Abstract
Free full text

High levels of estrogen enhance associative memory formation in ovariectomized females
Summary
The ovarian hormone estrogen is presumed to modulate processes of learning and memory in adulthood. In this study, we examined the effects of short-term estrogen replacement on associative memory formation. Adult ovariectomized female rats received two injections of estradiol (10, 20 or 40 µg) 24 h apart and were trained 4 h following each injection on the hippocampal-dependent task of trace eyeblink conditioning. Only the highest dose of estrogen, which produced plasma estradiol levels >250 pg/ml, enhanced conditioned responding. One day after the last injection, estrogen treated rats continued to exhibit elevated levels of conditioning and extinguished responding when the conditioned stimulus was no longer presented. Exposure to estrogen did not alter pain sensitivity or activity levels, but did greatly increase uterine weight. These results provide additional support to the view that that ovarian steroids are beneficial to the performance of certain forms of learning and memory tasks, albeit at supraphysiological doses. They are discussed with reference to hormone replacement and its effects on cognitive processes.
1. Introduction
The ovarian hormone estrogen has a profound influence on the morphological and electrophysiological properties of the hippocampus, a brain region implicated in certain forms of learning and memory. It has been shown that exposure to estrogen either exogenously or endogenously during proestrus greatly enhances the density of dendritic spines in area CA1 of the hippocampus (Gould et al., 1990; Woolley and McEwen, 1993; Shors et al., 2001). Over the 5-day estrous cycle of the rat, spine density can fluctuate as much as 30% (Woolley et al., 1990). Moreover, these changes in the dendritic spines have been shown to reflect changes in synapse density and to be accompanied by changes in astrocytic volume (Woolley and McEwen, 1992; Klintsova et al., 1995). In addition to these structural alterations, hippocampal electrophysiology is also sensitive to estrogen (Wong and Moss, 1992). For example, both in vivo and in vitro studies have shown that estrogen affects hippocampal excitability, as well as the induction of LTP (Warren et al., 1995; Cordoba Montoya and Carrer, 1997) and LTD (Desmond et al., 2000).
Such hormonally regulated changes in hippocampal synaptic plasticity may have important behavioral implications. Indeed, there are numerous studies that have examined the effects of estradiol on hippocampal-dependent learning. However, the results have been equivocal, with estrogen reported to impair, enhance or have no effect on performance. Studies examining performance in the spatial water maze task during the estrous cycle have reported small deficits (Frye, 1995; Warren and Juraska, 1997) or no difference (Berry et al., 1997) in females during exposure to proestrous levels of estrogen. In the radial arm maze, a test of spatial working memory, stable performance across the estrous cycle was demonstrated (Stackman et al., 1997). In contrast, our laboratory has observed that performance of both hippocampal-dependent and - independent types of classical eyeblink conditioning are enhanced during proestrus relative to other stages of estrus and to performance in males (Shors et al., 1998; Wood et al., 2001).
In hormone replacement studies, estrogen administration in ovariectomized rats enhances performance on some types of tasks but impairs performance on others. For example, estrogen improves spatial working memory aspects of the radial arm maze (Fader et al., 1999; Daniel et al., 1997; Luine et al., 1998; Bimonte and Denenberg, 1999; Gibbs, 1999; Korol and Kolo, 2002). Beneficial effects of estrogen replacement have been observed in spatial water maze tasks (O’Neal et al., 1996; Packard and Teather, 1997; Sandstrom and Williams, 2001). Estrogen has also been shown to adversely affect learning of conditioned place preference (Galea et al., 2001) and contextual fear memories (Markus and Zecevic, 1997; Gupta et al., 2001).
Although these studies differ with respect to route and duration of hormone administration, age of the animals and type of task used, circulating estradiol levels were within the physiological range, typically less than 100 pg/ml. However, there are several reports demonstrating that supraphysiological doses of estrogen have positive effects on cognition. For example, inhibitory avoidance performance is improved in rats treated with both physiological and supraphysiological doses of estradiol (Fugger et al., 2000; Frye and Rhodes, 2002). In humans, administration of estradiol after surgical menopause enhanced short-term memory when subjects were tested at a time when estradiol levels were supraphysiological (more than 4× that of their preoperative baseline) (Phillips and Sherwin, 1992). More recently, it was reported that postmenopausal women with Alzheimer’s disease treated with a high dose of estrogen exhibited enhanced attention and memory as compared to placebo treated controls (Asthana et al., 2001). High levels of plasma estrogen have also been shown to have a positive effect on mood in postmenopausal women (Klaiber et al., 1979; Sherwin, 1991; but see Schleifer et al., 2002).
Given the various reports that exogenous estrogen can affect learning processes, we evaluated the effects of both physiological and supraphysiological acute doses of estrogen on associative learning. Following ovariectomy, females were injected with differing doses of estradiol and trained on the hippocampal-dependent task of trace eyeblink conditioning. This task was chosen because it is affected by sex differences and stages of estrus (Solomon et al., 1986; Moyer et al., 1990; Shors, 1998; Beylin et al., 2001).
2. Materials and methods
2.1. Subjects and surgical procedures
Adult virgin female Sprague–Dawley rats (250–300 g) were obtained from Zivic Laboratories and housed individually prior to and following surgery in the Department of Psychology animal facility, Rutgers University. Rats had unlimited access to water and Purina Lab Chow (Ralston-Purina, St. Louis, MO) and were maintained on a 12:12 h light–dark cycle with light onset at 07:00 h. After at least a 1-week acclimation period, animals were anesthetized with 30 mg/kg pentobarbital injected intraperitoneally supplemented with isoflurane inhalant and bilaterally ovariectomized (OVX). Ovaries were removed through a small midline incision on the ventrum. After the removal of the ovaries, the muscle wall and skin were closed with absorbable suture. Immediately following OVX, rats underwent eyeblink surgery. In this surgery, a headstage with four electrodes was anchored to the skull with screws and acrylic. The electrodes were implanted subcutaneously to emerge through the ridge of the eyelid: two were used to deliver a periorbital shock to the eyelid and two detected eyeblinks by transmitting electromyographic activity. After surgery, 0.3 ml of penicillin (250,000 units/ml) was administered intramuscularly, and the rat kept warm until recovery from anesthesia. Postoperatively, rats were provided with 24 h access to acetaminophen (32 mg/ml; IDE, Interstate, Amityville, NY) diluted 1:100 in drinking water for two days, and allowed at least five days of recovery before behavioral training.
2.2. Conditioning procedure
Headstages were connected to a cable that allowed free movement within the conditioning chamber. All rats were acclimated to the conditioning apparatus for 1 h, with the ventilating fans and house lights operating and returned to their home cage immediately after acclimation. Twenty-four hours later, rats were returned to the conditioning chamber for measurement of spontaneous eyeblink activity, sensitization to an auditory cue and acquisition of the conditioned response. Spontaneous eyeblinks were recorded for thirty 500 ms periods at an interval of 25 ± 5 s. To detect any sensitized responding prior to training, responses to 10 white noise stimuli (250 ms, 83 dB, intertrial interval (ITI) 25 ± 5 s) were recorded. Eyeblinks during the first 100 ms of the white noise were recorded as sensitized responses. Acquisition of the conditioned response (CR) was evaluated using the trace paradigm. During trace conditioning, an 83 dB, 250 ms burst of white noise conditioned stimulus (CS) was separated from a 100 ms, 0.7 mA periorbital shock unconditioned stimulus (US) by a 500 ms trace interval. These stimulus parameters produce learning that is dependent on an intact hippocampus in rats (Beylin et al., 2001). Each block of trace conditioning consisted of 100 trials, with every 10-trial sequence composed of 1 CS-alone presentation, four paired presentations of the CS and US, a US-alone presentation, and four paired presentations of the CS and US. The ITI was 25 ± 5 s.
Eyelid EMG was bandpass filtered at 0.3–1.0 kHz, amplified (10K) with a differential AC amplifier, and passed to a 16 bit A/D card (Keithley-Metrabyte). To detect the occurrence of an eyeblink, the maximum EMG response occurring during a 250 ms prestimulus baseline recording period was added to four times its standard deviation. The responses that exceeded that value and had a width of at least 3 ms were considered eyeblinks. Eyeblinks were considered CRs if they occurred 500 ms prior to US onset.
2.3. Experiment 1
2.3.1. Methods
Adult virgin female rats underwent OVX and eyeblink surgery as described above. After at least five days of recovery, rats were injected subcutaneously with 10 µg (n = 7), 20 µg (n = 9) or 40 µg (n = 10) 17-β estradiol benzoate (Sigma) dissolved in sesame oil. Each group of estrogen treated rats was tested with a separate group of OVX rats injected with sesame oil vehicle (n = 8–10 rats/group). All rats were injected 4 h prior to undergoing 300 trials of trace eyeblink conditioning. Twenty-four hours later, rats were again injected with either the same dose of estradiol or vehicle and trained 4 h later for an additional 300 trials. Immediately following the last block of training, blood samples were collected via cardiac puncture for radioimmunoassay of hormone levels. Samples were added to the test tubes containing 0.1 ml heparin and centrifuged for 20 min at 3000 rpm. Plasma aliquots were stored at −20 °C and thawed before analysis. Circulating levels of estradiol were measured using a solid phase radioimmunoassay (RIA) system (Coat-A-Count; Diagnostic Products). Assay sensitivity for estradiol was 8 pg/ml and interassay variability was less than 7%.
2.3.2. Results
OVX females were categorized into three groups: lowest, moderate or highest on the basis of their circulating levels of estradiol (30–150 pg/ml, 151–250 pg/ml and over 250 pg/ml estradiol, respectively) as measured by RIA. This approach allowed us to directly associate blood levels of estrogen with performance on the trace conditioning task. ANOVA was used to analyze performance (percentage of CRs) over six blocks of 100 trials of trace conditioning with hormonal manipulation (estrogen versus vehicle) as the independent variable.
Administration of estradiol had a significant effect on conditioning in OVX females; however, the effect varied depending on the levels of plasma estradiol that were achieved by the two injections protocol (Fig. 1). There was no effect at the lowest [F(1, 13) = 0.73; p = 0.41] or moderate [F(1, 17) = 0.11; p = 0.75] levels of estradiol on percentage of CRs. However, there was an effect of the highest levels of estradiol in that OVX females with ~4× the physiological dose emitted more CRs than those injected with oil vehicle [F(1, 16) = 5.71; p = 0.03]. For all groups of ovariectomized females regardless of plasma estradiol levels, there was a main effect of training, with the percentage of CR increasing across blocks of trials [lowest: F(5, 65) = 17.45, p < 0.0001; moderate: F(5, 85) = 20.13, p < 0.0001; highest: F(5, 80) = 5.86, p < 0.001], suggesting that learning occurred in these groups. Also, estrogen treatment did not alter spontaneous blink rate [lowest: F(1, 13) = 0.01, p = 0.92; moderate: F(1, 17) = 0.02, p = 0.89; highest: F(1, 16) = 0, p = 1.0] or sensitized responses to the white noise stimulus prior to training [lowest: F(1, 13) = 0.81, p = 0.39; moderate: F(1, 17) = 0.35, p = 0.56; highest: F(1, 16) = 0.81, p = 0.38] at any of the estradiol levels, suggesting that estrogen did not enhance overall arousal or nonspecific responding to environmental stimuli.
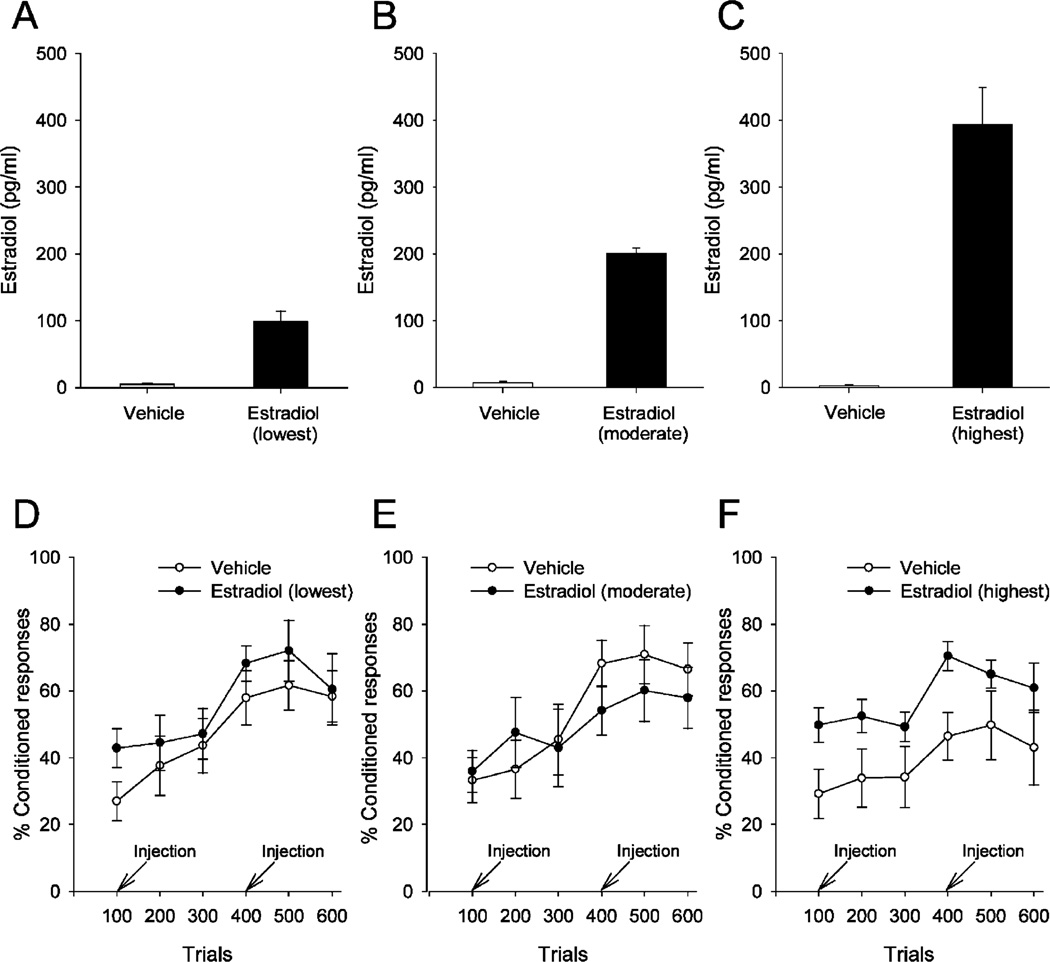
Effects of acute estrogen treatment on trace eyeblink conditioning in ovariectomized (OVX) female rats. Plasma estradiol (pg/ml) in OVX female rats were separated into three groups with levels in the range 30–150 pg/ml (lowest) (A), 151–250 pg/ml (moderate) (B) and >250 pg/ml (highest) (C). Four hours after the first injection of 17-β estradiol benzoate, rats were trained with the hippocampal-dependent task of trace eyeblink conditioning. Training continued the next day 4 h after a second injection. OVX females with the lowest (D) and moderate range (E) of plasma estradiol did not emit a greater percentage of CRs than the females injected with the oil vehicle (p > 0.05). However, females with the highest levels of estradiol (F) outperformed those injected with vehicle and thus emitted more conditioned responses (CRs) (p < 0.05). Error bars represent SEM.
2.4. Experiment 2
Our data from Experiment 1 suggest that acutely elevated circulating levels of estradiol enhance performance of the classically conditioned eyeblink response in OVX females. In Experiment 2, we examined whether the enhancing effect of estradiol persists 24 h after the last injection. In addition, we evaluated the effects of estradiol on extinction, activity and pain thresholds in a subset of animals following training. A separate subset of animals was used to examine the effects of estrogen treatment on uterine weight.
2.4.1. Methods
Adult virgin female rats underwent OVX and eyeblink surgery. Following recovery, rats received two subcutaneous injections of 40 µg 17-β estradiol benzoate (n = 14) or sesame oil vehicle (n = 11) 24 h apart in order to produce high circulating levels of estradiol. Four hours following each injection, OVX rats were exposed to 300 trials of trace conditioning. Twenty-four hours after the last injection, rats were trained for an additional 300 trials.
Following the last session of training, a subset of rats (estradiol, n = 10; vehicle, n = 8) were exposed to nonreinforced trials (no US) to assess whether estrogen affects extinction of the conditioned eyeblink response. Immediately after they had extinguished responding to the CS, we tested for potential effects of estradiol on gross motor activity and analgesic responses. For measurement of exploratory activity, rats were placed in a 30 cm2 Plexiglas chamber. The chamber was equipped with photobeams which detected beam crossings and horizontal movements of >4 cm and vertical movements (rearings) >15 cm. The measurements of analgesia, rats were gently held by the torso, and the tail positioned in a starting notch on a wooden board. A photobeam of increasing intensity was positioned above the tail, and the time to elicit a tailflick response was recorded four times and averaged for each rat. Another subset of animals (estradiol, n = 4; vehicle, n = 3) was overdosed immediately following the last training block and their uteri were removed and weighed while wet.
2.4.2. Results
In this experiment, OVX females were injected with the dose of estradiol that produced high plasma levels associated with enhanced conditioning in the first experiment. As before, injection with the high dose of estradiol increased the percentage of CRs across training [F(1, 23) = 6.47; p = 0.02], including during the last three blocks in which there was no estradiol injection [F(1, 23) = 4.78; p = 0.04] (Fig. 2A). Subgroups of animals were exposed to the CS alone after 900 training trials and decrease in CRs across trials was used as a measure of extinction. Although there was a general decrease in responses as groups were exposed to the CS alone [F(4, 64) = 4.24; p = 0.004], the groups injected with estradiol emitted more responses during extinction [F(1, 16) = 4.69; p = 0.04], an effect likely reflecting their enhanced rate of responding prior to extinction training. Thus, the group injected with estradiol emitted more CRs during training and achieved a higher level of responding; they also rapidly extinguished their responses. There was no effect of estradiol treatment on measures of pain sensitivity [F(1, 16) = 0.10; p = 0.75] (Fig. 2B) and horizontal activity [F(1, 16) = 0.04; p = 0.84] or rearing (vertical activity; [F(1, 16) = 1.3; p = 0.27]) (Fig. 2C). The remaining animals from each group were dissected and their uteri weighed. As expected, the uteri in females treated with two injections of 40 µg of estradiol were larger than in those injected with oil [F(1, 5) = 47:50; p < 0.001] (Fig. 2D).

High levels of estrogen are associated with enhanced trace conditioning. OVX rats received two injections of 40 µg 17-β estradiol benzoate and were trace conditioned 4 h after each injection as well as 24 h after the final injection. They were then extinguished with exposure to the CS alone. OVX females injected with 40 µg of estradiol emitted a greater percentage of CRs than those injected with oil vehicle (p < 0.05). More than 24 h after the second injection, estrogen treated rats continued to exhibit elevated levels of conditioning and extinguished responding when US was no longer presented with the CS (A). Acute exposure to estradiol did not alter pain sensitivity (B) or activity levels (C), but did increase uterine weight (D). Error bars represent SEM.
3. Discussion
In two experiments, we show that acute exposure to exogenous estrogen can enhance performance in ovariectomized female rats that are trained on the hippocampal-dependent learning task of trace eyeblink conditioning. In the first experiment, different levels of plasma estradiol were associated with different degrees of conditioning. Only those females with very high levels of estradiol (>250 pg/ml) emitted more learned responses than their respective oil-treated controls. The enhanced performance was evident 4 h after the first injection and remained elevated the next day 4 h after a second injection. In a second experiment, we found that injection of the highest dose of estradiol (40 µg) again increased the performance of the trace memory task. The enhanced responding persisted more than 24 h after the last injection. Also, both females treated with estrogen and those injected with the vehicle extinguished responding when the conditioned stimulus was no longer presented, with no difference in performance between the groups. Overall, the results from this study support others suggesting that ovarian steroids enhance performance during training on certain types of learning and memory tasks (O’Neal et al., 1996; Daniel et al., 1997; Packard and Teather, 1997; Bimonte and Denenberg, 1999; Gibbs, 1999; Sandstrom and Williams, 2001; Korol and Kolo, 2002), at least at high albeit supraphysiological doses (Fugger et al., 2000; Frye and Rhodes, 2002). However, it is important to note that while estrogen treated OVX females displayed significantly more learned responses during training, all the groups showed a significant increase in responding across training trials and a decrease in responding during extinction, including those with negligible levels of estradiol after OVX. Thus, it can be concluded that the removal of estrogen does not prevent females from acquiring the trace memory and extinguishing the learned association. Rather, it appears that exposure to very high levels of estradiol enhances trace conditioning and thus modulates the performance of this task. Modulatory effects of estrogen on spatial navigation in the Morris water maze have also been reported (Chesler and Juraska, 2000).
Our data suggest that estrogen modulates trace conditioning and perhaps learning itself. However, nonspecific effects on sensory/motor processes cannot be ruled out. We did not observe any effects of estradiol on blinking alone or eyeblink responses to the auditory stimulus alone prior to training. These data suggest that exposure to estradiol is not enhancing the overall response rate of animals or inducing sensitized responses to the CS. Moreover, estrogen treatment did not alter gross pain sensitivity or general activity 24 h after the last injection, a time period when conditioned responding was still elevated compared to vehicle-injected controls.
Our laboratory has previously reported females in proestrus outperform males and females in other stages of estrus. We have observed this effect during both the hippocampal-dependent task of trace conditioning and the hippocampal-independent task of delay conditioning (Shors et al., 1998; Wood et al., 2001). The present findings suggest that inducing proestrous levels of estrogen does not necessarily enhance performance and thereby suggests that estrogen is necessary but not sufficient for the greater levels of eyeblink conditioning observed during proestrus. There are several other modulators that could interact with estrogen to affect the learning processes. For example, proestrus is associated with high levels of progesterone, which has been shown to influence some types of memories either by itself or in combination with estrogen (Farr et al., 1995; Sturgis and Frye, 1995; Chesler and Juraska, 2000; Sandstrom and Williams, 2001). It is also possible that the cyclic nature of the estrus cycle allows for the changes in performance and without such variation, the positive association between the levels of estrogen and learning cannot be expressed. Finally, it is noted that the animals were trained relatively soon after the first injection—within hours—a time frame that may be too short for receptor-mediated effects at the nucleus. In fact, the rapidity with which estrogen enhanced acquisition of the conditioned eyeblink response suggests that the traditional genomic receptor mediated mechanism may not be operative, at least not during early acquisition. Instead, nongenomic effects through steroid membrane receptors may account for these effects on learning (Mermelstein et al., 1996; Fugger et al., 2000; Frye and Rhodes, 2002; Luine et al., 2003).
The hippocampus is one likely site for mediating these effects of estrogen on learning. The structure is critically involved in acquisition of the CR during trace conditioning (Solomon et al., 1986; Moyer et al., 1990; Beylin et al., 2001) and possesses a relatively high concentration of estrogen receptors, although the distribution of alpha and beta subtypes is still unclear (Shughrue et al., 1997). Probably the most compelling evidence that the hippocampus may be involved comes from studies in which direct injections of estradiol into the hippocampus improve performance on spatial navigation tasks (Packard and Teather, 1997). Similar effects have been reported for tasks that are not dependent on the hippocampus for learning itself (Packard, 1998; Frye and Rhodes, 2002). Others have suggested that estrogen affects performance by shifting the relative contribution of differing memory systems used to solve particular tasks (Packard, 1998; Korol and Kolo, 2002). Of course, it is possible that the modulation of conditioning by estrogen results from its effects on other brain regions. For example, the cerebellum is a region critical for learning the classically conditioned eyeblink response (McCormick and Thompson, 1984; Woodruff-Pak et al., 1985) and contains an abundance of mRNA for estrogen receptors (Shughrue et al., 1997). Moreover, the firing rates of cerebellar Purkinje cells vary across the estrous cycle indicating that estrogen modulates electrical activity within this region (Smith, 1994). There are also studies suggesting that estrogen can affect cognitive processes through interactions in the frontal cortex (Luine et al., 1998).
Exogenous administration of estradiol at the levels provided here can increase the density of dendritic spines in area CA1 of the hippocampus. These changes also occur across the estrous cycle (Gould et al., 1990; Woolley et al., 1990; Woolley and McEwen, 1993; Shors et al., 2001). The functional significance of these dendritic responses to estrogen is unknown although it is often speculated that they are involved in the formation of new memories. Recently, we found that associative memory formation did enhance spine density in the hippocampus (Leuner et al., 2003). This effect of learning was localized to basal dendrites in stratum oriens of area CA1. Given the apparent association between the presence of dendritic spines and learning, we anticipated that estrogen dosages that enhance spine density would also enhance learning. Such an enhancement did not occur, although we may have tested the animals too soon after the injection (4 h instead of >24 h which is when the increase in spine density is evident; Woolley and McEwen, 1993). Thus, we do not claim to have dissociated these two measures. The findings reported here may be more relevant to the studies in which very high doses of estrogen are provided to women during and after menopause or following hysterectomy. For example, there is a report that after surgical menopause, women exposed to a similar dose as used here exhibited a short-term memory enhancement (Phillips and Sherwin, 1992). Also, postmenopausal women with Alzheimer’s disease exhibit enhanced attention and memory following exposure to high doses of estrogen, although using a more chronic regimen than used in the present study (Asthana et al., 2001).
With these caveats in mind, the data presented here may provide insight into some of the discrepancies in the literature regarding estrogen’s presumed effects on cognition. First, estrogens do not universally affect learning processes and may even be unlikely to do so without other modulators and co-factors. Second, enhancing effects can be induced at doses not observed in nature but often observed in treated females. Finally, the very significant proliferative effect of the high dose of estrogen on uterine weight should temper any enthusiasm for using such doses to affect learning and memory performance in women. This last point is highlighted by recent findings from the Women’s Health Initiative showing increased risk for heart disease, stroke, breast cancer as well as dementia in postmenopausal women taking estrogen. In these studies, however, the women were also treated with progestin (Writing Group for the Women’s Health Initiative Investigators, 2002; Shumaker et al., 2003). We anxiously await the results of ongoing studies that are evaluating the effects of estrogen alone on women’s health, including their cognitive abilities.
Acknowledgements
We thank M. Slomovits for assistance with data collection. This work was supported by the National Institute of Mental Health (59970) and the National Alliance for Research on Schizophrenia and Depression (to T.J.S.). B.L. was supported by a predoctoral NRSA fellowship (MH63568).
References
- Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Rasking MA, Plymate SR. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001;57:605–612. [Abstract] [Google Scholar]
- Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav. Neurosci. 1997;111:267–274. [Abstract] [Google Scholar]
- Beylin AV, Gandhi CC, Wood G, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontiguity or task difficulty? Neurobiol. Learn. Mem. 2001;76:447–461. [Abstract] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. [Abstract] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Horm. Behav. 2000;38:234–242. [Abstract] [Google Scholar]
- Cordoba Montoya DA, Carrer HF. Estrogen facilitates induction of long-term potentiation in the hippocampus of awake rats. Brain Res. 1997;778:430–438. [Abstract] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm. Behav. 1997;32:217–225. [Abstract] [Google Scholar]
- Desmond NL, Zhang DX, Levy WB. Estradiol enhances the induction of homosynaptic long-term depression in the CA1 region of the adult ovariectomized rat. Neurobiol. Learn. Mem. 2000;73:180–187. [Abstract] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmamcol. Biochem. Behav. 1999;62:711–717. [Abstract] [Google Scholar]
- Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE. Effect of ovarian steroids on foot-shock avoidance and retention in female mice. Physiol. Behav. 1995;58:715–723. [Abstract] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol. Behav. 1995;57:5–14. [Abstract] [Google Scholar]
- Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956:285–293. [Abstract] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson JA, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–264. [Abstract] [Google Scholar]
- Galea LAM, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav. Brain Res. 2001;126:115–126. [Abstract] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm. Behav. 1999;36:222–233. [Abstract] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990;10:1286–1291. [Abstract] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats. Brain Res. 2001;888:356–365. [Abstract] [Google Scholar]
- Klaiber EL, Broverman EM, Vogel W, Kobayashi Y. Estrogen therapy for severe and persistent depressions in women. Arch. Gen. Psychiatry. 1979;36:550–554. [Abstract] [Google Scholar]
- Klintsova A, Levy WB, Desmond NL. Astrocytic volume fluctuates in the hippocampal CA1 region across the estrous cycle. Brain Res. 1995;690:269–274. [Abstract] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav. Neurosci. 2002;116:411–420. [Abstract] [Google Scholar]
- Leuner BL, Falduto JF, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003;23:659–665. [Europe PMC free article] [Abstract] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm. Behav. 1998;34:149–162. [Abstract] [Google Scholar]
- Luine V, Jacome LF, Maclusky N, Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. [Abstract] [Google Scholar]
- Markus EJ, Zecevic M. Sex differences and estrous cycle changes in hippocampus-dependent fear conditioning. Psychobiology. 1997;25:246–252. [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. [Abstract] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci. 1996;16:595–604. [Abstract] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace-eyeblink conditioning in rabbits. Behav. Neurosci. 1990;104:243–252. [Abstract] [Google Scholar]
- O’Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water escape working memory task. Psychoneuroendocrinology. 1996;21:51–65. [Abstract] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm. Behav. 1998;34:126–139. [Abstract] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997;8:3009–3013. [Abstract] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. [Abstract] [Google Scholar]
- Sandstrom NL, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci. 2001;115:384–393. [Abstract] [Google Scholar]
- Schleifer LA, Justice AJH, de Wit H. Lack of effects of acute estradiol on mood in postmenopausal women. Pharmacol. Biochem. Behav. 2002;71:71–77. [Abstract] [Google Scholar]
- Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J. Clin. Endocrinol. Metab. 1991;72:336–343. [Abstract] [Google Scholar]
- Shors TJ. Stress and sex effects on associative learning: for better or for worse. Neuroscientist. 1998;4:353–364. [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Matthew PR, Pickett J. Stages of estrus mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. [Abstract] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. [Abstract] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. [Abstract] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. J. Am. Med. Assoc. 2003;289:2651–2662. [Abstract] [Google Scholar]
- Smith SS. Female sex steroid hormones: from receptors to networks to performance—actions on the sensorimotor system. Prog. Neurobiol. 1994;44:55–86. [Abstract] [Google Scholar]
- Solomon PR, van der Schaaf ER, Weisz D, Thompson RF. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Neuroscience. 1986;100:729–744. [Abstract] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol. Learn. Mem. 1997;67:167–171. [Abstract] [Google Scholar]
- Sturgis JD, Frye CA. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobiol. Learn. Mem. 1995;64:83–96. [Abstract] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav. Neurosci. 1997;111:259–266. [Abstract] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. [Abstract] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electro-physiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J. Neurosci. 1992;12:3217–3225. [Abstract] [Google Scholar]
- Wood GE, Beylin A, Shors TJ. The contribution of adrenal and reproductive hormones to the sexually opposed effects of stress on trace conditioning. Behav. Neurosci. 2001;115:1–13. [Abstract] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar lesions but not lateral cerebellar cortex aspirations. Brain Res. 1985;2:249–260. [Abstract] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 1992;12:2549–2554. [Abstract] [Google Scholar]
- Woolley CS, McEwen BS. Role of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 1993;336:293–306. [Abstract] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10:4035–4039. [Abstract] [Google Scholar]
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. J. Am. Med. Assoc. 2002;288:321–333. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.psyneuen.2003.08.001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3289540?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Dimensional Affective Sensitivity to Hormones across the Menstrual Cycle (DASH-MC): A transdiagnostic framework for ovarian steroid influences on psychopathology.
Mol Psychiatry, 15 Aug 2024
Cited by: 1 article | PMID: 39143323
Review
The Impacts of Sex Differences and Sex Hormones on Fear Extinction.
Curr Top Behav Neurosci, 64:105-132, 01 Jan 2023
Cited by: 1 article | PMID: 37528309
Social ascent changes cognition, behaviour and physiology in a highly social cichlid fish.
Philos Trans R Soc Lond B Biol Sci, 377(1845):20200448, 10 Jan 2022
Cited by: 7 articles | PMID: 35000445 | PMCID: PMC8743896
Intranasal oxytocin compensates for estrus cycle-specific reduction of conditioned safety memory in rats: Implications for psychiatric disorders.
Neurobiol Stress, 14:100313, 10 Mar 2021
Cited by: 3 articles | PMID: 33778132 | PMCID: PMC7985696
Intact Female Mice Acquire Trace Eyeblink Conditioning Faster than Male and Ovariectomized Female Mice.
eNeuro, 8(2):ENEURO.0199-20.2021, 09 Mar 2021
Cited by: 3 articles | PMID: 33531367 | PMCID: PMC7986530
Go to all (65) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Differential effects of estrogen on hippocampal- and striatal-dependent learning.
Neurobiol Learn Mem, 84(2):132-137, 01 Sep 2005
Cited by: 69 articles | PMID: 16054404
Estrogen abolishes latent inhibition in ovariectomized female rats.
Brain Cogn, 66(2):156-160, 10 Aug 2007
Cited by: 19 articles | PMID: 17693005
Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation.
Endocrinology, 147(1):607-614, 20 Oct 2005
Cited by: 164 articles | PMID: 16239296
Funding
Funders who supported this work.
NIMH NIH HHS (7)
Grant ID: R01 MH059970-04
Grant ID: F31 MH063568-01A2
Grant ID: MH63568
Grant ID: F31 MH063568
Grant ID: R01 MH059970-03
Grant ID: R01 MH059970-02
Grant ID: R01 MH059970
PHS HHS (1)
Grant ID: 59970




