Abstract
Free full text

Myopathy caused by mammalian target of rapamycin complex 1 (mTORC1) inactivation is not reversed by restoring mitochondrial function
Associated Data
Abstract
Mammalian target of rapamycin complex 1 (mTORC1) is central to the control of cell, organ, and body size. Skeletal muscle-specific inactivation of mTORC1 in mice results in smaller muscle fibers, fewer mitochondria, increased glycogen stores, and a progressive myopathy that causes premature death. In mTORC1-deficient muscles, peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α), which regulates mitochondrial biogenesis and glucose homeostasis, is strongly down-regulated. Here we tested whether induction of mitochondrial biogenesis pharmacologically or by the overexpression of PGC-1α is sufficient to reverse the phenotype of mice deficient for mTORC1. We show that both approaches normalize mitochondrial function, such as oxidative capacity and expression of mitochondrial genes. However, they do not prevent or delay the progressive myopathy. In addition, we find that mTORC1 has a much stronger effect than PGC-1α on the glycogen content in muscle. This effect is based on the strong activation of PKB/Akt in mTORC1-deficient mice. We also show that activation of PKB/Akt not only affects glycogen synthesis but also diminishes glycogen degradation. Thus, our work provides strong functional evidence that mitochondrial dysfunction in mice with inactivated mTORC1 signaling is caused by the down-regulation of PGC-1α. However, our data also show that the impairment of mitochondria does not lead directly to the lethal myopathy.
Adaptations of skeletal muscle to changes in the environment have been shown to depend on insulin-like growth factor (IGF), PKB/Akt, and mammalian target of rapamycin (mTOR) signaling (1). mTOR is a highly conserved protein kinase that is known for its central role in the control of cell size through the regulation of protein synthesis (2). It is found in two distinct multiprotein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Mice with skeletal muscle-specific deletion of mTOR or rptor, essential components of mTORC1, suffer from progressive myopathy that causes premature death at the age of 4–6 mo (3, 4). Importantly, muscles of mTOR− (mTOR muscle-knockout) and raptor muscle-knockout (RAmKO) mice show impaired mitochondrial function and increased glycogen content. Moreover, both mTOR− and RAmKO mice show a striking increase in the phosphorylation of PKB/Akt at threonine 308 and serine 473; this increase most likely is based on the lack of feedback inhibition onto insulin receptor substrate 1 by S6 kinase (5). Although the increase in glycogen correlates with an increased inhibition of the PKB/Akt target glycogen synthase kinase 3β (GSK3β), the mitochondrial phenotype correlates with the strong reduction of peroxisome proliferator-activated receptor-γ (PPARγ) coactivator α (PGC-1α), which regulates mitochondrial biogenesis and glucose homeostasis in skeletal muscle and has been shown to associate with mTOR (6–8). Interestingly, similar to RAmKO and mTOR− mice, deletion of PGC-1α in skeletal muscle results in a myopathy (9), and overexpression of PGC-1α in mdx mice, a mouse model of Duchenne muscular dystrophy, has been shown to ameliorate the disease phenotype (10). These data suggest that both the mitochondrial and the myopathic phenotype of mTOR− and RAmKO mice could be based on a deregulation of PGC-1α.
In the current study, we tested this hypothesis by applying the PPAR pan-agonist bezafibrate and by transgenic overexpression of PGC-1α. We find that both experimental paradigms improve mitochondrial function but do not prevent the progressive myopathy in either mTOR− or RAmKO mice. In addition, we find that the increase in glycogen in both mTOR− and RAmKO mice is dominated by the activation of PKB/Akt and not by changes in PGC-1α.
Results
Bezafibrate Partially Restores Mitochondrial Function In Muscles in the Absence of mTOR.
Among the most striking phenotypes of mTOR− and RAmKO mice is a decrease in oxidative capacity and morphological changes of mitochondria (3, 4). In both mouse models, these phenotypes correlated with a decrease in the transcript levels of PGC-1α. Additionally, it has been demonstrated that mTORC1 controls mitochondrial gene expression through direct modulation of the transcriptional complex consisting of PGC-1α and yin-yang 1 (YY1) in vitro (8). PGC-1α in turn has been demonstrated to control its own expression through a feed-forward loop (11). These data suggest that the muscle pathology of mTOR− and RAmKO mice might be caused by mTORC1's effect on the expression of PGC-1α. To test this notion, we first used bezafibrate, a PPAR pan-agonist that activates the PPAR/PGC-1α pathway (12, 13). Administration of bezafibrate increases expression of PPARα and -δ and the coactivator PGC-1α in skeletal muscle (14). Importantly, the improvement of mitochondrial function by bezafibrate is sufficient to alleviate the progressive myopathy in mice deficient in cytochrome c oxidase (14). mTOR− mice were fed a diet containing 0.5% bezafibrate for 20 wk starting at age 5 wk. Quantitative real-time PCR (qRT-PCR) analysis showed that bezafibrate treatment significantly increased transcript levels of PGC-1α (Fig. 1A). Bezafibrate increased the expression of several mitochondrial genes, including medium-chain acyl coenzyme A dehydrogenase (MCAD), long chain acyl-coenzyme A dehydrogenase (LCAD), cytochrome c oxidase I and IV (COX I and IV), citrate synthase and muscle carnitine palmitoyltransferase I (mCPT1) (Fig. 1B), which are all known targets of PGC-1α (6). Most importantly, bezafibrate partially restored the activity of oxidative enzymes as shown by staining muscle cross-sections for succinate dehydrogenase (SDH) and cytochrome oxidase (COX) activities (Fig.1C and Fig. S1A). Significantly increased oxidative capacity of mTOR− muscles was confirmed further by measuring SDH activity directly (Fig. 1D).
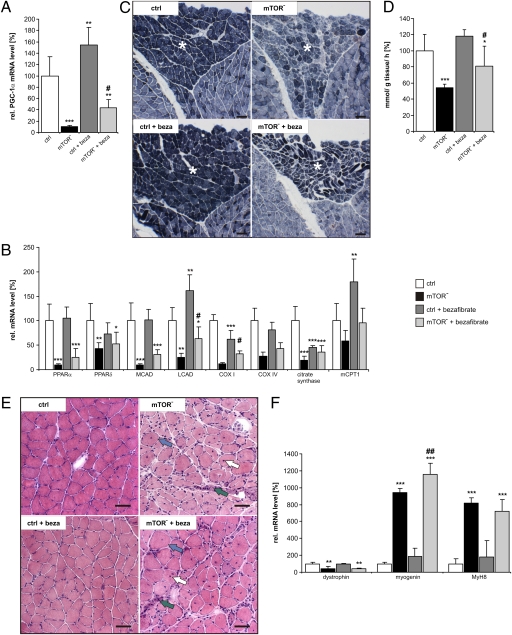
Bezafibrate partially restores mitochondrial function in muscles in the absence of mTOR but does not prevent the myopathy. (A) Relative mRNA levels of PGC-1α were determined by qRT-PCR in control (ctrl), mTOR-deficient (mTOR−), and bezafibrate-treated control (ctrl + beza) and mTOR− (mTOR− + beza) soleus muscle. Data represent mean ± SEM (n ≥ 3 mice). (B) Relative mRNA levels of PPARα and PPAR δ, MCAD, LCAD, COX I and COX IV, citrate synthase and mCPT1 in the soleus muscle of indicated mice. Note that bezafibrate increases expression of most genes. Data represent mean ± SEM (n ≥ 3 mice). (C) Oxidative properties of hind leg muscle increase upon bezafibrate treatment. Pictures show representative cross-sections stained for SDH activity (dark blue). The soleus muscle is marked by an asterisk. (Scale bar, 100 μm.) (D) Quantification of SDH activity as detected by an enzymatic assay (for details see SI Materials and Methods). Data represent mean ± SD (n ≥ 5 mice). (E) H&E staining of cross-sections of soleus muscle. In the muscle of mTOR− and mTOR− + beza mice, some large (blue arrows) but also small fibers are present. Both groups of mice also show centralized nuclei (white arrows) and many mononuclear cells (green arrows). (Scale bar, 50 μm.) (F) Relative mRNA levels of the indicated proteins in soleus muscle as determined by qRT-PCR. Data represent mean ± SEM (n ≥ 3 mice). In all experiments shown, mice were 20-wk-old males. Significant differences between control and experimental groups are indicated by *; significant changes between mTOR− and mTOR− + beza mice are indicated by #. */#P < 0.05; **/##P < 0.01; ***/###P < 0.001.
Improved Mitochondrial Function Does Not Reverse Myopathy in mTOR− Mice.
Mice deficient for mTORC1 display muscle atrophy and progressive myopathy resulting in early death (3, 4). Thus, we tested whether treatment with bezafibrate would ameliorate the myopathy in the mTOR− mice. H&E staining of muscle cross-sections showed that the myopathy was still present after bezafibrate treatment (Fig. 1E). In addition, expression of dystrophin, which is reduced in mTOR− mice (3), was still low, and expression of two markers for muscle regeneration, myogenin and the perinatal form of myosin heavy-chain MyH8, were still increased (Fig. 1F). Moreover, bezafibrate decreased rather than increased muscle mass and total force in both fast- (tibialis anterior, TA) and slow-twitch (soleus) muscles (Table S1) of 140-d-old mTOR− mice. Finally, kyphosis in mTOR− mice was not prevented or delayed by bezafibrate (Fig. S1B).
Transgenic Overexpression of PGC-1α Restores Mitochondrial Function in RAmKO Mice.
Because our results show that bezafibrate improved mitochondrial function but not the myopathy in mTOR− mice, we next tested directly whether overexpression of PGC-1α would be beneficial for the mutant mice. Indeed, PGC-1α was shown to improve the muscular dystrophy in mdx mice, a mouse model for Duchenne muscular dystrophy (10), and has been implicated in preventing muscle atrophy upon denervation (15) and during aging (16). For our experiments, we used PGC-1α transgenic mice that overexpress the protein specifically in skeletal muscle using the muscle creatine kinase (MCK) promoter (17). These mice were mated with RAmKO mice (4), which have largely the same phenotype as mTOR− mice (3) but allow any contri-bution of mTORC2 to be ruled out. The resulting RAmKO mice that also are transgenic for PGC-1α (herein called “RAmKO-PGC1α-TG”) expressed increased levels of PGC-1α mainly in the fast-twitch extensor digitorum longus (EDL) muscle (Fig. 2A). Expression of PGC-1α in RAmKO-PGC1α-TG mice also was increased in the slow-twitch soleus muscle (Fig. S2A), although at considerably lower extent, consistent with much greater activity of the MCK promoter in fast- as compared with slow-twitch muscle fibers (17). Therefore we used the fast-twitch EDL and TA muscles in the subsequent analysis. Like the mRNA, the protein level of PGC-1α in EDL was lower in RAmKO and was strongly increased in PGC-1α-TG mice. Interestingly, despite the overexpression, PGC-1α protein levels were only doubled compared with controls (Fig. S2B and quantification in Fig. 2B).
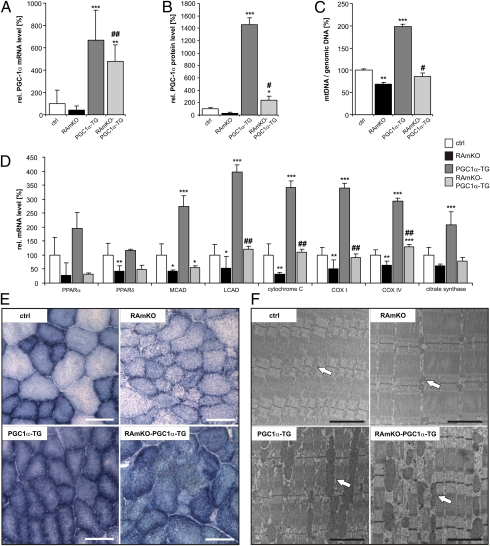
Transgenic expression of PGC-1α in RAmKO mice normalizes mitochondrial properties. (A) Relative mRNA levels of PGC-1α in EDL muscle of 90-d-old mice of different genotypes as determined by qRT-PCR (n ≥ 3 mice). (B) Quantification of PGC-1α protein levels. Western blot analysis was performed from lysates of EDL muscle from 80-d-old mice. Values represent average of gray values (n ≥ 3 mice; see also Fig. S2B). (C) Ratio between mtDNA and genomic DNA in EDL muscle of 140-d-old mice as determined by qRT-PCR (n ≥ 2 mice). (D) Relative mRNA levels of the indicated genes in EDL muscle of 90-d-old mice as determined by qRT-PCR (n ≥ 3 mice). Abbreviations are as described in the legend of Fig. 1. (E) Activity of oxidative enzymes examined by NADH-TR staining (blue precipitate) in TA muscle of 130-d-old mice. (Scale bar, 100 μm.) (F) Electron micrographs of longitudinal sections of EDL muscle of 140-d-old mice. (Scale bar, 200 nm.) Mitochondria are indicated by white arrows. Values in A–D represent mean ± SD. Significant differences between control and genetically modified mice are indicated by *; significant changes between RAmKO and RAmKO-PGC1α-TG mice are indicated by #. */#P < 0.05; **/##P < 0.01; ***/###P < 0.001.
To estimate whether PGC-1α affected mitochondria number in the mice, we used qRT-PCR to determine the copy number of mtDNA (D-loop region) relative to the amount of genomic DNA [using sequences for NADH dehydrogenase (ubiquinone) flavoprotein 1 (Ndufv1)] (18). As expected, the ratio between mtDNA and genomic DNA was decreased in RAmKO and increased in PGC1α-TG mice (Fig. 2C). Importantly, the ratio was restored to wild-type levels in RAmKO-PGC1α-TG mice (Fig. 2C). A restoration of mitochondrial function in RAmKO-PGC1α-TG mice also was indicated by the normalization of transcripts encoding mitochondrial proteins (Fig. 2D). These data thus show that in RAmKO-PGC1α-TG mice the expression and the function of PGC-1α are restored to control levels. The measurement of the oxidative property of skeletal muscle by NADH-tetrazolium reductase (NADH-TR) confirmed the improvement of mitochondrial function (Fig. 2E). Finally, examination of the muscle by electron microscopy showed that the shape and size of the mitochondria in RAmKO-PGC-1α-TG mice was similar to that in PGC-1α-TG mice and was clearly distinct from RAmKO mice (Fig. 2F). These results therefore are strong evidence that overexpression of PGC-1α is sufficient to restore mitochondrial function in RAmKO mice to that of control mice.
mTORC1 Regulates Glycogen Content Independently of PGC-1α Through PKB/Akt.
Recent evidence has implicated PGC-1α in the regulation of glucose metabolism in muscle (7). Because RAmKO and mTOR− mice have an approximately fivefold increase in glycogen in skeletal muscle (3, 4), we also investigated whether PGC-1α expression would affect this parameter. To do so, we stained muscle cross-sections with periodic acid-Schiff (PAS) and quantified the amount of glycogen by an enzymatic assay. As described previously (4, 7), the glycogen content was increased in RAmKO and PGC1α-TG mice (Fig. 3 A and B), but the increase was much more pronounced in RAmKO than in PGC1α-TG mice. Interestingly, the effect of PGC-1α overexpression and rptor deletion were additive (RAmKO-PGC1α-TG mice; Fig. 3B), suggesting that the two factors affect independent signaling pathways controlling glycogen storage. One mechanism responsible for the high levels of glycogen in RAmKO mice might be activation of PKB/Akt attested by the hyperphosphorylation of the T308 and S473 sites (Fig. 3C and quantification in Table S2). Surprisingly, overexpression of PGC-1α increased the total amount of PKB/Akt, but this increase did not affect phosphorylation at T308 or S473 (Fig. 3C and Table S2).

Glycogen content is increased in all genetically modified mouse models. (A) PAS staining of cross-sections of TA muscle of 90-d-old mice. The reaction product (magenta color) is indicative of the amount of glycogen in the tissue. (Scale bar, 50 μm.) (B) Quantification of the glycogen concentration in TA muscle from 90-d-old mice as determined by an enzymatic assay (for details see SI Materials and Methods). (C) Western blot analysis of EDL muscle from 80-d-old mice using antibodies directed against the proteins indicated. An equal amount of protein was loaded in each lane. An antibody against α-actinin was used as loading control. For quantification see Table S2. (D) Relative mRNA levels of glycogen phosphorylase in EDL muscle of 90-d-old mice as determined by qRT-PCR (n ≥ 3 mice). Values in B and D represent mean ± SD. Significant differences between control and genetically modified mice are indicated by *; significant changes between RAmKO and RAmKO-PGC1α-TG mice are indicated by #. */#P < 0.05; **/##P < 0.01; ***/###P < 0.001.
A well-described target of PKB/Akt is GSK3β, which inhibits glycogen synthase (19). As a consequence of increased PKB/Akt signaling, inhibitory phosphorylation of GSK3β was increased in mTORC1-deficient muscle (Fig. 3C and Table S2). Thus, inhibition of GSK3β leads to higher synthesis of glycogen in the mutant mice. It has been suggested that the increase in glycogen stores in the PGC1α-TG mice is caused by a down-regulation of glycogen phosphorylase (7). Indeed, the levels of glycogen phosphorylase were lower at the mRNA and protein level in PGC1α-TG mice (Fig. 3 C and D and Table S2). Strikingly, the same down-regulation of glycogen phosphorylase also was seen in RAmKO mice although the levels of PGC-1α are low in those mice (Fig. 3 C and D and Table S2). In summary, these data indicate that hyperactivation of PKB/Akt is sufficient to regulate both glycogen synthesis and degradation independently of PGC-1α.
Increased PGC-1α Expression Does Not Prevent Myopathy of RAmKO Mice.
To exclude the possibility that the failure of bezafibrate to ameliorate the myopathy in mTOR− mice resulted from insufficient activation of PGC-1α, we also examined whether muscle mass and integrity were restored in the RAmKO-PGC1α-TG mice. However, the loss in muscle mass of the RAmKO mice was not prevented (Table S3), nor was the significant reduction of the whole-body weight (Fig. 4A). Moreover, H&E staining of muscle cross-sections of RAmKO-PGC1α-TG mice detected clear signs of myopathy (Fig. 4B). For example, the muscles contained more small fibers (blue arrows in Fig. 4B), as reflected in the doubling of the number of fibers that have a mean fiber feret between 20 and 30 μm in RAmKO and RAmKO-PGC1α-TG mice compared with controls (Fig. 4C). Additionally, mononuclear cells were found (green arrows in Fig. 4B), as were centralized nuclei in several muscle fibers (white arrows in Fig. 4B and quantification in Fig. 4D). Expression of PGC-1α also did not affect muscle force in RAmKO mice (Table S4). There also was no significant improvement in the physical activity of the RAmKO mice by the overexpression of PGC-1α (Fig. 4E), and the mice still developed a kyphosis (Fig. 4F). In fact, we did not detect a significant change in the mean age of onset of the kyphosis (RAmKO: 15 ± 2 wk; RAmKO-PGC1α-TG: 15 ± 3 wk; mean ± SD, n = 7 for each genotype). Finally, the age at which the disease became so severe that the mice needed to be killed was similar for RAmKO and RAmKO-PGC1α-TG mice. These results show that an increase in PGC-1α levels, despite the restoration of mitochondrial function and number, fails to rescue the myopathy of RAmKO mice.
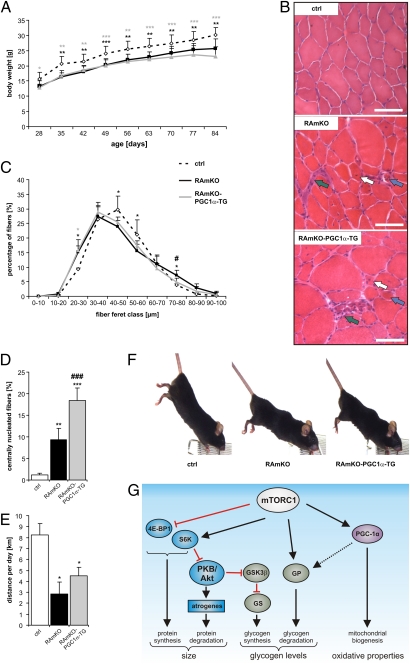
Increased PGC-1α levels do not prevent progressive myopathy in RAmKO mice. (A) Mice of each genotype were weighed every week (n ≥ 5 mice). (B) H&E staining of cross-sections of TA muscle of 130-d-old mice. In the muscle of RAmKO and RAmKO-PGC1α-TG mice some large (blue arrows) but also small fibers are present. Both genotypes also show centralized nuclei (white arrows) and many mononuclear cells (green arrows). (C) Distribution of fiber size in the TA muscle of 14-d-old mice (n = 2 for control; n = 4 for RAmKO; n = 5 for RAmKO-PGC-1α-TG mice). (D) Percentage of muscle fibers with centralized nuclei in TA muscle of 90-d-old mice (n ≥ 5 mice). All fibers (~2,000) of one midbelly cross-section were analyzed in experiments shown in C and D. (E) Average distance run voluntarily per day between the ages of 95 d and 105 d (n ≥ 3 mice). (F) Photographs of 140-d-old littermates. The RAmKO and RAmKO-PGC1α-TG mice are leaner and suffer from a kyphosis. (G) Proposed model for the contribution of different signaling pathways to the disease phenotype in mTORC1-deficient skeletal muscle. The balance between protein synthesis [via eIF4E-Binding Protein 1 (4E-BP1) and S6 kinase (S6K)] and protein degradation contributes to muscle size (Left). The balance between glycogen synthesis [via PKB/Akt; GSK3β, and glycogen synthase (GS)] and glycogen degradation [via glycogen phosphorylase (GP)] regulates glycogen levels (Center). PGC-1α has only a minor contribution to the glycogen levels but is the main regulator of the oxidative properties (Right). Values in A, C, D, and E represent mean ± SD. Significant differences between control and genetically modified mice are indicated by *; significant changes between RAmKO and RAmKO-PGC1α-TG mice are indicated by #. */#P < 0.05; **/##P < 0.01; ***/###P < 0.001.
Discussion
This work provides direct evidence that the mitochondrial deficits in mTOR− and RAmKO mice are the consequence of decreased expression of PGC-1α (Fig. 4G). Furthermore, our study shows that both the high glycogen content and the severe myopathy observed in mTORC1-deficient mice are not strongly affected by restoring PGC-1α function (Fig. 4G). We chose to examine this question by using (i) bezafibrate, because it reaches all muscles, and (ii) mice transgenic for PGC-1α (17), where the transgene is driven by the MCK promoter that is expressed mainly in fast-twitch muscle fibers. Bezafibrate is used in clinical trials and was used successfully in the Δcox10 mouse model to delay the onset of the mitochondrial myopathy (14). The PGC-1α–mediated rescue of mitochondrial function in mTORC1-deficient muscles, characterized by a normalization of the mtDNA content, increased levels of key mitochondrial enzymes and of tissue oxidative capacity. However, despite the normalization of the oxidative capacity in RAmKO-PGC1α-TG mice, overall running activity was still low, similar to that of RAmKO mice. This result suggests that the myopathic pathology, but not the metabolic changes in RAmKO muscle, prevents the mice from running as far as control littermates.
mTORC1 influences the expression of PGC-1α and therefore mitochondrial biogenesis through formation of a complex with PGC-1α and the transcription factor YY1. Inhibition of mTORC1 disrupts the interaction between YY1 and PGC-1α, thereby decreasing the expression of mitochondrial target genes including PGC-1α itself (8). Interestingly, our data revealed that the function of PGC-1α in driving mitochondrial biogenesis does not require functional mTORC1. However, we noted that the expression levels of PGC-1α and its downstream targets, such as MCAD, LCAD, COX I and IV, and cytochrome c, were consistently lower in RAmKO-PGC1α-TG than in PGC1α-TG mice. Our data indicate that both mRNA and protein expression of PGC-1α are less efficient in mTORC1-deficient than in wild-type muscle, because the levels always were much lower in RAmKO-PGC1α-TG than in PGC1α-TG mice. Another reason for the lower activity of PGC-1α in mTORC1-deficient muscle could be the hyperactivation of PKB/Akt, which was shown to inhibit PGC-1α directly by phosphorylation in liver (20).
The amount of glycogen stored in the muscles was 2–15 times higher in all the genetically modified mice studied than in controls. We also found that PGC-1α-TG and RAmKO-PGC1α-TG mice had increased levels of glycogen compared with their littermate controls, a finding that is in agreement with the finding that PGC-1α inhibits the transcription of glycogen phosphorylase (7). However, in the RAmKO mice, glycogen phosphorylase levels are low despite the low levels of PGC-1α. Most likely, these low levels are the result of the strong inhibitory effect by activated PKB/Akt, as demonstrated in hepatocytes (21). In addition, our data also show that hyperactivation of PKB/Akt in RAmKO mice is sufficient to overrule the effect of PGC-1α on glycogen phosphorylase. Activated PKB/Akt also regulates glycogen synthase through inhibition of GSK3β (19). Thus, the pronounced glycogen accumulation in muscles of mTORC1-deficient mice probably is based on the simultaneous stimulation of glycogen synthesis and inhibition of glycogen degradation by PKB/Akt (Fig. 4G).
Finally, our data show that increased levels of PGC-1α and improved mitochondrial function are not sufficient to reverse any aspects of the severe myopathy in mTORC1-deficient mice. It has been reported that PGC-1α counteracts denervation-induced atrophy and age-related muscle loss (15, 16). However, we could not observe a beneficial effect of higher PGC-1α levels on muscle mass in the atrophic mTORC1-deficient mice. Of course, PGC-1α may ameliorate only certain types of muscle pathologies. In addition, although PGC-1α is increased in RAmKO-PGC1α-TG mice, the disruption of the mTORC1/YY1/PGC-1α complex may prevent the restoration of muscle in the mTORC1-deficient mice.
Because restoration of mitochondrial function does not affect the myopathy in mTORC1-deficient muscle, a possible scenario is that alterations in the balance between protein synthesis and protein degradation are responsible for this pathology. Such changes might involve proteasomal and autophagic protein degradation and overall protein synthesis (Fig. 4G). Indeed, recent evidence shows that such a shift in the catabolic–anabolic balance can result in muscle pathology. For example, inactivation of autophagy directly or by chronic hyperactivation of PKB/Akt results in severe muscle phenotypes that cause the death of the animals at the age of approximately 1 y (22, 23). Thus, a change in the homeostasis between protein synthesis and degradation as observed in RAmKO and mTOR− mice is likely to affect the health of skeletal muscle.
In summary, our data show that mTORC1 regulates mitochondrial activity through PGC-1α and regulates glycogen levels through PKB/Akt. Intriguingly, the lethal myopathy resulting from the loss of mTORC1 is independent of mitochondrial activity and the PGC-1α pathway.
Materials and Methods
Mice.
RAmKO, mTOR−, and PGC1α-TG mice have been described previously (3, 4, 17). The RAmKO-PGC1α-TG mice were generated by crossing RAmKO with PGC1α-TG mice. Mice were maintained in a conventional facility with a fixed light–dark cycle. Body weight was measured weekly. Moribund mice were killed before natural death. Studies were carried out according to criteria outlined for the care and use of laboratory animals and with approval of the Swiss (for experiments with RAmKO, PGC1α-TG, and RAmKO-PGC1α-TG mice) and French (for experiments with mTOR− mice) authorities, respectively.
Acknowledgments
We thank Arnaud Ferry from the Université Pierre et Marie Curie-Paris for measurement of muscle force of the mTOR− mice. We are indebted to the animal (Le Plateau de Biologie Expérimental de la Souris) and microscopy (Plateau Technique Imagerie/Microscopie) facilities of the Institut Fédératif de Recherche 128 Biosciences. This work was supported by grants from the Cantons of Basel-Stadt and Baselland, the Swiss Foundation for Research on Muscle Disease, and Swiss Life (to M.A.R.) and from the Association Française Contres les Myopathies (to M.A.R., L.S., and Y.-G.G.) and the Agence Nationale de la Recherche (to L.S. and Y.-G.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/10.1073/pnas.1111448109/-/DCSupplemental.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.1111448109
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/108/51/20808.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.1111448109
Article citations
mTORC1 in energy expenditure: consequences for obesity.
Nat Rev Endocrinol, 20(4):239-251, 15 Jan 2024
Cited by: 1 article | PMID: 38225400
Review
Adult skeletal muscle peroxisome proliferator-activated receptor γ -related coactivator 1 is involved in maintaining mitochondrial content.
Am J Physiol Regul Integr Comp Physiol, 324(4):R470-R479, 30 Jan 2023
Cited by: 2 articles | PMID: 36717166 | PMCID: PMC10026983
Thyroid hormone regulates glutamine metabolism and anaplerotic fluxes by inducing mitochondrial glutamate aminotransferase GPT2.
Cell Rep, 38(8):110409, 01 Feb 2022
Cited by: 7 articles | PMID: 35196498 | PMCID: PMC8889437
AKT controls protein synthesis and oxidative metabolism via combined mTORC1 and FOXO1 signalling to govern muscle physiology.
J Cachexia Sarcopenia Muscle, 13(1):495-514, 09 Nov 2021
Cited by: 24 articles | PMID: 34751006 | PMCID: PMC8818654
Berberine attenuates fructose-induced insulin resistance by stimulating the hepatic LKB1/AMPK/PGC1α pathway in mice.
Pharm Biol, 58(1):385-392, 01 Dec 2020
Cited by: 8 articles | PMID: 32393087 | PMCID: PMC7269079
Go to all (30) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy.
Cell Metab, 8(5):411-424, 01 Nov 2008
Cited by: 440 articles | PMID: 19046572
Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake.
PLoS One, 6(12):e28290, 08 Dec 2011
Cited by: 100 articles | PMID: 22174785 | PMCID: PMC3234261
mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex.
Nature, 450(7170):736-740, 01 Nov 2007
Cited by: 929 articles | PMID: 18046414
PGC-1 coactivators and skeletal muscle adaptations in health and disease.
Curr Opin Genet Dev, 18(5):426-434, 07 Sep 2008
Cited by: 155 articles | PMID: 18782618 | PMCID: PMC2629557
Review Free full text in Europe PMC





