Abstract
Free full text

The N-terminal part of the E.coli DNA binding protein FIS is essential for stimulating site-specific DNA inversion but is not required for specific DNA binding.
Abstract
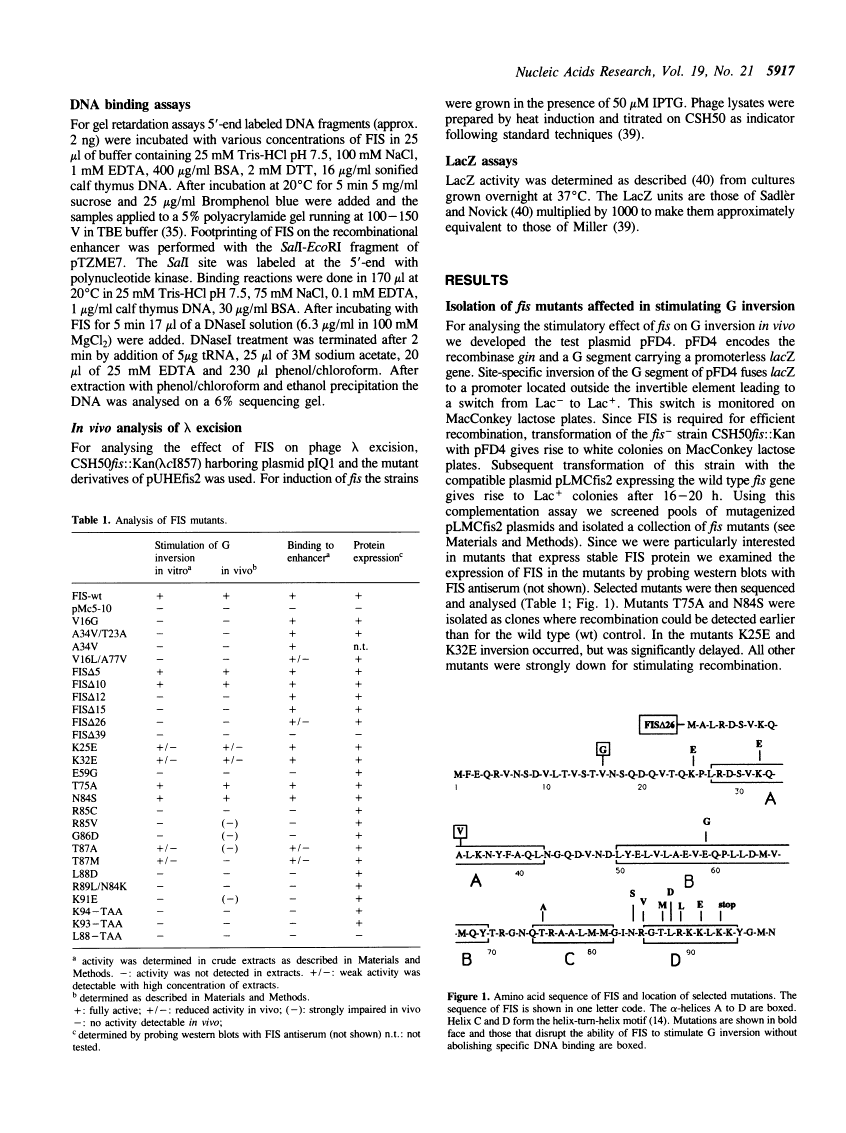
FIS protein is involved in several different cellular processes stimulating site-specific recombination in phages Mu and lambda as well as transcription of stable RNA operons in E.coli. We have performed a mutational analysis of fis and provide genetic and biochemical evidence that a truncated version of FIS lacking the N-terminal region is sufficient for specific DNA binding and for stimulating lambda excision. These mutants also retain their ability to autoregulate fis gene expression. Such mutant proteins, however, cannot stimulate the enhancer dependent DNA inversion reaction.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig NL. The mechanism of conservative site-specific recombination. Annu Rev Genet. 1988;22:77–105. [Abstract] [Google Scholar]
- Kahmann R, Rudt F, Koch C, Mertens G. G inversion in bacteriophage Mu DNA is stimulated by a site within the invertase gene and a host factor. Cell. 1985 Jul;41(3):771–780. [Abstract] [Google Scholar]
- Johnson RC, Simon MI. Hin-mediated site-specific recombination requires two 26 bp recombination sites and a 60 bp recombinational enhancer. Cell. 1985 Jul;41(3):781–791. [Abstract] [Google Scholar]
- Mertens G, Klippel A, Fuss H, Blöcker H, Frank R, Kahmann R. Site-specific recombination in bacteriophage Mu: characterization of binding sites for the DNA invertase Gin. EMBO J. 1988 Apr;7(4):1219–1227. [Europe PMC free article] [Abstract] [Google Scholar]
- Bruist MF, Glasgow AC, Johnson RC, Simon MI. Fis binding to the recombinational enhancer of the Hin DNA inversion system. Genes Dev. 1987 Oct;1(8):762–772. [Abstract] [Google Scholar]
- Hübner P, Arber W. Mutational analysis of a prokaryotic recombinational enhancer element with two functions. EMBO J. 1989 Feb;8(2):577–585. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson RC, Glasgow AC, Simon MI. Spatial relationship of the Fis binding sites for Hin recombinational enhancer activity. Nature. 1987 Oct 1;329(6138):462–465. [Abstract] [Google Scholar]
- Johnson RC, Ball CA, Pfeffer D, Simon MI. Isolation of the gene encoding the Hin recombinational enhancer binding protein. Proc Natl Acad Sci U S A. 1988 May;85(10):3484–3488. [Europe PMC free article] [Abstract] [Google Scholar]
- Koch C, Vandekerckhove J, Kahmann R. Escherichia coli host factor for site-specific DNA inversion: cloning and characterization of the fis gene. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4237–4241. [Europe PMC free article] [Abstract] [Google Scholar]
- Harrison SC, Aggarwal AK. DNA recognition by proteins with the helix-turn-helix motif. Annu Rev Biochem. 1990;59:933–969. [Abstract] [Google Scholar]
- Kostrewa D, Granzin J, Koch C, Choe HW, Raghunathan S, Wolf W, Labahn J, Kahmann R, Saenger W. Three-dimensional structure of the E. coli DNA-binding protein FIS. Nature. 1991 Jan 10;349(6305):178–180. [Abstract] [Google Scholar]
- Thompson JF, Landy A. Empirical estimation of protein-induced DNA bending angles: applications to lambda site-specific recombination complexes. Nucleic Acids Res. 1988 Oct 25;16(20):9687–9705. [Europe PMC free article] [Abstract] [Google Scholar]
- Hübner P, Haffter P, Iida S, Arber W. Bent DNA is needed for recombinational enhancer activity in the site-specific recombination system Cin of bacteriophage P1. The role of FIS protein. J Mol Biol. 1989 Feb 5;205(3):493–500. [Abstract] [Google Scholar]
- Kanaar R, van de Putte P, Cozzarelli NR. Gin-mediated DNA inversion: product structure and the mechanism of strand exchange. Proc Natl Acad Sci U S A. 1988 Feb;85(3):752–756. [Europe PMC free article] [Abstract] [Google Scholar]
- Heichman KA, Johnson RC. The Hin invertasome: protein-mediated joining of distant recombination sites at the enhancer. Science. 1990 Aug 3;249(4968):511–517. [Abstract] [Google Scholar]
- Thompson JF, Moitoso de Vargas L, Koch C, Kahmann R, Landy A. Cellular factors couple recombination with growth phase: characterization of a new component in the lambda site-specific recombination pathway. Cell. 1987 Sep 11;50(6):901–908. [Abstract] [Google Scholar]
- Ball CA, Johnson RC. Efficient excision of phage lambda from the Escherichia coli chromosome requires the Fis protein. J Bacteriol. 1991 Jul;173(13):4027–4031. [Europe PMC free article] [Abstract] [Google Scholar]
- Bétermier M, Lefrère V, Koch C, Alazard R, Chandler M. The Escherichia coli protein, Fis: specific binding to the ends of phage Mu DNA and modulation of phage growth. Mol Microbiol. 1989 Apr;3(4):459–468. [Abstract] [Google Scholar]
- Nilsson L, Vanet A, Vijgenboom E, Bosch L. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 1990 Mar;9(3):727–734. [Europe PMC free article] [Abstract] [Google Scholar]
- Ross W, Thompson JF, Newlands JT, Gourse RL. E.coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990 Nov;9(11):3733–3742. [Europe PMC free article] [Abstract] [Google Scholar]
- Klippel A, Cloppenborg K, Kahmann R. Isolation and characterization of unusual gin mutants. EMBO J. 1988 Dec 1;7(12):3983–3989. [Europe PMC free article] [Abstract] [Google Scholar]
- Kramer W, Drutsa V, Jansen HW, Kramer B, Pflugfelder M, Fritz HJ. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. [Europe PMC free article] [Abstract] [Google Scholar]
- Lanzer M, Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8973–8977. [Europe PMC free article] [Abstract] [Google Scholar]
- Bujard H, Gentz R, Lanzer M, Stueber D, Mueller M, Ibrahimi I, Haeuptle MT, Dobberstein B. A T5 promoter-based transcription-translation system for the analysis of proteins in vitro and in vivo. Methods Enzymol. 1987;155:416–433. [Abstract] [Google Scholar]
- Stanssens P, Opsomer C, McKeown YM, Kramer W, Zabeau M, Fritz HJ. Efficient oligonucleotide-directed construction of mutations in expression vectors by the gapped duplex DNA method using alternating selectable markers. Nucleic Acids Res. 1989 Jun 26;17(12):4441–4454. [Europe PMC free article] [Abstract] [Google Scholar]
- Klippel A, Mertens G, Patschinsky T, Kahmann R. The DNA invertase Gin of phage Mu: formation of a covalent complex with DNA via a phosphoserine at amino acid position 9. EMBO J. 1988 Apr;7(4):1229–1237. [Europe PMC free article] [Abstract] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. [Abstract] [Google Scholar]
- Koch C, Kahmann R. Purification and properties of the Escherichia coli host factor required for inversion of the G segment in bacteriophage Mu. J Biol Chem. 1986 Nov 25;261(33):15673–15678. [Abstract] [Google Scholar]
- Choe HW, Labahn J, Itoh S, Koch C, Kahmann R, Saenger W. Crystallization of the DNA-binding Escherichia coli protein FIS. J Mol Biol. 1989 Jul 5;208(1):209–210. [Abstract] [Google Scholar]
- Mertens G, Fuss H, Kahmann R. Purification and properties of the DNA invertase gin encoded by bacteriophage Mu. J Biol Chem. 1986 Nov 25;261(33):15668–15672. [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Tabor S, Richardson CC. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. [Europe PMC free article] [Abstract] [Google Scholar]
- Derbyshire KM, Salvo JJ, Grindley ND. A simple and efficient procedure for saturation mutagenesis using mixed oligodeoxynucleotides. Gene. 1986;46(2-3):145–152. [Abstract] [Google Scholar]
- SADLER JR, NOVICK A. THE PROPERTIES OF REPRESSOR AND THE KINETICS OF ITS ACTION. J Mol Biol. 1965 Jun;12:305–327. [Abstract] [Google Scholar]
- Garner MM, Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. [Europe PMC free article] [Abstract] [Google Scholar]
- Fried M, Crothers DM. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu HM, Crothers DM. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. [Abstract] [Google Scholar]
- Travers AA. DNA conformation and protein binding. Annu Rev Biochem. 1989;58:427–452. [Abstract] [Google Scholar]
- Johnson RC, Bruist MF. Intermediates in Hin-mediated DNA inversion: a role for Fis and the recombinational enhancer in the strand exchange reaction. EMBO J. 1989 May;8(5):1581–1590. [Europe PMC free article] [Abstract] [Google Scholar]
- Osuna R, Finkel SE, Johnson RC. Identification of two functional regions in Fis: the N-terminus is required to promote Hin-mediated DNA inversion but not lambda excision. EMBO J. 1991 Jun;10(6):1593–1603. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/19.21.5915
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC329047
Citations & impact
Impact metrics
Article citations
Testing mechanisms of DNA sliding by architectural DNA-binding proteins: dynamics of single wild-type and mutant protein molecules in vitro and in vivo.
Nucleic Acids Res, 49(15):8642-8664, 01 Sep 2021
Cited by: 9 articles | PMID: 34352099 | PMCID: PMC8421229
Crystal structure of the nucleoid-associated protein Fis (PA4853) from Pseudomonas aeruginosa.
Acta Crystallogr F Struct Biol Commun, 76(pt 5):209-215, 29 Apr 2020
Cited by: 0 articles | PMID: 32356522 | PMCID: PMC7193516
Cooperative DNA binding by proteins through DNA shape complementarity.
Nucleic Acids Res, 47(16):8874-8887, 01 Sep 2019
Cited by: 10 articles | PMID: 31616952 | PMCID: PMC7145599
DNA Sequence Determinants Controlling Affinity, Stability and Shape of DNA Complexes Bound by the Nucleoid Protein Fis.
PLoS One, 11(3):e0150189, 09 Mar 2016
Cited by: 28 articles | PMID: 26959646 | PMCID: PMC4784862
Site-specific DNA Inversion by Serine Recombinases.
Microbiol Spectr, 3(3):1-36, 01 Feb 2015
Cited by: 16 articles | PMID: 25844275 | PMCID: PMC4384473
Go to all (44) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Identification of two functional regions in Fis: the N-terminus is required to promote Hin-mediated DNA inversion but not lambda excision.
EMBO J, 10(6):1593-1603, 01 Jun 1991
Cited by: 84 articles | PMID: 1851089 | PMCID: PMC452825
Escherichia coli host factor for site-specific DNA inversion: cloning and characterization of the fis gene.
Proc Natl Acad Sci U S A, 85(12):4237-4241, 01 Jun 1988
Cited by: 83 articles | PMID: 2837762 | PMCID: PMC280402
The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli.
Nucleic Acids Res, 19(15):4167-4172, 01 Aug 1991
Cited by: 118 articles | PMID: 1870971 | PMCID: PMC328557
The Fis protein: it's not just for DNA inversion anymore.
Mol Microbiol, 6(22):3257-3265, 01 Nov 1992
Cited by: 206 articles | PMID: 1484481
Review