Abstract
Free full text

Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2
Abstract
Interest in how the gut microbiome can influence the metabolic state of the host has recently heightened. One postulated link is bacterial fermentation of “indigestible” prebiotics to short-chain fatty acids (SCFAs), which in turn modulate the release of gut hormones controlling insulin release and appetite. We show here that SCFAs trigger secretion of the incretin hormone glucagon-like peptide (GLP)-1 from mixed colonic cultures in vitro. Quantitative PCR revealed enriched expression of the SCFA receptors ffar2 (grp43) and ffar3 (gpr41) in GLP-1–secreting L cells, and consistent with the reported coupling of GPR43 to Gq signaling pathways, SCFAs raised cytosolic Ca2+ in L cells in primary culture. Mice lacking ffar2 or ffar3 exhibited reduced SCFA-triggered GLP-1 secretion in vitro and in vivo and a parallel impairment of glucose tolerance. These results highlight SCFAs and their receptors as potential targets for the treatment of diabetes.
Targeting the release of anorectic and antidiabetic gut peptides is the focus of many ongoing drug development programs, as evidence is accumulating that enhanced secretion of glucagon-like peptide (GLP)-1 and peptide YY (PYY) from intestinal L cells may translate into beneficial effects in subjects with diabetes and obesity (1). Already, the diabetes field has witnessed the impact of therapeutic GLP-1 mimetics and dipeptidyl peptidase (DPP)4 inhibitors, which, respectively, mimic endogenous active GLP-1 and slow its enzymatic degradation in the circulation (2). The injectable GLP-1 mimetics, in particular, are associated not only with improved blood glucose control and reduced incidence of hypoglycemia but also with significant weight reduction (3). The observed correlation between elevated postprandial GLP-1 levels and improved glucose homeostasis in patients after bariatric surgery indicates that the body has excess capacity in the GLP-1 axis that can be recruited in obese diabetic patients, with downstream beneficial effects on food intake and diabetes control (4). Identifying and validating pharmaceutical strategies to enhance GLP-1 secretion are central to many ongoing L cell–targeting research programs.
L cells are a component of the enteroendocrine system, diffusely located along the length of the intestinal epithelium (5). They make contact with the gut lumen via apical processes and are believed to respond directly to luminal signals. Among the best characterized triggers of GLP-1 secretion are sugars, amino acids, and long-chain fatty acids, which stimulate L cells by a variety of pathways including transporter-associated uptake, metabolism, and G-protein–coupled receptor activation (5,6). L cells are, however, found in highest density in the colonic epithelium, where these nutrients are unlikely to reach significant concentrations (7). Short-chain fatty acids (SCFAs), derived from bacterial fermentation of macrofibrous material reaching the distal gut, by contrast are known to reach high concentrations under physiological conditions in the colons of healthy subjects. Intraluminal concentrations beyond 100 mmol/L, comprising ~60% acetate (C2), 25% propionate (C3), and 15% butyrate (C4), have been reported (8). Plasma levels of SCFA are also dominated by acetate but are generally below ~200 μmol/L unless elevated by ethanol metabolism (9,10). Nondigestible and fermentable dietary fiber, as well as SCFAs themselves, has been shown to increase GLP-1 secretion in humans (11,12) and rodents (13,14), and enhanced PYY release has been proposed as a link between luminal SCFAs and altered gut motility (15,16).
SCFAs act as a local nutrient source but can also trigger cell-specific signaling cascades by activation of the G-protein–coupled free fatty acid receptor (FFAR)2 (GPR43) and FFAR3 (GPR41) (17,18). These two receptors share ~40% amino acid sequence similarity and are conserved across several mammalian species (17–19). Both receptors respond to SCFAs containing two to five carbons, although a preference of FFAR2 for C2 and C3 fatty acids and of FFAR3 for C3–C5 carbon chain lengths has been reported (17,18,20). The receptors differ in their intracellular signaling capabilities, with FFAR2 reportedly coupling to either Gq or Gi/o and FFAR3 exclusively activating Gi/o pathways (17,18,20). The finding that both receptors are located in colonic L cells by immunostaining (21–23) suggests that SCFAs may use this pathway to modulate L-cell function. Experimental data in support of this idea are, however, still lacking.
The aim of the current study was to establish and explore the link between SCFAs and GLP-1 secretion, making use of our transgenic mouse model (GLU-Venus) in which L cells are identifiable by their expression of a yellow fluorescent protein derivative, Venus (24).
RESEARCH DESIGN AND METHODS
All animal procedures were approved by the local ethics review committees and conformed to Home Office regulations. Experiments were performed using mice on a C57B/6 background, except in the case of the ffar2−/− and ffar3−/− mice and littermate controls, which were on a 129/SvEv background.
Generation of knockout mice.
For generation of the targeting vectors, homology arms (ffar3, 1,250 base pairs [bp] 5′ and 3,914 bp 3′; ffar2, 1,761 bp 5′ and 4,199 bp 3′) were PCR amplified from genomic DNA and cloned into a vector containing internal ribosomal entry site β-gal reporter gene, neomycin selection marker, and two thymidine kinase negative selection markers (Supplementary Figs. 1 and 2). The linearized vector was electroporated into embryonic stem cells (129/SvEv), and homologous recombination was detected by screening PCR and Southern blot. Chimeras were generated by blastocyst injection, bred with 129/SvEv animals, and maintained inbred in this background.
Glucose-stimulated GLP-1 secretion in vivo.
Experiments were performed on 3- to 4-month-old ffar2−/− and ffar3−/− mice or wild-type littermate control 129/SvEv mice. No significant differences in body weight were observed between the wild-type and knockout groups. Mice were fasted for 4 h and dosed per os with 20 mg/kg DPP4 inhibitor (cat. no. KR-62436; Sigma). Thirty minutes later, mice were dosed by gavage with 1.5 g/kg glucose solution (Fisher Scientific). For initial GLP-1 measurement, 150 μL blood was collected from awake mice via tail bleed into EDTA-coated capillary tubes (Bilbate) before glucose dosing. Thirty minutes after glucose dosing, mice were killed by CO2 inhalation and blood was collected via cardiac puncture into tubes containing aprotinin (0.6 trypsin inhibiting units/mL). Blood samples were centrifuged immediately, and plasma was frozen on dry ice before assay in duplicate for active GLP-1 (MesoScale, Gaithersburg, Maryland).
Oral glucose tolerance test.
Three- to four-month-old ffar2−/−, ffar3−/−, and wild-type littermate control 129/SvEv mice were fasted for 14 h and then dosed by gavage with 1.5 g/kg glucose. Blood glucose was measured using a handheld glucometer (OneTouch Ultra) via tail bleed. Samples for insulin measurements were taken via tail bleed from awake mice into heparinized capillary tubes (Bilbate) and assayed by ELISA (Crystalchem Ultra sensitive mouse insulin ELISA). Mice undergoing the procedure were of similar body weight: ffar2−/−, 22.5 ± 0.4 g (n = 11), wild type, 21.5 ± 0.5 g (n = 8); ffar3−/−, 22.7 ± 1.5 g (n = 6), and wild type, 20.8 ± 0.6 g (n = 7).
Insulin tolerance test.
Three- to four-month-old ffar2−/−, ffar3−/−, and wild-type littermate control 129/SvEv mice were fasted for 4 h and then dosed with 0.75 units/kg insulin i.p. (Actrapid insulin, supplied by our Named Veterinary Surgeon). Blood glucose was measured using a handheld glucometer (OneTouch Ultra) via tail bleed from awake mice.
Colonic tissue and cell preparation.
Mice aged 6–26 weeks were killed by cervical dislocation, and colons were collected in ice-cold Leibovitz-15 medium. Cultures for secretion and calcium imaging experiments were prepared as previously described (24). Colonic tissue used for RNA and protein extraction was washed in PBS and placed in RNA later or a protein lysis buffer, respectively, and frozen until processed.
GLUTag cells were cultured in Dulbecco’s modified Eagle’s medium (5.5 mmol/L glucose) supplemented with 10% FBS, 2 mmol/L L-glutamine, 100 units/mL penicillin, and 0.1 mg/mL streptomycin.
RNA extraction and quantitative PCR.
Total RNA from cells sorted with fluorescence-activated cell sorter (FACS) prepared from GLU-Venus transgenic mice (24) was isolated using a microscale RNA isolation kit (Ambion). Total RNA from GLUTag cells and murine colonic tissue was prepared following the Tri-Reagent protocol (Sigma). All samples were reverse transcribed according to standard protocols. Quantitative RT-PCR was performed with a 7900 HT Fast Real-Time PCR system (Applied Biosystems) using Taqman probes for β-actin, gcg, pyy, ffar2, and ffar3 from Applied Biosystems. Expression was compared with that of β-actin measured in parallel on the same sample, giving a ΔCt for β-actin minus the test gene. If the test gene was undetectable, it was assigned a Ct value of 40. Means ± SE were calculated and statistics were performed for the ΔCt data and only converted to relative expression levels (2ΔCt) for presentation in the Figures.
Colonic protein analysis.
Tissue was mechanically homogenized in lysis buffer. Active GLP-1 was assessed by ELISA (Millipore, Watford, U.K.) and expressed relative to total protein content, measured using a Bradford assay (Sigma).
Secretion from primary mixed colonic culture.
Secretion studies were performed 24–36 h after culture preparation. Where applicable, cultures were preincubated with 0.2 μg/mL pertussis toxin for 18 h. Cultures were washed with standard saline and incubated with test substances for 2 h at 37°C. Secreted and cellular GLP-1 was extracted as previously described (24), and active GLP-1 was quantified by ELISA (Millipore). GLP-1 secretion was expressed as a fraction of the total hormone (secreted plus extracted) and normalized to basal secretion measured in the same set of experiments.
Ca2+ imaging.
Experiments were performed 4–7 days postplating, using colonic tissue from GLU-Venus transgenic mice with L cell–specific Venus expression (24). Cells were loaded with 7 μmol/L Fura2-AM (Invitrogen, Paisley, U.K.), 0.01% pluronic F127, and 300 μmol/L eserine in standard saline solution for 30 min at 37°C. Single-cell imaging was performed using an inverted fluorescence microscope (Olympus IX71; Southall, U.K.) with a 40× oil-immersion objective. Fura2 was excited at 340/6 and 380/4 nm and Venus at 475/10 nm, using a 75-W xenon arc lamp and a monochromator (Cairn Research, Faversham, U.K.) controlled by MetaFluor software (Molecular Devices, Wokingham, U.K.). Emission was recorded with an Orca ER CCD camera (Hamamatsu, Welwyn Garden City, U.K.) using a dichroic mirror and a 510-nm long-pass filter. Test substances were added to the bath solution and perfused at ~1 mL/min. Fura2 fluorescence measurements were taken every 2 s, background corrected, and expressed as the 340:380 nm ratio. Average fluorescence ratios from individual cells were determined over 20-s periods before addition and during perfusion of a test agent. Peak responses to a test agent were expressed as the mean ratio of the test agent divided by the averaged ratios measured prior to drug application.
Solutions.
Standard in vitro saline solution contained (in millimoles per liter) 4.5 KCl, 138 NaCl, 4.2 NaHCO3, 1.2 NaH2PO4, 2.6 CaCl2, 1.2 MgCl2, 10 glucose, and 10 HEPES, pH 7.4 (NaOH). Essentially fatty acid–free BSA (0.1%) was added to solutions used for static secretion experiments. SCFAs were dissolved directly in saline solution and pH corrected if necessary. All other drugs for in vitro experiments were prepared as 1,000× stocks. The protein and secretion lysis buffer contained 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% IGEPAL-CA 630, 0.5% deoxycholic acid, and one tablet of complete EDTA-free protease inhibitor cocktail (Roche). All reagents were supplied by Sigma (Poole, U.K.) unless otherwise indicated. The synthetic GPR43 agonist (S)-2-(4-chlorophenyl)-N-(5-fluorothiazol-2-yl)-3-methylbutanamide (CFMB) (25) was synthesized, its identity was confirmed by nuclear magnetic resonance analysis, and it was dissolved in DMSO as a 1,000× stock.
Data analysis.
Comparisons between conditions were made using Student t test (Microsoft Excel) or by one- or two-way ANOVA with post hoc Bonferroni correction or Dunnett test (Prism5; GraphPad), as indicated, with a threshold for significance of P < 0.05. All data are expressed as means ± SEM.
RESULTS
SCFAs enhance GLP-1 release.
In primary murine colonic cultures, acetate and propionate (1 mmol/L) significantly stimulated GLP-1 secretion over a 2-h incubation period (Fig. 1A). Secretion was further enhanced in the presence of 100 μmol/L phosphodiesterase inhibitor isobutyl methyl xanthine (IBMX), and under these conditions a significant stimulation by 1 mmol/L butyrate was also observed. To mimic the higher SCFA content of the colonic lumen, we also examined the effect of a 140 mmol/L mixture containing 80 mmol/L acetate, 20 mmol/L butyrate, and 40 mmol/L propionate, which was added to the standard bath solution and compared with 140 mmol/L additional NaCl as an osmotic control. The effect of the high-SCFA mixture was similar to that triggered by the 1 mmol/L concentrations, enhancing GLP-1 secretion ~1.3-fold compared with the osmotic control (n = 5–6, P < 0.01) (Fig. 1B). By contrast, GLUTag cells were not found to secrete GLP-1 in response to 1 or 10 mmol/L SCFA (data not shown).
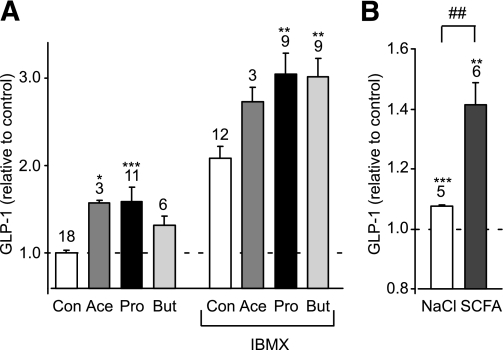
SCFAs stimulate GLP-1 secretion. A: Acute stimulation of GLP-1 secretion. Mixed primary cultures from murine colon were incubated for 2 h in 10 mmol/L glucose (Con) or in the additional presence of acetate (Ace) (1 mmol/L), propionate (Pro) (1 mmol/L), or butyrate (But) (1 mmol/L) with or without IBMX (100 μmol/L) as indicated. GLP-1 secretion in each well is expressed relative to the basal secretion (Con) measured in parallel on the same day. Data represent the means ± SEM of the number of wells indicated above each bar. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with their respective controls in the absence or presence of IBMX by one-way ANOVA with post hoc Dunnett test. B: GLP-1 secretion from primary colonic cultures triggered by 140 mmol/L cocktail of SCFAs and an osmotic control of 140 mmol/L NaCl. GLP-1 secretion in each well is expressed relative to the basal secretion measured in parallel on the same day. Data represent the means ± SEM of the number of wells indicated above each bar. **P < 0.01 and ***P < 0.001 compared with baseline and ##P < 0.01 compared with NaCl by Student t test.
SCFA-induced calcium responses in primary mouse colonic L cells.
L cells in mixed cultures were identified by their expression of Venus (Fig. 2B), and the calcium concentration, [Ca2+]i, was monitored in both L cells and their nonfluorescent neighbors after loading of the monolayer with Fura2-AM (Fig. 2A). [Ca2+]i is represented in the figures by the 340:380 fluorescence ratio. Propionate (1 mmol/L) triggered a transient Ca2+ response (Fig. 2A, C, and D), with a mean 1.5 ± 0.1-fold increase (n = 17, P < 0.01) above baseline. Acetate (1 mmol/L) triggered a comparable [Ca2+]i response of 1.3 ± 0.1-fold (n = 6, P < 0.05) (Fig. 2D). The SCFA-induced Ca2+ mobilization appeared specific to L cells, as intracellular [Ca2+]i in the non–L-cell population was unaffected by acetate and only marginally elevated by propionate (Fig. 2D).
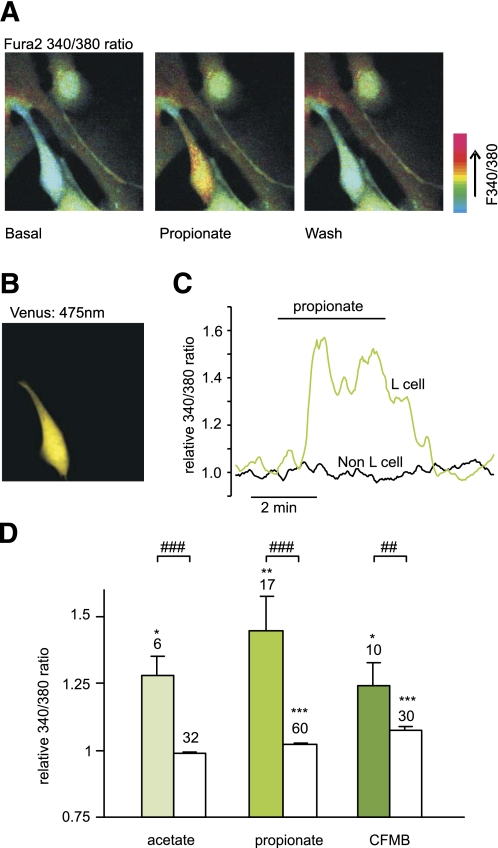
SCFAs raise intracellular calcium in identified colonic L cells. A: Mixed colonic cultures were loaded with fura2-AM. Pseudocolor images of fura2 340:380 nm fluorescence ratio (reflecting [Ca2+]i) shown prior to (basal) and during the application of propionate (1 mmol/L), and after washing with saline. B: Identification of an L cell in the field of view shown in A identified by the fluorescence of Venus (475 nm excitation). C: A representative response of an L and a non–L cell recorded as in A. D: Mean calcium changes in L cells (filled bars) and non–L cells (open bars) after the addition of acetate (1 mmol/L), propionate (1 mmol/L), or CFMB (30 μmol/L) as indicated. Ratios (340:380) in the presence of the test agent were normalized to the mean of the background ratios of each cell measured before addition and after washout of the test compound. Data represent the means ± SEM of the number of cells indicated above each bar. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with baseline and ##P < 0.01 and ###P < 0.001 compared with non–L cells by Student t test. (A high-quality digital representation of this figure is available in the online issue.)
ffar2 and ffar3 expression is enriched in primary L cells.
Expression of ffar2 and ffar3 was investigated by quantitative RT-PCR using mRNA from FACS-sorted intestinal L cells from transgenic GLU-Venus mice (24). Nonfluorescent cells collected in parallel were used to represent the mixed non–L-cell population. As shown in Fig. 3, ffar2 and ffar3 were expressed in small intestinal and colonic L cells and significantly enriched in L-cell compared with non–L-cell populations (P < 0.05). GLUTag cells expressed ffar3 but had barely detectable levels of ffar2.
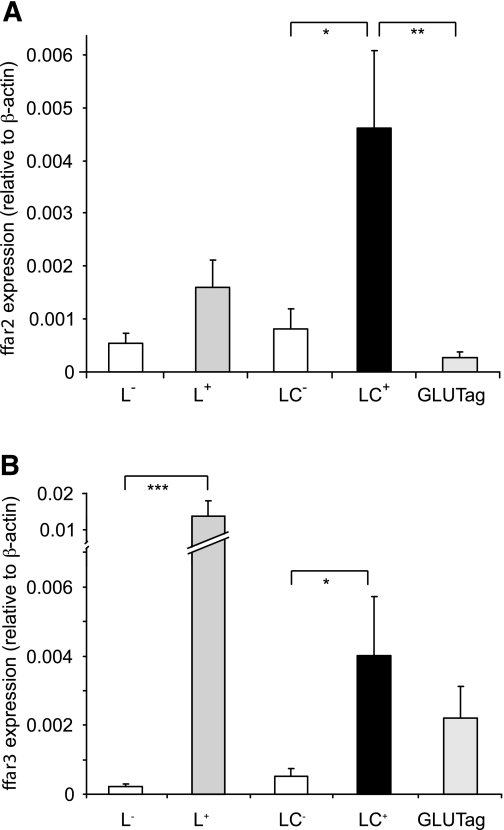
SCFA receptors FFAR2 and FFAR3 are expressed in L cells. Relative expression of ffar2 (A) and ffar3 (B) mRNAs relative to β-actin assessed by RT-PCR in FACS-sorted L cells and non–L cells from the small intestine (L+ and L−, respectively) and colon (LC+ and LC−) and the GLUTag model L-cell line. Data are presented as geometric means ± the upper SEM calculated from the log(base 2) data (n = 3 each). Significance comparisons between L cells and non–L cells were calculated by one-way ANOVA with a post hoc Bonferroni correction test performed on the log(base 2) data: *P < 0.05, **P < 0.01, and ***P < 0.001.
Acute SCFA-induced GLP-1 secretion is not mediated via a Gi-signaling pathway.
The findings that SCFAs trigger Ca2+ elevation in L cells and enhance GLP-1 secretion are consistent with the involvement of a Gq-mediated pathway, potentially, therefore, implicating FFAR2. Hence, we tested whether a synthetic phenylacetamide agonist for FFAR2, CFMB (25), could also mobilize Ca2+ and found that 30 μmol/L CFMB indeed elevated intracellular Ca2+ in L cells (Fig. 2D). A smaller but significant increase was also observed in the non–L-cell population. To evaluate any opposing contribution from Gi signaling, we examined GLP-1 release in the presence of the Gi inhibitor pertussis toxin. Whereas SCFAs were stimulatory in the presence of IBMX (Fig. 1B), somatostatin (100 nmol/L), used as a positive control for Gi-coupled pathways, abolished GLP-1 secretion under these conditions (n = 3, P < 0.001) (Fig. 4A), an effect that was partially reversed by preincubation with 0.2 μg/mL pertussis toxin (n = 3, P < 0.001). By contrast, pertussis toxin did not have a significant effect on the response to 1 mmol/L propionate (Fig. 4B), suggesting that there is little signaling by SCFA through Gi-coupled pathways during 2-h secretion studies in vitro.
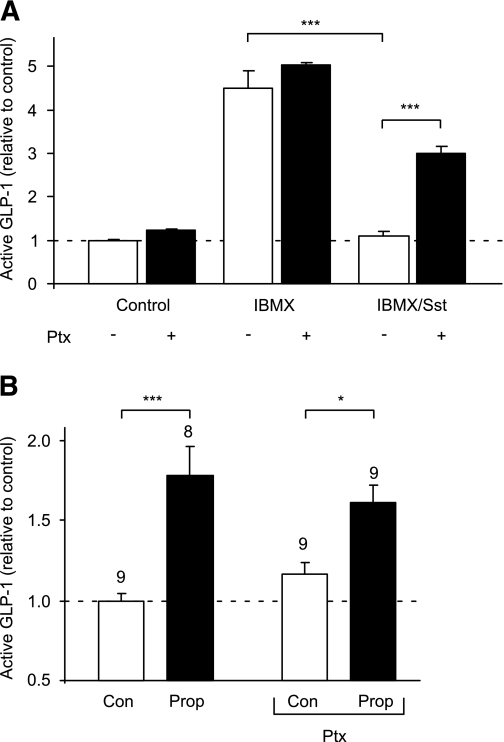
Propionate (Prop) responses are not sensitive to pertussis toxin (Ptx). A: GLP-1 secretion from primary colonic cultures treated with IBMX (100 μmol/L) with or without somatostatin (Sst) (100 nmol/L) in the absence (■) or presence (□) of 0.2 μg/mL pertussis toxin (all n = 3). B: GLP-1 secretion from primary colonic cultures triggered by propionate (1 mmol/L) in the absence and presence of pertussis toxin (0.2 μg/mL). The number of wells is indicated above the bars. Mixed primary cultures from the colon were incubated in bath solution containing reagents as indicated. GLP-1 secretion in each well is expressed relative to the basal secretion (control), measured in parallel on the same day. Data represent the means ± SEM of the number of wells indicated. Statistical significance was assessed by one-way ANOVA with a post hoc Bonferroni correction test: *P < 0.05 and ***P < 0.001.
FFAR2 mediates SCFA-induced GLP-1 release.
To evaluate the relative contributions of FFAR2 and FFAR3 to SCFA-triggered secretion, we examined the effects of propionate and acetate on primary colonic cultures from mice with knockout of either ffar2 (ffar2−/−) or ffar3 (ffar3−/−). While GLP-1 secretion was triggered by 1 mmol/L propionate and acetate in cultures from wild-type littermate controls (Fig. 5A), the response to propionate was reduced by 70% (P < 0.001) and the response to acetate was abolished (P < 0.001) in the ffar2 knockout tissue. Knockout of ffar3 also impaired secretory responses to both acetate and propionate (P < 0.01) (Fig. 5A), although to a lesser extent than ffar2 knockout. Similar, but more pronounced, effects were observed in the presence of 100 μmol/L IBMX (Fig. 5A). The higher concentration of mixed SCFA (140 mmol/L, as described above) also failed to enhance GLP-1 secretion significantly in both the ffar2 and ffar3 knockout mouse models (Fig. 5B).
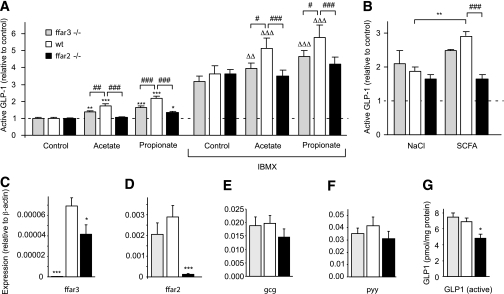
ffar2 and ffar3 knockout impairs SCFA-triggered GLP-1 secretion. A: GLP-1 secretion from primary colonic cultures from wild-type, ffar2−/−, and ffar3−/− mice. Mixed primary cultures from the colon from wild-type, ffar3−/−, and ffar2−/− mice were incubated in bath solution containing 10 mmol/L glucose together with acetate (1 mmol/L), propionate (1 mmol/L), and IBMX (100 μmol/L) as indicated (all n = 6). B: GLP-1 secretion from primary colonic cultures from wild-type, ffar2−/−, and ffar3−/− mice triggered by a 140 mmol/L cocktail of SCFAs and an osmotic control of 140 mmol/L NaCl. GLP-1 secretion in each well is expressed relative to the basal secretion (control) measured in parallel on the same day, and error bars represent 1 SEM. Effects of SCFAs in the absence (*P < 0.05, **P < 0.01, and ***P < 0.001) or presence (ΔΔP < 0.01 and ΔΔΔP < 0.001) of IBMX and effects of genotype (#P < 0.05, ##P < 0.01, and ###P < 0.001) were assessed for significance by two-way ANOVA with post hoc Bonferroni correction test. C–F: Expression of ffar3 (C), ffar2 (D), gcg (E), and pyy (F) mRNA in colonic tissue isolated from ffar3−/− (n = 5) and ffar2−/− (n = 5) mice and wild-type littermates (n = 6). Expression was normalized to that of β-actin in the same sample. Data are presented as geometric means, and the error bar was calculated from the log(base 2) data. Significance comparisons between genotypes were calculated by one-way ANOVA with a post hoc Dunnett test performed on the log(base 2) data: *P < 0.05 and ***P < 0.001. G: Content of active GLP-1 peptide in colonic tissue isolated from ffar3−/− and ffar2−/− mice and wild-type littermates. Active GLP-1 in colonic extracts was assessed by enzyme-linked immunosorbent assay and is expressed relative to sample protein assessed with a Bradford assay. Significance comparisons between genotypes (n = 6 each) were calculated by one-way ANOVA with a post hoc Dunnett test: *P < 0.05.
Analysis of mRNA expression in whole colonic tissue extracts from wild-type and knockout mice revealed that, as expected, ffar2−/− mice lacked ffar2 mRNA and ffar3−/− mice lacked ffar3 (Fig. 5C and D). ffar3 expression was also significantly decreased in ffar2−/− mice, but, although we also observed a trend toward a reduced ffar2 expression in ffar3−/− mice, this did not reach statistical significance.
ffar2 knockout decreases GLP-1 content.
To examine whether the receptors have additional effects on L cells operating over a longer time scale, we examined whether colonic tissue from mice lacking ffar2 or ffar3 had altered levels of mRNA for glucagon (gcg) (which includes the coding sequence for GLP-1), pyy, or active GLP-1 peptide. Knockout of ffar3 did not have a significant effect on colonic gcg mRNA or active GLP-1. There was a trend toward reduced colonic gcg and pyy mRNA expression in the ffar2 receptor knockout model that did not reach statistical significance (Fig. 5E and F). ffar2-deficient mice, however, had significantly reduced colonic GLP-1 protein content (n = 6, P < 0.05) (Fig. 5G).
FFAR2 and FFAR3 affect GLP-1 release in vivo.
To evaluate whether the reduced in vitro GLP-1 secretory capacity of ffar2- and ffar3-deficient mice translates into impaired hormone secretion in vivo, we examined plasma GLP-1–level responses to oral glucose administration. Basal levels of active GLP-1 were reduced by ~40% in ffar2-deficient mice (P < 0.01) (Fig. 6A) and were also lower, although not significantly, in ffar3-deficient mice compared with those in wild-type littermate controls. Following an oral glucose load, more pronounced impairments of plasma GLP-1 responses were observed in both ffar2 and ffar3 knockout models (n = 5, P < 0.05) (Fig. 6A). Coinciding with the reduced circulating GLP-1 concentrations, ffar2- and ffar3-deficient mice also exhibited impaired glucose tolerance when tested with gavage administration of 1.5 g/kg glucose (Fig. 6B and C). This correlated with reduced plasma insulin levels, although again this reached significance in mice lacking ffar2 but not ffar3 when compared with their respective littermate controls (Fig. 6D and E). To test for possible differences in insulin sensitivity, ffar2−/− and ffar3−/− mice and littermate controls were injected with 0.75 units/kg insulin after a 4-h fast. No significant differences were found between the different genotypes (Fig. 6F and G).
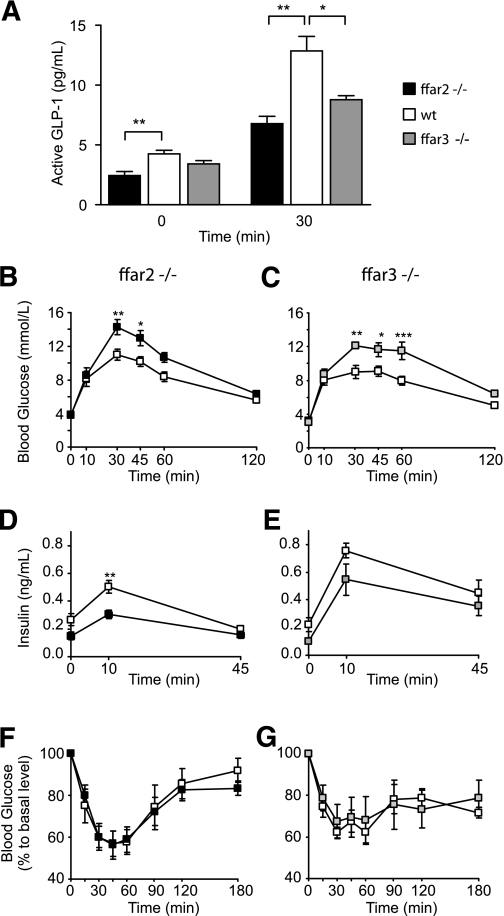
ffar2 and ffar3 knockout mice have impaired glucose tolerance. A: Glucose stimulated GLP-1 secretion in vivo. ffar2−/− and ffar3−/− mice and wild-type littermates (n = 5 each) were dosed with DPP4 inhibitor at a dose of 20 mg/kg per os after a 4-h fast. Thirty minutes post–DPP4 inhibitor dosing, mice were dosed with 1.5 g/kg glucose per os Plasma active GLP-1 was assessed by a MesoScale assay at 0 and 30 min of the oral glucose tolerance test. Data represent means ± 1 SEM, and statistical significance was assessed by Student t test: *P < 0.05 and **P < 0.01. B–E: Oral glucose tolerance test in ffar2−/− mice (n = 8) (left panel) and wild-type littermates (n = 11) (B and D) or ffar3−/− mice (n = 7) (right panel) and wild-type littermates (n = 6) (C and E). Following an overnight fast, mice were given 1.5 g/kg glucose per os, and blood glucose (B and C) and insulin (D and E) were measured at the time points indicated. F and G: Insulin tolerance test in ffar2−/− mice (n = 11) (left panel) and wild-type littermates (n = 6) (F) or in ffar3−/− mice (n = 7) (right panel) and wild-type littermates (n = 7) (G). Following a 4-h fast, mice were given 0.75/kg insulin intraperitoneal, and blood glucose was measured at the time points indicated. No significant differences between genotypes were observed. Data in B–G represent means ± 1 SEM, and statistical significance was assessed by two-way ANOVA with repeated measures: * P < 0.05, ** P < 0.01, and *** P < 0.001.
DISCUSSION
Our results demonstrate a direct link between SCFA activation of FFAR2, elevation of intracellular Ca2+ in L cells, and enhanced GLP-1 secretion from primary colonic cultures. A stimulatory role for FFAR2 in vivo is supported by the finding that knockout of ffar2 lowers both basal and glucose-stimulated GLP-1 concentrations.
By quantitative PCR, we could demonstrate that expression of mRNA for ffar2 and ffar3 is enriched in L cells, consistent with the detection of these receptors in PYY- and GLP-1–positive cells in rat and human colon by immunohistochemistry (21–23). Interestingly, ffar2 expression was very low in the GLP-1–secreting cell line GLUTag, perhaps explaining the poor responsiveness of GLUTag cells to SCFAs (data not shown) and emphasizing the importance of studying primary L cells in parallel with cell line models.
FFAR2 reportedly couples to Gq- or Gi/o-signaling pathways and FFAR3 exclusively to Gi. The finding that GLP-1 secretion is enhanced, rather than inhibited, by SCFAs suggests that Gq-coupled pathways predominate over any Gi activation, and indeed, we did not observe any evidence that Gi coupling blunts SCFA-triggered GLP-1 secretion either in secretion studies with added pertussis toxin or after knockout of ffar3. Somatostatin, by contrast, inhibited IBMX-triggered GLP-1 release in a pertussis toxin–sensitive manner, indicating that downstream Gi-coupled pathways are globally functional in L cells.
The idea that FFAR2 is coupled to Gq-signaling pathways in L cells is suggested by the Ca2+ imaging data, which showed that acetate and propionate triggered intracellular Ca2+ responses in the L-cell population. In support of this idea, knockout of ffar2 abolished SCFA-triggered GLP-1 secretion in vitro. The explanation for the blunted GLP-1 secretory response to SCFA in cultures from ffar3−/− mice is less clear, as FFAR3 has not been reported to couple to stimulatory Gq- or Gs-signaling pathways. One contributing factor could be the reduced ffar2 expression that was observed in ffar3−/− mice, although this did not reach statistical significance in our samples. Reduced expression of ffar2 has, however, previously been reported in adipose tissue of ffar3 knockout mice (26). Alternatively, there may be a previously unidentified component to FFAR3 signaling in L cells that also contributes to SCFA-triggered GLP-1 secretion. This is not, however, supported by the observation that stimulation of GLP-1 release was not observed in GLUTag cells, which express ffar3 but very little ffar2. A dominant role of FFAR2 over FFAR3 in SCFA-triggered L-cell activation is further suggested by the acute Ca2+-elevations seen in primary L cells in response to the FFAR2-specific agonist CFMB, which reportedly does not have activity against FFAR3 (25).
It is not known whether FFAR2 and FFAR3 reside on the apical or basolateral membrane of L cells or whether they primarily detect luminal or plasma SCFA. SCFA levels in the colonic lumen are in the area of 100 mmol/L. Although this is considerably above the half maximal effective concentrations (EC50s) of FFAR2 and FFAR3 for SCFA (0.5–1 mmol/L) (17,18,20), SCFA concentrations may be lower in the immediate vicinity of the L cells as a result of the diffusional barrier provided by the mucous layer and active uptake by neighboring enterocytes. Circulating plasma SCFA concentrations are in the range of 100–200 μmol/L (10), which is closer to the working range of the receptors, and while most studies have examined how luminally applied SCFAs affect GLP-1 release (14,27,28) it has also been shown that systemically infused acetate can enhance GLP-1 secretion (28). As luminal SCFA concentrations are not predicted to change markedly in response to acute food ingestion, it is possible that SCFAs produced by colonic fermentation provide a chronic stimulatory tone on L cells via apical or basolaterally located SCFA receptors. This could account for the circulating levels of active GLP-1 that are detectable even in the fasting state and amplify the responses to ingested nutrients, mediated, for example, via neurohormonal signals triggered by nutrient arrival higher up the gastrointestinal tract.
Consistent with the impaired responsiveness of ffar2−/− and ffar3−/− colonic cultures to SCFAs in vitro, we also observed lower GLP-1 levels in the knockout mice in vivo. As ffar2−/− mice exhibited both reduced colonic GLP-1 content and impaired SCFA-triggered secretion in vitro, we cannot conclude from our data which of these components underlies the reduced basal and glucose-triggered GLP-1 secretory responses in vivo. In ffar3−/− mice, however, the colonic GLP-1 content was not reduced, suggesting that the lower plasma GLP-1 levels reflect the impaired SCFA-triggered secretory response.
In addition to having low circulating GLP-1 levels, ffar2−/− and ffar3−/− mice exhibited impaired glucose tolerance. The reduced GLP-1 concentrations are likely to contribute to the impaired glucose homeostasis but may not be the only cause because ffar2 and ffar3 are not exclusively expressed in intestinal cells. In fact, they are better characterized as mediators of SCFA effects on immune cells and adipocytes (17,18,20), where they have been implicated in modulating neutrophil activation (18,20), reducing inflammation by inhibition of cytokine and chemokine expression, enhancing leptin production (29), stimulating adipogenesis, and inhibiting lipolysis (30,31). They are also expressed in pancreatic β and α cells (G. Tolhurst, H. Parker, A. Habib, F. Reimann, and F. Gribble, unpublished observations). Global knockout of these receptors may therefore affect adipocyte function, inflammation, or pancreatic β-cell function, which would themselves impact on glucose tolerance. The fact that we observed reduced insulin levels in the ffar2−/− mice during an oral glucose tolerance test but did not observe differences in insulin tolerance indicates that the observed impaired glucose tolerance in our model reflects an impairment of insulin secretion, possibly in part due to an impaired incretin axis. Another ffar2−/− mouse model was recently reported to exhibit increased food intake but had lower body weight and insulin levels compared with wild-type controls after a prolonged period on a high-fat diet (32). No explanation was found for the increased food intake, but our data suggest that it may be related to reduced secretion of L-cell peptides like GLP-1 and PYY. Unlike the findings of our study, those of Bjursell et al. (32) did not show a significant difference in glucose tolerance between chow-fed ffar2−/− and control mice. The apparent differences between the two knockout models may reflect the fact that the mice were maintained on different genetic backgrounds (C57B/6JOlaHsd vs. 129/SvEv) or that they were studied at different ages.
Interest in the gut microbiome has heightened recently, with the recognition that our complement of colonic bacteria may not simply reflect factors such as diet and illness but can also in return influence food intake and metabolism. Probiotics, prebiotics, and high-fiber diets are promoted for their potential beneficial effects on obesity, diabetes, inflammation, and immunity, as well as local effects on the health and integrity of the gut. One potential link between colonic microflora and systemic activity are SCFAs, which are produced by bacterial fermentation of fiber and which not only act as a local nutrient source but also trigger the release of anorectic hormones like GLP-1 and PYY. Our data suggest that SCFAs produced by bacterial fermentation in the gut can directly influence L cells to enhance the release of peptides such as GLP-1 and PYY. Whether this interrelationship is altered by metabolic conditions such as diabetes and obesity remains to be determined. FFAR2 should perhaps be added to the list of target receptors that may be exploitable for the pharmacological stimulation of the enteroendocrine system.
ACKNOWLEDGMENTS
This work was funded by Wellcome Trust grants to F.M.G. and F.R. (WT088357 and WT084210, respectively) and the European Union Seventh Framework Programme (FP7/2007-2013, grant agreement no. 266408).
The in vivo experiments on the knockout and control mice were performed at Takeda Cambridge Ltd, and the knockout mice used were originally created for Takeda Cambridge Ltd. The ffar2 selective agonist CFMB used in the study was synthesized for Takeda Cambridge Ltd. No other potential conflicts of interest relevant to this article were reported.
G.T., H.H., Y.S.L., H.E.P., A.M.H., E.D., and J.C. researched data. J.G. designed the study. F.R. and F.M.G. designed the study and wrote the manuscript.
The authors thank Keith Burling and the Medical Research Council Centre for Obesity and Related Disorders (Cambridge, U.K.) for performing plasma GLP-1 assays. Daniel Drucker (Samuel Lunenfeld Research Institute, Toronto, Canada) kindly provided the GLUTag cell line.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/10.2337/db11-1019/-/DC1.
G.T. and H.H. contributed equally to this work.
REFERENCES
Articles from Diabetes are provided here courtesy of American Diabetes Association
Full text links
Read article at publisher's site: https://doi.org/10.2337/db11-1019
Read article for free, from open access legal sources, via Unpaywall:
https://diabetesjournals.org/diabetes/article-pdf/61/2/364/559450/364.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.2337/db11-1019
Article citations
Unveiling the Longevity Potential of Natural Phytochemicals: A Comprehensive Review of Active Ingredients in Dietary Plants and Herbs.
J Agric Food Chem, 72(45):24908-24927, 31 Oct 2024
Cited by: 0 articles | PMID: 39480905 | PMCID: PMC11565747
Review Free full text in Europe PMC
Bitter taste receptors as sensors of gut luminal contents.
Nat Rev Gastroenterol Hepatol, 28 Oct 2024
Cited by: 0 articles | PMID: 39468215
Review
Harnessing Prebiotics to Improve Type 2 Diabetes Outcomes.
Nutrients, 16(20):3447, 11 Oct 2024
Cited by: 0 articles | PMID: 39458444 | PMCID: PMC11510484
Review Free full text in Europe PMC
What defines a healthy gut microbiome?
Gut, 73(11):1893-1908, 07 Oct 2024
Cited by: 0 articles | PMID: 39322314 | PMCID: PMC11503168
Review Free full text in Europe PMC
Microbiota-derived short chain fatty acids in pediatric health and diseases: from gut development to neuroprotection.
Front Microbiol, 15:1456793, 08 Oct 2024
Cited by: 0 articles | PMID: 39439941 | PMCID: PMC11493746
Review Free full text in Europe PMC
Go to all (1,078) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon.
Am J Physiol Gastrointest Liver Physiol, 315(1):G53-G65, 01 Mar 2018
Cited by: 161 articles | PMID: 29494208
The nuclear receptor FXR inhibits Glucagon-Like Peptide-1 secretion in response to microbiota-derived Short-Chain Fatty Acids.
Sci Rep, 10(1):174, 13 Jan 2020
Cited by: 34 articles | PMID: 31932631 | PMCID: PMC6957696
GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes.
Endocrinology, 154(10):3552-3564, 24 Jul 2013
Cited by: 282 articles | PMID: 23885020
Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release.
Digestion, 89(1):31-36, 20 Jan 2014
Cited by: 78 articles | PMID: 24458110
Review
Funding
Funders who supported this work.
European Commission FP7 (1)
Grant ID: FP7_266408
Medical Research Council (2)
Grant ID: G0600717B
MRC Centre for Translational Research in Obesity and related Metabolic Diseases
Prof Sir Stephen O'Rahilly, University of Cambridge
Grant ID: G0600717
Wellcome Trust (2)
Grant ID: WT084210
Enteroendocrine cell function in diabetes and obesity.
Prof Fiona Gribble, University of Cambridge
Grant ID: 088357





