Abstract
Free full text

PROLIFERATIVE CAPACITY OF CORNEAL ENDOTHELIAL CELLS
Abstract
The corneal endothelial monolayer helps maintain corneal transparency through its barrier and ionic “pump” functions. This transparency function can become compromised, resulting in a critical loss in endothelial cell density (ECD), corneal edema, bullous keratopathy, and loss of visual acuity. Although penetrating keratoplasty and various forms of endothelial keratoplasty are capable of restoring corneal clarity, they can also have complications requiring re-grafting or other treatments. With the increasing worldwide shortage of donor corneas to be used for keratoplasty, there is a greater need to find new therapies to restore corneal clarity that is lost due to endothelial dysfunction. As a result, researchers have been exploring alternative approaches that could result in the in vivo induction of transient corneal endothelial cell division or the in vitro expansion of healthy endothelial cells for corneal bioengineering as treatments to increase ECD and restore visual acuity. This review presents current information regarding the ability of human corneal endothelial cells (HCEC) to divide as a basis for the development of new therapies. Information will be presented on the positive and negative regulation of the cell cycle as background for the studies to be discussed. Results of studies exploring the proliferative capacity of HCEC will be presented and specific conditions that affect the ability of HCEC to divide will be discussed. Methods that have been tested to induce transient proliferation of HCEC will also be presented. This review will discuss the effect of donor age and endothelial topography on relative proliferative capacity of HCEC, as well as explore the role of nuclear oxidative DNA damage in decreasing the relative proliferative capacity of HCEC. Finally, potential new research directions will be discussed that could take advantage of and/or improve the proliferative capacity of these physiologically important cells in order to develop new treatments to restore corneal clarity.
1. Introduction
The corneal endothelium helps maintain corneal transparency via its barrier and ionic “pump” functions. To maintain transparency, endothelial cell density (ECD) must remain above a critical number—usually 400-500 cells/mm2. Morphometric analyses of ECD in fetal and adult endothelium (Murphy et al. 1984; Bourne et al. 1997; Hollingsworth et al. 2001) indicate that, following formation of the endothelial monolayer during corneal development, human corneal endothelial cells (HCEC) do not normally divide in vivo at a rate sufficient to replace dead or injured cells. This results in an average cell loss of 0.3 – 0.6% per year. The response of the endothelium to this gradual cell loss, as well as to larger wounds, normally involves spreading and/or migration of neighboring cells to cover the wound area (Laing et al. 1976; Honda et al. 1982; Matsuda et al. 1985). The result of this form of wound healing is an increase in overall cell size and an alteration from a hexagonal to a pleomorphic shape. Unfortunately, ECD can be significantly decreased as the result of accidental or surgical trauma, refractive surgery, previous penetrating or endothelial keratoplasty, stress caused by certain diseases such as diabetes or glaucoma, or endothelial dystrophies. If the density of endothelial cells is too low, barrier function is lost and more fluid enters the cornea than can be removed through the activity of the ionic “pumps”. Loss of endothelial barrier function results in corneal edema, development of bullous keratopathy, and loss of visual acuity. Current treatments, such as penetrating or endothelial keratoplasty to restore visual acuity generally work well, but can have complications requiring re-grafting or other treatments (Rahman et al. 2010; Lass et al. 2010; Terry et al. 2008; Clements et al. 2011; Shulman et al. 2009). In addition, there is an increasing worldwide shortage of donor corneas that are considered acceptable for transplant purposes and the aging of the “baby boomer” generation will bring a greater need to find new therapies to restore corneal clarity that is lost due to endothelial dysfunction. One approach to develop new therapies to prevent or treat excessive corneal endothelial cell loss is to explore the relative ability of HCEC to divide. This review will present information regarding the positive and negative regulation of the cell cycle and discuss results of studies exploring the proliferative capacity of HCEC.
2. The Cell Cycle
Figure 1 presents a simplified diagram of the positive and negative regulation of the cell cycle. Additional information related to the cell cycle, but not emphasized here, can be found in recent reviews (Ozaki and Nakagawara, 2011; Kim et al. 2011; Ma and Poon, 2011; and Rieder, 2011). Non-dividing cells normally exist in a “resting” (G0) state in which DNA is present in an unduplicated (2N) form. Mitogenic stimulation induces G1-phase entry, which prepares cells for DNA duplication in S-phase. Movement of cells from G1- into S-phase is highly regulated and involves control of the activity of the E2F transcription factor, which activates genes required for DNA synthesis (Leone et al. 1999). In quiescent cells, E2F is tightly associated with the retinoblastoma tumor suppressor, pRb, which prevents its activation. To inhibit E2F activity, pRb must be in a hypo-phosphorylated state. Hypo-phosphorylation of pRb is maintained, in part, by the activity of the cyclin-dependent kinase inhibitors (CKIs), p27Kip1, p21Cip1, and p16INK4a. Mitogenic stimulation induces a reduction in the protein level of these CKIs due to transcriptional inhibition and/or to increased degradation by the ubiquitin-proteasome pathway. Mitogenic stimulation also induces synthesis of the positive G1-phase regulatory protein, cyclin D (Sherr, 1993), which binds to cyclin-dependent kinase (CDK)-4, forming an active kinase complex that specifically phosphorylates pRb. This hyper-phosphorylation alters the interaction of pRb with E2F, promoting activation of E2F and leading to S-phase entry. Cyclin E is synthesized late in G1-phase upon E2F activation. Cyclin E binding to CDK2 helps activate this kinase complex and, in part, promotes continued hyper-phosphorylation of pRb and movement into S-phase. In S-phase, DNA is duplicated under highly controlled conditions, moving DNA from the 2N to a 4N state. Cyclin A synthesis begins in late G1-phase. Cyclin A binds to CDK2 and the activity of this complex down-regulates E2F activity by facilitating its degradation, thus promoting forward progression from S- to G2-phase. Cyclin B synthesis is activated at the end of S-phase. In G2-phase, cyclin B binds to and activates the kinase activity of CDK1, which prepares the cell for M-phase (mitosis), in which cells divide, forming daughter cells, each of which contains 2N DNA.
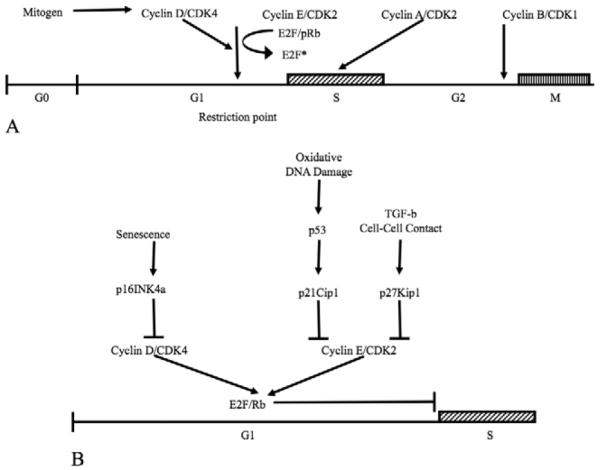
Diagrams illustrating the positive (A) and negative (B) regulation of the cell cycle. Springer-Verlag is the original copyright holder.
Negative regulation of G1-phase involves inhibition of the kinase activity of the G1-phase cyclin/CDK complexes by CKIs. There are two CKI families. The “INK” family includes p16INK4a, which specifically binds to free CDK4 and prevents its association with cyclin D to form an active complex (Serrano et al., 1993). p16INK4a also competes with cyclin D for binding to CDK4 in existing complexes, thus dissociating the complex. Inhibition of cyclin D/CDK4 kinase activity by p16INK4a prevents the initial downstream hyper-phosphorylation of pRb that is required for E2F activation and S-phase entry. The “Cip/Kip” family includes p21Cip1 and p27Kip1 (Harper et al., 1993; Polyak et al., 1994). Both these inhibitors bind G1-phase cyclin/CDK complexes, inhibiting their kinase activity. In the presence of p16INK4a, the “Cip/Kip” proteins mainly bind and inhibit the activity of cyclin E/CDK2 complexes. Synthesis of p21Cip1 is induced by the transcription factor, p53, which can be activated by a number of factors, including oxidative DNA damage (Helton and Chen, 2007). Transforming growth factor-β (TGF-β) and formation of mature cell-cell contacts (Polyak et al., 1994) increase the cellular level of p27Kip1. The inhibitory function of all the G1-phase CKIs is extremely important, because it prevents unscheduled entry into S-phase and inappropriate DNA synthesis.
3. Cell Cycle Status of HCEC In Vivo
To understand why HCEC do not proliferate in vivo, this laboratory conducted studies to determine the cell cycle status of endothelial cells in ex vivo donor human corneas (Joyce et al., 1996a; Joyce et al., 1996b). This was accomplished by observing the relative staining intensity and subcellular localization of a battery of key cell cycle proteins in transverse corneal sections using immunofluorescence microscopy. Together, results strongly suggest that HCEC in vivo are arrested in G1-phase of the cell cycle. Several mechanisms have been identified that contribute to the maintenance of G1-phase arrest in HCEC in vivo. These mechanisms will be discussed below.
3.1. Cell-Cell Contact-Dependent Inhibition
Studies (Wulle and Lerche, 1969; Wulle, 1972; Hay, 1980) indicate that corneal endothelium is formed from neural crest-derived mesenchymal cells located at the periphery of the presumptive cornea. During eye development, these cells both proliferate and migrate centrally to form the endothelial monolayer (Nuttall, 1976). Analysis of transmission electron micrographs indicates that proliferation of the presumptive endothelial cells ceases upon formation of cell-cell contacts (Wulle and Lerche, 1969; Wulle, 1972). This same mechanism also plays a role in inhibition of proliferation in the mature endothelium. Studies of the role of cell-cell contacts in the inhibition of proliferation were conducted in this laboratory using corneas obtained from neonatal rats (Joyce et al., 1998), since maturation of rat corneal endothelial cells (RCEC) does not take place until after birth. Results indicated a correlation between the time at which endothelial cell division ceased and the time that mature cell-cell contacts were formed. This timing also correlated with an increase in the expression of p27Kip1 protein, implicating a role for this CKI in mediating contact inhibition in corneal endothelium. Western blot studies showed that the protein level of p27Kip1 in confluent, contact-inhibited cultures of RCEC was 20-fold higher than in subconfluent cultures. When confluent cultures were treated with ethylenediamine tetraacetic acid (EDTA) to release cell-cell contacts, the level of p27Kip1 was greatly reduced, providing additional evidence that p27Kip1 plays a role in mediating contact inhibition in these cells (Joyce et al., 2002). Similar results have been obtained in studies of developing mouse corneal endothelium (Yoshida et al., 2004) and in HCEC. In ex vivo human corneas, release of endothelial cell-cell contacts by mechanical wounding (Senoo and Joyce, 2000) or by treatment with EDTA (Senoo et al., 2000) promoted cell division upon exposure to mitogens.
3.2. Lack of Effective Growth Factor Stimulation
In vivo, cell division does not appear to occur in HCEC following wounding, even though cell-cell contacts would have been disrupted. This suggests that there may not be sufficient paracrine or autocrine growth factor stimulation to induce cells to divide. Low levels of positive growth factors have been detected in aqueous humor from normal eyes. These include acidic and basic fibroblast growth factor (FGF) (Schulz et al., 1993), insulin-like growth factor-I and –II (Arnold et al., 1993), and hepatocyte growth factor (HGF) (Araki-Sasaki et al., 1997). Descemet’s membrane, the thick extracellular matrix secreted by corneal endothelial cells, appears to bind a number of growth factors (Gospodarowicz et al., 1980; Blake et al., 1997). In addition, corneal endothelial cells themselves appear to synthesize a number of growth factors and their receptors, including epidermal growth factor (EGF) and its receptor, acidic and basic FGF and their receptors, TGF-β1, TGF-α, HGF and its receptor, keratinocyte growth factor and its receptor (Wilson and Lloyd, 1991; Wilson et al., 1993a, 1993b, 1994), and platelet-derived growth factor receptor (Hoppenreijs, et al., 1993). Although endothelial cells may have access to growth factors, it is possible that they are not present in sufficient concentration, are in inactive forms, or do not bind effectively enough to HCEC receptors to induce and/or sustain a positive mitogenic signal in injured endothelium.
3.3. TGF-β2 Suppression of S-Phase Entry
TGF-β2 is present mainly in latent form in aqueous humor; however, HCEC express proteins, such as thrombospondin-1 (Hiscott et al, 1997), which activate latent TGF-β2 (Schultz-Cherry et al., 1994). HCEC also express mRNA and protein for TGF-β receptors-I, II, and III (Joyce and Zieske, 1997)—all three of which are needed for optimal TGF-β-induced signal transduction. Studies in cultured rabbit (Harris and Joyce, 1999) and rat corneal endothelial cells (Chen et al., 1999) indicate that exogenous active TGF-β2 and activated TGF-β2 from aqueous humor suppress S-phase entry. In rabbit corneal endothelial cells, TGF-β2 down-regulates the expression of CDK4 and prevents nuclear export of p27Kip1 for degradation, thus maintaining a high level of this G1-phase inhibitory protein (Kim et al., 2001). Evidence also suggests that the TGF-β2 suppressive effect may be due to its stimulation of the synthesis and secretion of prostaglandin E2, which is capable of inhibiting rabbit corneal endothelial cell proliferation in a dose-dependent manner (Chen et al., 2003).
4. Efforts to Stimulate Corneal Endothelial Proliferation
A number of methods have been tested to take advantage of the existing proliferative capacity of HCEC as a means of increasing ECD. These will be discussed in more detail below.
4.1. Release of Cell-Cell Contacts and Growth Factor Stimulation
HCEC in ex vivo corneas and in culture are able to enter and complete the cell cycle upon release of cell-cell contacts in the presence of mitogens. Figure 2A shows that nuclei of HCEC in the wounded area of ex vivo corneas stain positively for Ki67, a marker of actively cycling cells (Gerdes et al., 1983), whereas there is no positive staining in cells more peripheral to the wound. HCEC can also be isolated from donor corneas, successfully grown in culture, and passaged a limited number of times (Baum et al., 1979; Engelmann and Friedl, 1989; Chen et al., 2001; Li et al., 2007). Figure 2B shows a subconfluent culture of HCEC with nuclei stained positively for Ki67, indicating that cells are actively proliferating. This data not only provides evidence that HCEC retain proliferative capacity, but also suggests potential methods by which donor corneas with low ECD could be treated to increase cell numbers prior to transplantation.
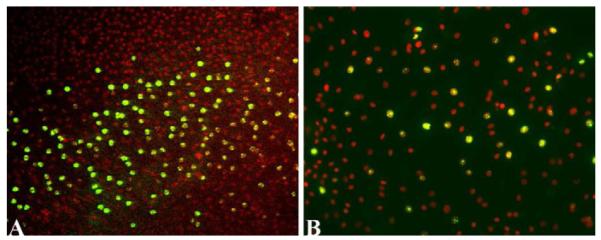
Evidence that HCEC in the wound area of ex vivo corneas (A) and in culture (B) can actively proliferate. Endothelium in the donor cornea in (A) was scrape-wounded, incubated in culture medium, immunostained for Ki67 (green) and counterstained with DAPI (red) to detect nuclei. Nuclei within the wound area are positively stained for Ki67, whereas nuclei in cells in the unwounded part of the endothelial monolayer are not stained for Ki67. HCEC cultured from a 67-year old donor show positive Ki67 staining. Orig. mag.= 16X (A) and 20X (B). Lippincott Williams & Wilkins is the original copyright holder of the image in (B).
4.2. Sustaining Growth Factor-Induced Stimulation of Proliferation
Stimulation of proliferation by EGF involves binding of EGF to its receptor and the subsequent autophosphorylation of specific tyrosine residues in the receptor intracellular domain (Wells, 1999). This autophosphorylation initiates a signaling cascade that leads to cell cycle entry (Marshall, 1995). The protein tyrosine phosphatase, PTP1B, interacts with and dephosphorylates a number of growth factor receptors, including EGF, thereby attenuating EGF-induced downstream signaling (Haj et al., 2003). Studies conducted in both RCEC (Harris and Joyce, 2007) and HCEC (Ishino et al., 2008) have demonstrated that specific inhibition of PTP1B activity was able to sustain EGF-induced tyrosine autophosphorylation of the EGF receptor and increase the number of cells entering the cell cycle, indicating the possibility that the number of proliferating HCEC could be increased by suppressing the down-regulation of growth factor signaling.
4.3. Overcoming G1-Phase Inhibition
Studies have been conducted to test whether HCEC can be induced to divide by overcoming G1-phase inhibition. Ectopic expression of either the SV40 large-T antigen (Wilson et al., 1993; Aboalchamat et al., 1999) or the human papilloma virus type 16 oncoproteins, E6/E7 (Wilson et al., 1995) in cultured HCEC results in multiple rounds of cell division. Although these treatments have been successful in inducing proliferation of HCEC, questions remain regarding their long-term safety as a treatment to increase ECD. Another possible method to overcome G1-phase inhibition is to lower the level of one or more cyclin-dependent kinase inhibitors, thereby removing the constraint to S-phase entry. Studies were conducted to determine whether treatment of HCEC with p27Kip1 siRNA would promote proliferation (Kikuchi et al., 2006). Interestingly, siRNA-induced reduction of p27Kip1 protein levels only promoted a significant increase in cell numbers in HCEC cultured from young donors. SiRNA-induced reduction of the protein levels of both p21Cip1 and p16INK4a increased the number of cells entering the cell cycle, as well as total cell numbers in HCEC cultured from both young (< 30 years old) and older donors (>50 years old) (Joyce and Harris, 2010). Together, results of these studies indicate that it is possible to overcome G1-phase inhibition and promote HCEC division by reducing the expression of CKIs. The results also suggest that there is a difference in the relative response of HCEC to these treatments depending on donor age. (See further discussion below.)
4.4. Bypassing G1-Phase Inhibition
Studies have tested the effect of ectopically expressing E2F2 in rabbit corneal endothelium (Joyce et al., 2004) and in HCEC in ex vivo corneas (McAlister et al., 2005). E2F2 is one of the isoforms of E2F that is responsible for activating genes necessary for S-phase entry (DeGregori et al. 1997). For studies in HCEC, the endothelium of ex vivo corneas from both young and older donors was transfected with an adenoviral vector containing the full-length gene for E2F2. Ectopic expression of E2F2 was able to induce S-phase entry, as determined by immunostaining for bromodeoxyuridine, a recognized marker of DNA synthesis. The effect of E2F2 expression on cell division was demonstrated by a significant increase in ECD compared with vector controls. Together, these studies indicate that it is possible to stimulate division in HCEC by bypassing G1-phase inhibition.
5. Effect of Donor Age on HCEC Proliferative Capacity
Of importance is the fact that, although HCEC retain an ability to divide, their relative proliferative capacity is affected by donor age. Baum, et al. (1979) first described age-related differences in the proliferation of cultured HCEC. In their studies, cells from donors less than 20 years old grew well in culture, whereas, cells from older donors were either difficult to grow or did not grow at all. Results of those early studies have been confirmed and expanded using ex vivo cornea and tissue culture models. The kinetics of cell cycle traverse in HCEC from young (<30 years old) and older donors (>50 years old) were first compared using an ex vivo corneal endothelial scrape wound (Senoo and Joyce, 2000). Semi-quantitative analysis of immunostaining for Ki67 in the wound area revealed a significant decrease in the rate of cell cycle entry and in the relative number of dividing HCEC in corneas from older compared with younger donors (Figure 3A). Direct counting of cultured HCEC (Joyce, 2005; Zhu and Joyce, 2004) showed very similar age-related changes in proliferative capacity to those observed using the ex vivo cornea wound model (Figure 3B). Calculations from the log phase of growth indicate that the average doubling time for HCEC cultured from older donors was 90.25 hours compared with 46.25 hours for cells cultured from young donors (Joyce and Zhu, 2004).
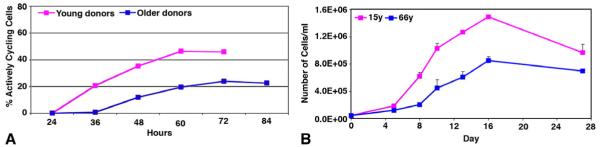
Growth curves demonstrating age-related differences in relative proliferative capacity in HCEC in an ex vivo cornea scrape wound (A) and in culture (B). Graph in (A) presents the average percent of actively cycling cells in the wound area from at least 3 corneas per age-group. Graph in (B) presents direct cell counts at various times after initiation of the culture. Adapted from Joyce. 2005. Exp. Eye Res. 81: 629-638. Springer-Verlag is the original copyright holder of this image.
5.1. Age-Related Changes in the Positive Regulation of G1-Phase of the Cell Cycle
As indicated above, mitogenic stimulation leads to the hyper-phosphorylation of pRb by specific G1-phase cyclin/CDK complexes, resulting in the activation of E2F and the subsequent increased expression of proteins required for S-phase entry. Semi-quantitative analysis (Enomoto et al., 2006) of Western blots of HCEC cultured from young (<30 years old) and older donors (>50 years old) indicated that the overall expression of pRb is very similar in HCEC, regardless of donor age. The same blots indicated that the kinetics of pRb hyper-phosphorylation were much slower and the relative amount of hyper-phosphorylated pRb was significantly lower in HCEC cultured from older compared with younger donors.
Formation of origin-recognition complexes on DNA is required for S-phase entry. These complexes associate with DNA during G1-phase and are present at sites on chromatin that are at or near future sites of initiation of DNA replication. Binding of these complexes to DNA makes chromatin competent for replication (Stoeber et al., 2001). Minichromosome maintenance-2 (MCM2) protein is a member of the origin-recognition complex and its expression is a reliable marker to identify replication-competent cells (Wharton et al., 2001). Studies using the ex vivo cornea wound model compared the relative number of replication-competent HCEC in corneas obtained from young (<30 years old) and older donors (>50 years old) (Mimura and Joyce, 2006) by counting cells immunostained for MCM2. The percentage of MCM2-positive cells in the endothelium of corneas from older donors was significantly less than in the endothelium of younger corneas, indicating that more HCEC from young donors were competent to replicate. In addition, the relative time at which cells became competent to replicate occurred sooner after mitogenic stimulation in HCEC from young compared with older donors.
5.2. Age-Related Differences in the Expression of G1-Phase Inhibitors
The above data provide strong evidence that HCEC from older donors are slower to respond to mitogenic stimulation and exhibit an overall reduced proliferative capacity compared with HCEC from young donors. Since p27Kip1, p21Cip1, and p16INK4a are important negative regulators of G1-phase of the cell cycle, Western blot studies were conducted to compare the relative expression of these proteins in primary cultures of HCEC from young (<30 years old) and older donors (>50 years old) (Enomoto, et al., 2006). Results of the semi-quantitative analysis indicated that the relative expression of p27Kip1 does not differ significantly between age-groups; however, a statistically significant increase in the expression of both p16INK4a and p21Cip1 was detected in HCEC cultured from older donors. Together, results from these studies strongly suggest that p27Kip1, which plays an important role in mediating contact inhibition, does not significantly contribute to the age-related difference in relative proliferative capacity observed in HCEC. On the other hand, the significant increase in p21Cip1 and p16INK4a protein levels in HCEC from older donors provides evidence that both these CKIs help mediate the observed age-related decrease in proliferative capacity.
6. Effect of Endothelial Topography on HCEC Proliferative Capacity
The proliferative capacity of HCEC also differs with the position of cells within the endothelial monolayer. Bednarz, et al. (1998) found that HCEC cultured from the central 6.5 mm diameter of the endothelium had little-to-no mitogenic activity, whereas, cells cultured from the 6.5 – 9.0 mm peripheral rim exhibited greater mitogenic activity. Studies from this laboratory further explored the effect of endothelial topography on the replicative competence of HCEC using an ex vivo cornea wound model (Mimura and Joyce, 2006). The endothelium was divided into two topographic areas: a 6.0 mm diameter central area and a 6.0 mm-to-9.5 mm peripheral rim. A mechanical scrape wound was made across the endothelium, including both the center and the peripheral rim. Corneas were incubated in mitogen-containing medium, immunostained for MCM2, and the number of MCM2-positive cells counted. Results showed that the number of MCM2-positive cells, and therefore the number of cells competent to replicate, was consistently lower in central endothelium compared with the peripheral rim in both age-groups. Interestingly, the relative percent of replication-competent cells was significantly lower in the central area of older donors compared with young donors. Results from other laboratories have confirmed the existence of topographical differences related to the relative ability of HCEC to divide (Paull and Whikehart, 2005; Yamagami et al., 2007; Patel and Bourne, 2009).
General characteristics of senescent cells include decreased saturation density, slower cell cycle kinetics, stable arrest with a 2N DNA content, and increased expression of p21Cip1 and p16INK4a protein (Christofalo, 1988; Campisi, 1996). Staining for beta-galactosidase (SA-β-Gal) at pH 6.0 is a marker of cellular senescence (Dimri et al., 1995). Since HCEC from older donors exhibit a number of senescence-like characteristics, studies were conducted to identify senescent cells in ex vivo corneas by staining for SA-β-Gal (Mimura and Joyce, 2006). Scoring of the endothelium for the relative intensity of SA-β-Gal staining showed few-to-no senescent cells in either the central or peripheral area of corneas from young donors. In corneas from older donors, SA-β-Gal staining was detectable at low-to-moderate levels in the periphery, but moderate-to-intense levels were observed in cells within the central area, indicating a greater percentage of senescent cells in central endothelium of older donors. Similar age-related differences in SA-β-Gal staining in HCEC from young and older donors were observed in studies by Song et al. (2008).
Comparative studies using senescence-resistant and senescence-prone strains of mice (Xiao et al., 2009) have indicated that the signaling pathway of the G1-phase inhibitor, p16INK4a, may play a key role in the early stages of senescence in corneal endothelial cells, while the signaling pathway involving the p53 transcription factor and its downstream activation of p21Cip1 may be active in the later stages of senescence in these cells. These results generally correlate with findings discussed above indicating an up-regulation in the expression of both p21Cip1 and p16INK4a in HCEC cultured from older donors (Enamoto et al., 2006).
7. Molecular Basis for Decreased Proliferative Capacity
Together, accumulated data strongly suggest that there is both an age-related and topographical difference in the relative proliferative capacity of HCEC and that this difference is related to cellular senescence. Researchers in the field have identified two forms of cellular senescence: replicative senescence and stress-induced premature senescence. Below is a description of studies conducted to identify the mechanisms responsible for the decreased proliferative capacity observed in HCEC.
7.1. Test for Critically Short Telomeres
Replicative senescence results from the successive shortening of telomeres that occurs during DNA replication (Wright and Shay, 1992). Once telomeres have eroded to a critically short length, the senescence program is activated and cells become irreversibly inhibited from dividing (Ben-Porath and Weinberg, 2005). Egan, et al. (1998) measured telomere restriction fragment (TRF) lengths in HCEC isolated from donors 5 weeks to 84 years old and found that the mean TRF length was 12.2 kb, regardless of donor age. This length is sufficient to support several additional rounds of cell division prior to the formation of critically short telomeres. This laboratory confirmed and extended these findings using a fluorescent probe that specifically binds to telomere repeats (Konomi and Joyce, 2007). Semi-quantitative analysis of the intensity of telomere staining in ex vivo corneas and in HCEC freshly isolated from donor corneas showed no statistical age-related or topographic difference indicative of a difference in telomere length. Together, these data strongly suggest that HCEC retain the potential to divide based on telomere length and that the observed decrease in proliferative capacity is NOT due to “replicative senescence”.
7.2. Role of Nuclear Oxidative DNA Damage
Stress-induced premature senescence is considered to be “premature” because cells lose the ability to proliferate prior to telomere exhaustion. Thus, in this form of senescence, cells retain proliferative potential based on telomere length, but stop dividing due to inhibitory mechanisms activated by stress-induced damage pathways. Corneal endothelium is very metabolically active and lies directly in the light-path, making it potentially vulnerable to oxidative stress and its negative effects on cellular function. This effect on function has been observed in other ocular cells that lie within the light-path, including retinal pigment epithelial cells (Burke and Soref, 1988; Hjelmeland et al., 1999) and lens epithelial cells (Chylack, 1984). Studies were conducted in this laboratory to determine whether there is a relationship between oxidative stress, oxidative nuclear DNA damage, and reduced proliferative capacity in HCEC (Joyce et al., 2009). Oxidized DNA damage was first quantified by a competitive ELISA assay using an antibody directed against 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidized DNA (Beckman and Ames, 1997; Melov, 2000). The average concentration of 8-OHdG per nanogram of DNA was found to be higher in cells isolated from older donors and this difference was mainly contributed by a statistically significant increase in 8-OHdG within central endothelium. Immunostaining for 8-OHdG in the endothelium of ex vivo corneas revealed the presence of oxidative DNA damage in mitochondria, regardless of donor age or topographical location. Nuclei were much more intensely stained in central endothelium than in the periphery in corneas from older donors, whereas relatively little nuclear staining was observed in either area of corneas from young donors. Together, these results provide evidence for the existence of nuclear oxidative DNA damage mainly in the central endothelium of older donors. To test whether there is a relationship between oxidative stress and proliferative capacity in HCEC, a study was conducted in which subconfluent HCEC cultured from young donors were exposed to increasing concentrations of hydrogen peroxide (H2O2), a known oxidative stressor, and then tested for their ability to divide. The graph in Figure 4 shows that HCEC not exposed to H2O2 and cells exposed to a low concentration (25 μM) of H2O2 showed similar robust growth curves. With increasing concentrations of H2O2, the growth of HCECdecreased, making the growth kinetics of HCEC from young donors closely resemble those observed in HCEC from older donors (Compare graph in Fig. 4 with graphs in Fig. 2). Together, these results provide strong evidence the reduced proliferative capacity observed in HCEC from older donors is due to oxidative stress-induced premature senescence.
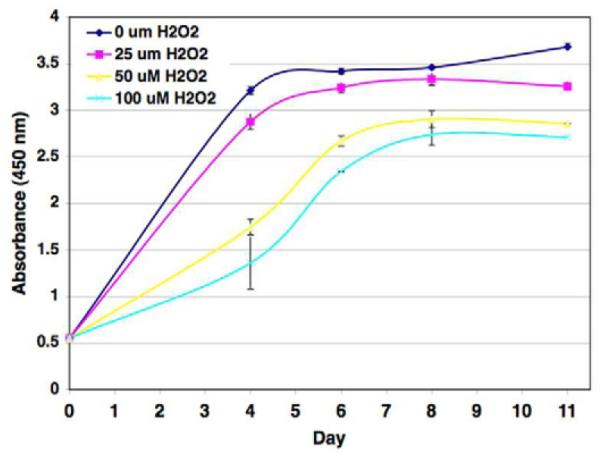
Effect of mild oxidative stress on the proliferative response of HCEC. Subconfluent HCECs cultured from a 26-year old donor were incubated in the presence of 0, 25, 50, or 100 mM H2O2 as the oxidative stressor. Cell numbers were determined using a WST-8 spectrophotometric assay. Results are presented as absorbance at 450 nm. The Association for Research in Vision and Ophthalmology is the original copyright holder.
Comparative proteomic analysis of a subset of proteins expressed in HCEC from young (<30 years old) and older donors (>50 years old) (Zhu et al., 2008) found that HCEC from older donors exhibit reduced expression of proteins that support important cellular functions such as metabolism, antioxidant protection, protein folding, and protein degradation. Immunostaining for the phosphorylated histone, H2AX-Ser139, which is associated with DNA damage foci (Paull et al., 2000), revealed significant positive staining in the in the central endothelium of older donors. This result indicates that HCEC are capable of responding to oxidative nuclear DNA damage; however, PCR-based microarray analysis of genes involved in cellular response to oxidative stress and DNA damage showed little change in expression with age, indicating that HCEC do not vigorously defend against or repair oxidative DNA damage by up-regulating the expression of multiple oxidative stress or DNA damage-signaling genes (Joyce et al., 2011).
8. Potential New Research Directions
It is clear that, although HCECs do not normally proliferate in vivo, they do possess proliferative capacity. These studies provide “proof of principle” that it should be possible to take advantage of the existing proliferative capacity of healthy HCECs to increase ECD in vivo, in ex vivo donor corneas to be used for transplantation, and in cultured cells to be used for corneal bioengineering. Clearly, these methods require further development and testing prior to any effort at clinical application.
The correlation of donor age and endothelial topography with increasing oxidative stress and reduced proliferative capacity is important. These results are consistent with results found in other differentiated cell types (Gaubatz and Tan, 1994; Fortini and Dogliotti, 2010). It is suggested that cells with more extensive DNA damage become arrested in G1-phase, thereby preventing duplication of damaged DNA and subsequent proliferation. These cells would then be able to concentrate on repair of DNA within the actively transcribed genome, permitting them to survive and function, but not to divide. Together, this information presents challenges, as well as suggests possible new directions, for basic research to increase ECD. It may be possible to prevent loss of proliferative capacity by treating HCEC with antioxidants to reduce the negative affect of oxidative stress. In addition, it should be possible to ectopically induce expression of specific antioxidant enzymes and/or DNA repair enzymes that could help prevent and /or repair nuclear oxidative DNA damage that would otherwise lead to reduced proliferative capacity. Another approach would be to identify methods to differentiate stem cells to form functional corneal endothelium in vivo. This might overcome the chronic need to obtain donor corneal tissue, thereby significantly increasing the number of individuals who could be treated to restore corneal transparency that has been lost due to the dysfunction of these physiologically important cells.
It is important to emphasize that any treatment designed to increase ECD in vivo, in ex vivo corneas, or for corneal bioengineering needs to be highly controlled. Besides demonstration of the ability of any new treatment to induce cell division, it will be important to demonstrate that the treatment does not negatively affect the karyotype of HCEC, as shown by Miyai et al. (2008). Any treatment would also need to demonstrate that cell division was induced only transiently and could not be re-stimulated. This safeguard is necessary to prevent over-replication of HCEC, which could result in multi-layering or interfere with the aqueous outflow pathway.
ACKNOWLEDGEMENTS
The author wishes to recognize a number of individuals who have contributed thought, time, and effort to conduct the studies reported from this laboratory. These include Deshea L. Harris, M.S., Tadashi Senoo, M.D., Ko-Hua Chen, M.D., Kikuko Enomoto, M.D., James McAlister, M.D., Tatsuya Mimura, M.D. and Cheng Zhu, M.D.
Funding sources: NEI R01 EY05756, NEI R01 EY12700
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
REFERENCES
- Aboalchamat B, Engelmann K, Böhnke M, Eggli P, Bednarz J. Morphological and functional analysis of immortalized human corneal endothelial cells after transplantation. Exp. Eye Res. 1999;69:547–553. [Abstract] [Google Scholar]
- Araki-Sasaki K, Danjo S, Kawaguchi S, Hosohata J, Tano Y. Human hepatocyte growth factor (HGF) in the aqueous humor. Jpn. J. Ophthalmol. 1997;41:409–413. [Abstract] [Google Scholar]
- Arnold DR, Moshayedi P, Schoen TJ, Jones BE, Chader GJ, Waldbillig RJ. Distribution of IGF-I and -II binding proteins (IGFBPs) and EGFBP mRNA in ocular fluids and tissues: potential sites of synthesis of IGFBPs in aqueous and vitreous. Exp. Eye Res. 1993;56:555–565. [Abstract] [Google Scholar]
- Baum JL, Niedra R, Davis C, Yue BY. Mass culture of human corneal endothelial cells. Arch. Ophthalmol. 1979;97:1136–1140. [Abstract] [Google Scholar]
- Beckman KB, Ames BN. Oxidative decay of DNA. J. Biol. Chem. 1997;272:19633–19636. [Abstract] [Google Scholar]
- Bednarz J, Rodokanaki-von Schrenck A, Engelmann K. Different characteristics of endothelial cells from central and peripheral human cornea in primary culture and after subculture. In Vitro Cell Dev. Biol. Anim. 1998;34:149–153. [Abstract] [Google Scholar]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005;37:961–976. [Abstract] [Google Scholar]
- Blake DA, Yu H, Young DL, Caldwell DR. Matrix stimulates the proliferation of human corneal endothelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1997;38:1119–1129. [Abstract] [Google Scholar]
- Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest. Ophthalmol. Vis. Sci. 1997;38:779–782. [Abstract] [Google Scholar]
- Burke JM, Soref C. Topographical variation in growth in cultured bovine retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1988;29:1784–1788. [Abstract] [Google Scholar]
- Campisi J. Replicative senescence: An old lives’ tale? Cell. 1996;84:497–500. [Abstract] [Google Scholar]
- Chen KH, Azar D, Joyce NC. Transplantation of adult human corneal endothelium ex vivo. Cornea. 2001;20:731–737. [Abstract] [Google Scholar]
- Chen KH, Harris DL, Joyce NC. TGF-beta2 in aqueous humor suppresses S-phase entry in cultured corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 1999;40:2513–2519. [Abstract] [Google Scholar]
- Chen KH, Hsu WM, Chiang CC, Li YS. Transforming growth factor-beta2 inhibition of corneal endothelial proliferation mediated by prostaglandin. Curr. Eye Res. 2003;26:363–370. [Abstract] [Google Scholar]
- Chylack LT., Jr. Mechanisms of senile cataract formation. Ophthalmol. 1984;91:596–602. [Abstract] [Google Scholar]
- Clements JL, Bouchard CS, Lee WB, Dunn SP, Mannis MJ, Reidy JJ, John T, Hannush SB, Goins KM, Wagoner MD, Adi MA, Rubenstein JB, Udell IJ, Babiuch AS. Retrospective review of graft dislocation rate associated with Descemet stripping automated endothelial keratoplasty after primary failed penetrating keratoplasty. Cornea. 2011;30:414–418. [Abstract] [Google Scholar]
- Cristofalo VJ. Cellular biomarkers of aging. Exp. Gerontol. 1988;23:297–305. [Abstract] [Google Scholar]
- DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:7245–7250. [Europe PMC free article] [Abstract] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. [Europe PMC free article] [Abstract] [Google Scholar]
- Egan CA, Savre-Train I, Shay JW, Wilson SE, Bourne WM. Analysis of telomere lengths in human corneal endothelial cells from donors of different ages. Invest. Ophthalmol. Vis. Sci. 1998;39:648–653. [Abstract] [Google Scholar]
- Engelmann K, Friedl P. Optimization of culture conditions for human corneal endothelial cells. In Vitro Cell Dev. Biol. 1989;25:1065–1072. [Abstract] [Google Scholar]
- Enomoto K, Mimura T, Harris DL, Joyce NC. Age-related differences in cyclin-dependent kinase inhibitor expression and retinoblastoma hyperphosphorylation in human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2006;47:4330–4340. [Abstract] [Google Scholar]
- Fortini P, Dogliotti E. Mechanisms of dealing with DNA damage in terminally differentiated cells. Mutat. Res. 2010;685:38–44. [Abstract] [Google Scholar]
- Gaubatz JW, Tan BH. Aging affects the levels of DNA damage in postmitotic cells. Ann. N.Y. Acad. Sci. 1994;719:1213–1221. [Abstract] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer. 1983;31:13–20. [Abstract] [Google Scholar]
- Gospodarowicz D, Delgado D, Vlodavsky I. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc. Natl. Acad. Sci. USA. 1980;77:4094–4098. [Europe PMC free article] [Abstract] [Google Scholar]
- Haj FG, Markova B, Klaman LD, Bohmer FD, Neel BG. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J. Biol. Chem. 2003;278:739–744. [Abstract] [Google Scholar]
- Harper JW, Adami GF, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1-cyclin-dependent kinases. Cell. 1993;75:805–816. [Abstract] [Google Scholar]
- Harris DL, Joyce NC. Transforming growth factor-beta suppresses proliferation of rabbit corneal endothelial cells in vitro. J. Interferon Cytokine Res. 1999;19:327–334. [Abstract] [Google Scholar]
- Harris DL, Joyce NC. Protein tyrosine phosphatase, PTP1B, expression and activity in rat corneal endothelial cells. Mol. Vis. 2007;13:785–796. [Europe PMC free article] [Abstract] [Google Scholar]
- Hay ED. Development of the vertebrate cornea. Internatl. Rev. Cytol. 1980;63:263–322. [Abstract] [Google Scholar]
- Helton ES, Chen X. p53 modulation of the DNA damage response. J. Cell. Biochem. 2007;100:883–896. [Abstract] [Google Scholar]
- Hiscott P, Seitz B, Schlötzer-Schrehardt U, Naumann GO. Immunolocalisation of thrombospondin 1 in human, bovine and rabbit cornea. Cell Tissue Res. 1997;289:307–310. [Abstract] [Google Scholar]
- Hjelmeland LM, Cristofalo VJ, Funk W, Rakoczy E, Katz ML. Senescence of the retinal pigment epithelium. Mol. Vis. 1999;5:33. [Abstract] [Google Scholar]
- Hollingsworth J, Perez-Gomez I, Mutalib HA, Efron N. A population study of the normal cornea using an in vivo slit-scanning confocal microscope. Optom. Vis. Sci. 2001;78:706–711. [Abstract] [Google Scholar]
- Honda H, Ogita Y, Higuchi S, Kani K. Cell movements in a living mammalian tissue: long-term observation of individual cells in wounded corneal endothelial of cats. J. Morphol. 1982;174:25–39. [Abstract] [Google Scholar]
- Hoppenreijs VP, Pels E, Vrensen GF, Felten PC, Treffers WF. Platelet-derived growth factor: receptor expression in corneas and effects on corneal cells. Invest. Ophthalmol. Vis. Sci. 1993;34:637–649. [Abstract] [Google Scholar]
- Ishino Y, Zhu C, Harris DL, Joyce NC. Protein tyrosine phosphatase-1B (PTP1B) helps regulate EGF-induced stimulation of S-phase entry in human corneal endothelial cells. Mol. Vis. 2008;14:61–70. [Europe PMC free article] [Abstract] [Google Scholar]
- Joyce NC. Cell cycle status in human corneal endothelium. Exp. Eye. Res. 2005;81:629–638. [Abstract] [Google Scholar]
- Joyce NC, Harris DL. Decreasing expression of the G1-phase inhibitors, p21Cip1 and p16INK4a, promotes division of corneal endothelial cells from older donors. Mol. Vis. 2010;16:897–906. [Europe PMC free article] [Abstract] [Google Scholar]
- Joyce NC, Harris DL, McAlister J, Ali RR, Larkin DFP. Effect of overexpressing the transcription factor E2F2 on cell cycle progression in rabbit corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2004;45:1340–1348. [Abstract] [Google Scholar]
- Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: Contact inhibition and TGF-b2. Invest. Ophthalmol. Vis. Sci. 2002;43:2152–2159. [Abstract] [Google Scholar]
- Joyce NC, Harris DL, Zieske JD. Mitotic inhibition of corneal endothelium in neonatal rats. Invest. Ophthalmol. Vis. Sci. 1998;39:2572–2583. [Abstract] [Google Scholar]
- Joyce NC, Harris DL, Zhu CC. Age-related gene response of human corneal endothelium to oxidative stress and DNA damage. Invest. Ophthalmol. Vis. Sci. 2011;52:1641–1649. [Europe PMC free article] [Abstract] [Google Scholar]
- Joyce NC, Meklir B, Joyce SJ, Zieske JD. Cell cycle protein expression and proliferative status in human corneal cells. Invest. Ophthalmol. Vis. Sci. 1996a;37:645–655. [Abstract] [Google Scholar]
- Joyce NC, Navon SE, Roy S, Zieske JD. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest. Ophthalmol. Vis. Sci. 1996b;37:1566–1575. [Abstract] [Google Scholar]
- Joyce NC, Zieske JD. Transforming growth factor-beta receptor expression in human cornea. Invest. Ophthalmol. Vis. Sci. 1997;38:1922–1928. [Abstract] [Google Scholar]
- Joyce NC, Zhu CC. Human corneal endothelial cell proliferation. Potential for use in regenerative medicine. Cornea. 2004;23(Suppl. 1):S8–S19. [Abstract] [Google Scholar]
- Joyce NC, Zhu CC, Harris DL. Relationship among oxidative stress, DNA damage, and proliferative capacity in human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2009;50:2116–2122. [Abstract] [Google Scholar]
- Kikuchi M, Zhu C, Senoo T, Obara Y, Joyce NC. p27kip1 siRNA induces proliferation in corneal endothelial cells from young, but not older donors. Invest. Ophthalmol. Vis. Sci. 2006;47:4803–4809. [Abstract] [Google Scholar]
- Kim DH, Kundu JK, Surh YJ. Redox modulation of p53: Mechanisms and functional significance. Molec. Carcinogen. 2011;50:222–234. [Abstract] [Google Scholar]
- Kim TY, Kim WI, Smith RE, Kay ED. Role of p27(Kip1) in cAMP- and TGF-beta2-mediated antiproliferation in rabbit corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2001;42:3142–3149. [Abstract] [Google Scholar]
- Konomi K, Joyce NC. Age and topographical comparison of telomere lengths in human corneal endothelial cells. Mol. Vis. 2007;13:1251–1258. [Abstract] [Google Scholar]
- Laing RA, Sandstrom MM, Berrospi AR, Leibowitz HM. Changes in the corneal endothelium as a function of age. Exp. Eye Res. 1976;22:587–594. [Abstract] [Google Scholar]
- Lass JH, Sugar A, Benetz BA, Beck RW, Dontchev M, Gal RL, Kollman C, Gross R, Heck E, Holland EJ, Mannis MJ, Raber I, Stark W, Stulting RD, Cornea Donor Study Investigator Group Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch. Ophthalmol. 2010;128:63–69. [Europe PMC free article] [Abstract] [Google Scholar]
- Leone G, DeGregori J, Jakoi L, Cook JG, Nevins JR. Collaborative role of E2F transcriptional activity and G1 cyclin dependent kinase activity in the induction of S phase. Pro. Natl. Acad. Sci .USA. 1999;96:6626–6631. [Europe PMC free article] [Abstract] [Google Scholar]
- Li W, Sabater AL, Chen YT, Hayashida Y, Chen SY, He H, Tseng SC. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2007;48:614–620. [Europe PMC free article] [Abstract] [Google Scholar]
- Ma HT, Poon RYC. How protein kinases co-ordinate mitosis in animal cells. Biochem. J. 2011;435:17–31. [Abstract] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. [Abstract] [Google Scholar]
- Matsuda M, Sawa M, Edelhauser HF, Bartels SP, Neufeld AH, Kenyon KR. Cellular migration and morphology in corneal endothelial wound repair. Invest. Ophthalmol. Vis. Sci. 1985;26:443–449. [Abstract] [Google Scholar]
- McAlister JC, Joyce NC, Harris DL, Ali RR, Larkin DF. Induction of replication in human corneal endothelial cells by E2F2 transcription factor cDNA transfer. Invest. Ophthalmol. Vis. Sci. 2005;46:3597–3603. [Abstract] [Google Scholar]
- Melov S. Mitochondrial oxidative stress: Physiologic consequences and potential for a role in aging. Ann. N.Y. Acad. Sci. 2000;908:219–225. [Abstract] [Google Scholar]
- Mimura T, Joyce NC. Replication competence and senescence in central and peripheral human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2006;47:1387–1396. [Abstract] [Google Scholar]
- Miyai T, Maruyama Y, Osakabe Y, Nejima R, Miyata K, Amano S. Karyotype changes in cultured human corneal endothelial cells. Mol. Vis. 2008;14:942–950. [Europe PMC free article] [Abstract] [Google Scholar]
- Murphy C, Alvarado J, Juster R, Maglio M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest. Ophthalmol. Vis. Sci. 1984;25:312–322. [Abstract] [Google Scholar]
- Nuttall RP. DNA synthesis during the development of the chick cornea. J. Exp. Zool. 1976;198:193–208. [Abstract] [Google Scholar]
- Ozaki T, Nakagawara A. p53: The attractive tumor suppressor in the cancer research field. J. Biomed. Biotech. 2011:603925. 2011. Epub 2010 Dec. 6. [Europe PMC free article] [Abstract] [Google Scholar]
- Patel SP, Bourne WM. Corneal endothelial cell proliferation: a function of cell density. Invest. Ophthalmol. Vis. Sci. 2009;50:2742–2746. [Europe PMC free article] [Abstract] [Google Scholar]
- Paull AC, Whikehart DR. Expression of the p53 family of proteins in central and peripheral human corneal endothelial cells. Mol. Vis. 2005;11:328–334. [Abstract] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000;10:886–895. [Abstract] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. P27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-b and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. [Abstract] [Google Scholar]
- Rahman I, Huang MC, Carley F, Hillarby MC, Vasileiadis GT, Tullo A. The influence of donor and recipient factors in allograft rejection of the human cornea. Eye (Lond) 2010;24:334–339. [Abstract] [Google Scholar]
- Rieder CL. Mitosis in vertebrates: the G2/M and M/A transitions and their associated checkpoints. Chrom. Res. 2011;19:291–306. [Abstract] [Google Scholar]
- Schulz MW, Chamberlain CG, De Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–126. [Abstract] [Google Scholar]
- Schultz-Cherry S, Lawler J, Murphy-Ullrich JE. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J. Biol. Chem. 1994;269:26783–26788. [Abstract] [Google Scholar]
- Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest. Ophthalmol.Vis. Sci. 2000;41:660–616. [Abstract] [Google Scholar]
- Senoo T, Obara Y, Joyce NC. EDTA: A promoter of proliferation in human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2000;41:2930–2935. [Abstract] [Google Scholar]
- Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. [Abstract] [Google Scholar]
- Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. [Abstract] [Google Scholar]
- Shulman J, Kropinak M, Ritterband DC, Perry HD, Seedor JA, McCormick SA, Milman T. Failed descemet-stripping automated endothelial keratoplasty grafts: a clinicopathologic analysis. Am. J. Ophthalmol. 2009;148:752–759. [Abstract] [Google Scholar]
- Song Z, Wang Y, Xie L, Zang X, Yin H. Expression of senescence-related genes in human corneal endothelial cells. Mol. Vis. 2008;14:161–170. [Europe PMC free article] [Abstract] [Google Scholar]
- Stoeber K, Tlsty TD, Happerfield I, Thomas GA, Romanov S, Bobrow L, Williams ED, Williams GH. DNA replication licensing and human cell proliferation. J. Cell Sci. 2001;114:2027–2041. [Abstract] [Google Scholar]
- Terry MA, Shamie N, Chen ES, Hoar KL, Phillips PM, Friend DJ. Endothelial keratoplasty: the influence of preoperative donor endothelial cell densities on dislocation, primary graft failure, and 1-year cell counts. Cornea. 2008;27:1131–1137. [Abstract] [Google Scholar]
- Wells A. EGF receptor. Int. J. Biochem. Cell Biol. 1999;31:637–643. [Abstract] [Google Scholar]
- Wharton SB, Chan KK, Anderson JR, Stoeber K, Williams GH. Replicative Mcm2 protein as a novel proliferation marker in oligo-dendrogliomas and its relationship to Ki67 labelling index, histological grade and prognosis. Neuropathol. Appl. Neurobiol. 2001;27:305–313. [Abstract] [Google Scholar]
- Wilson SE, Lloyd SA. Epidermal growth factor and its receptor, basic fibroblast growth factor, transforming growth factor-beta1 and interleukin-1 alpha messenger RNA production in human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 1991;32:2747–2756. [Abstract] [Google Scholar]
- Wilson SE, Lloyd SA, He YG. Fibroblast growth factor-1 receptor messenger RNA expression in corneal cells. Cornea. 1993a;12:249–254. [Abstract] [Google Scholar]
- Wilson SE, Lloyd SA, He YG, McCash CS. Extended life of human corneal endothelial cells transfected with the SV40 large T antigen. Invest. Ophthalmol. Vis. Sci. 1993;34:2112–2123. [Abstract] [Google Scholar]
- Wilson SE, Schultz GS, Chegini N, Weng J, He YG. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp. Eye Res. 1994;59:63–71. [Abstract] [Google Scholar]
- Wilson SE, Walker JW, Chwang EL, He Y-G. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest. Ophthalmol. Vis. Sci. 1993b;34:2544–2561. [Abstract] [Google Scholar]
- Wilson SE, Weng J, Blair S, He YG, Lloyd S. Expression of E6/E7 or SV40 large T antigen-coding oncogenes in human corneal endothelial cells indicates regulated high-proliferative capacity. Invest. Ophthalmol. Vis. Sci. 1995;36:32–40. [Abstract] [Google Scholar]
- Wright WE, Shay JW. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 1992;8:193–197. [Abstract] [Google Scholar]
- Wulle KG. Electron microscopy of the fetal development of the corneal endothelium and Descemet’s membrane of the human eye. Invest. Ophthalmol. 1972;11:897–904. [Abstract] [Google Scholar]
- Wulle KG, Lerche W. Electron microscopic observations of the early development of the human corneal endothelium and Descemet’s membrane. Ophthalmologica. 1969;157:451–61. [Abstract] [Google Scholar]
- Xiao X, Wang Y, Gong H, Chen P, Xie L. Molecular evidence of senescence in corneal endothelial cells of senescence-accelerated mice. Mol. Vis. 2009;15:747–761. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamagami S, Yokoo S, Mimura T, Takato T, Araie M, Amano S. Distribution of precursors in human corneal stromal cells and endothelial cells. Ophthalmology. 2007;114:433–439. [Abstract] [Google Scholar]
- Yoshida K, Kase S, Nakayama K, Nagahama H, Harada T, Ikeda H, Harada C, Imaki J, Ohgami K, Shiratori I, Ilieva IB, Ohno S, Nishi S, Nakayama KI. Involvement of p27KIP1 in the proliferation of the developing corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2004;45:2163–2167. [Abstract] [Google Scholar]
- Zhu CC, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest. Ophthalmol. Vis. Sci. 2004;45:1743–1751. [Abstract] [Google Scholar]
- Zhu C, Rawe I, Joyce NC. Differential protein expression in human corneal endothelial cells cultured from young and older donors. Mol. Vis. 2008;14:1805–1814. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.exer.2011.08.014
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3261346?pdf=render
Citations & impact
Impact metrics
Article citations
[Innovative surgical treatment approaches for endothelial dysfunction : Descemet stripping only (DSO) and endothelial cell injection].
Ophthalmologie, 121(10):796-802, 11 Oct 2024
Cited by: 0 articles | PMID: 39392519
Tissue-specific TCF4 triplet repeat instability revealed by optical genome mapping.
EBioMedicine, 108:105328, 14 Sep 2024
Cited by: 0 articles | PMID: 39278108 | PMCID: PMC11419830
TCF4 trinucleotide repeat expansions and UV irradiation increase susceptibility to ferroptosis in Fuchs endothelial corneal dystrophy.
Redox Biol, 77:103348, 10 Sep 2024
Cited by: 0 articles | PMID: 39332053 | PMCID: PMC11470242
Effect of Ablation Depth on the Endothelial Status of Eyes of Myopic Patients Undergoing Transepithelial Photorefractive Keratectomy: A Retrospective Study in Saudi Arabia.
Cureus, 16(7):e64527, 14 Jul 2024
Cited by: 0 articles | PMID: 39139351 | PMCID: PMC11321596
Sleep deprivation induces corneal endothelial dysfunction by downregulating Bmal1.
BMC Ophthalmol, 24(1):268, 21 Jun 2024
Cited by: 1 article | PMID: 38907352 | PMCID: PMC11191275
Go to all (164) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Graft survival and endothelial outcomes in the new era of endothelial keratoplasty.
Exp Eye Res, 95(1):40-47, 15 Jun 2011
Cited by: 50 articles | PMID: 21689649 | PMCID: PMC3902807
Review Free full text in Europe PMC
Engineering of Human Corneal Endothelial Grafts.
Curr Ophthalmol Rep, 3(3):207-217, 27 Jun 2015
Cited by: 11 articles | PMID: 26509105 | PMCID: PMC4617200
[Cultured human corneal endothelial cell transplantation].
Nippon Ganka Gakkai Zasshi, 110(11):879-897, 01 Nov 2006
Cited by: 0 articles | PMID: 17134036
Review
Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models.
Exp Eye Res, 95(1):60-67, 03 Nov 2011
Cited by: 66 articles | PMID: 22067130
Review
Funding
Funders who supported this work.
NEI NIH HHS (4)
Grant ID: R01 EY012700-09
Grant ID: R01 EY012700
Grant ID: R01 EY005767
Grant ID: R01 EY005767-19





