, and 1.2 mm KH2PO4 containing 0.5 mg/ml BSA] supplemented with 2.8 mm glucose, followed by 1 h incubation in either 2.8 or 16.7 mm glucose. Insulin secreted into the buffer and insulin content in the corresponding islet lysates was quantified by Rat Insulin RIA (Millipore).
Statistical analysis
All quantitated data are expressed as mean ± se. Data were evaluated using Student's t test and considered significant if P < 0.05.
Acknowledgments
We thank Dr. Eunjin Oh for her assistance and technical expertise and Dr. Jenna Jewell, Dr. Vinnie Tagliabracci, and Latha Ramalingam for many helpful discussions. MIN6 cells and the modified pIRES-GFP-3X flag vector were kindly provided by Dr. John Hutton (Denver, CO) and Dr. Raj Khanna (Indianapolis, IN), respectively.
This work was supported by National Institutes of Health Grants DK076614; and DK067912; (to D.C.T.), DK087811; (to D.A.W.), and CTSI-KL2 RR025760 (to Z.W.); the American Heart Association predoctoral fellowship 10PRE3040010 (to M.A.K.); and the NIH predoctoral fellowship T32DK64466 (to M.A.K.).
Disclosure Summary: The authors have nothing to disclose.
†Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
Abbreviations:
- GFP
- Green fluorescent protein
- GFP-39–70-Ad
- adenovirus encoding GFP-39–70
- GFP-Scr
- GFP fused to scrambled peptide
- GST
- glutathione S-transferase
- KATP
- ATP-sensitive potassium channel
- LAT
- latrunculin B
- MKRBB
- modified Krebs-Ringer bicarbonate buffer
- NP-40
- Nonidet P-40
- PAK1
- p21-activated kinase-1
- PM
- plasma membrane
- PVDF
- polyvinylidene fluoride
- SNARE
- soluble N-ethylmaleimide-sensitive factor attachment receptor
- Syn
- syntaxin
- TM
- transmembrane domain
- t-SNARE
- target membrane-SNARE
- VAMP2
- vesicle-associated membrane protein-2.
References
Articles from Molecular Endocrinology are provided here courtesy of The Endocrine Society
Full text links
Read article at publisher's site: https://doi.org/10.1210/me.2011-1112
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/mend/article-pdf/26/1/128/8932755/mend0128.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Architecture of androgen receptor pathways amplifying glucagon-like peptide-1 insulinotropic action in male pancreatic β cells.
Cell Rep, 42(5):112529, 17 May 2023
Cited by: 5 articles | PMID: 37200193 | PMCID: PMC10312392
Combining mass spectrometry and machine learning to discover bioactive peptides.
Nat Commun, 13(1):6235, 20 Oct 2022
Cited by: 12 articles | PMID: 36266275 | PMCID: PMC9584923
DOC2b Enhances β-Cell Function via a Novel Tyrosine Phosphorylation-Dependent Mechanism.
Diabetes, 71(6):1246-1260, 01 Jun 2022
Cited by: 3 articles | PMID: 35377441 | PMCID: PMC9163558
Proteomics Studies in Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis.
J Clin Med, 11(10):2737, 12 May 2022
Cited by: 6 articles | PMID: 35628864 | PMCID: PMC9143836
Angiopoietins stimulate pancreatic islet development from stem cells.
Sci Rep, 11(1):13558, 30 Jun 2021
Cited by: 9 articles | PMID: 34193893 | PMCID: PMC8245566
Go to all (32) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Stimulus-induced S-nitrosylation of Syntaxin 4 impacts insulin granule exocytosis.
J Biol Chem, 286(18):16344-16354, 10 Mar 2011
Cited by: 38 articles | PMID: 21393240 | PMCID: PMC3091240
Transduction of MIN6 beta cells with TAT-syntaxin SNARE motif inhibits insulin exocytosis in biphasic insulin release in a distinct mechanism analyzed by evanescent wave microscopy.
J Biol Chem, 277(52):50805-50811, 21 Oct 2002
Cited by: 15 articles | PMID: 12393909
Syntaxin 2 Acts as Inhibitory SNARE for Insulin Granule Exocytosis.
Diabetes, 66(4):948-959, 23 Jan 2017
Cited by: 15 articles | PMID: 28115395 | PMCID: PMC5860373
Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis.
Diabetes Obes Metab, 19 Suppl 1:115-123, 01 Sep 2017
Cited by: 41 articles | PMID: 28880475
Review
Funding
Funders who supported this work.
NCRR NIH HHS (2)
Grant ID: CTSI-KL2 RR025760
Grant ID: KL2 RR025760
NIDDK NIH HHS (8)
Grant ID: R01 DK076614
Grant ID: DK076614
Grant ID: K01 DK087811
Grant ID: DK067912
Grant ID: DK087811
Grant ID: T32 DK064466
Grant ID: R01 DK067912
Grant ID: T32DK64466






