Abstract
Free full text

Immunosuppressive exosomes from TGF-β1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells
Associated Data
Dear Editor,
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease, is generally believed to originate from the complicated interactions between genetic factors and commensal flora. This leads to the breakdown of innate immunity and the aberrant activation of the immune system, which is largely responsible for tissue damages in patients and animal models 1. A new CD4+ T cell lineage, Th17, which predominantly produces IL-17, has been revealed recently to play a key role in the development of IBD. Studies in IL-17 receptor A (IL-17RA)-knockout mice have demonstrated that IL-17 is necessary for the development of IBD. Blockade of IL-17 signaling by an IL-17RA-IgG1 fusion protein significantly attenuated IBD 2. It has also been shown that the CD4+ T cells that express Foxp3 (Tregs) are the major regulatory T cells that help maintain intestinal homeostasis 3. Despite the further understanding of the mechanisms of IBD, effective treatment is still lacking 4. Therefore, it is necessary to explore new clues and targets for therapeutic interventions.
Exosomes are small vesicles released by various live cells with a structure of lipid bilayer and diameter ranging from 50 to 100 nm. They are formed by the fusion of multivesicular bodies with the plasma membrane followed by exocytosis 5. It has been demonstrated that human tumor-derived exosomes can selectively impair lymphocyte responses to IL-2 and then impair their effector functions 6. In addition, exosomes from immune inhibitory gene-modified bone marrow-derived dendritic cell (BMDC) have been found to suppress inflammation and collagen-induced arthritis 7. Therefore, it is conceivable that exosomes may be used as vehicles for therapeutic intervention of autoimmune diseases.
In the present study, we were interested in whether exosomes derived from the TGF-β1 gene-modified BMDC (TGF-β1-EXO) have the immunosuppressive function and play a protective role during IBD development. First, we identified the profile of exosomes that we prepared. Under the electron microscope, the exosomes displayed typical characteristic of round morphology with a diameter of 50-100 nm. Immunoblotting results demonstrated that all types of exosomes contained the exosome-associated proteins Hsp70, Tsg101 and CD9, whereas ER-residing protein Grp94 was absent in exosomes (Supplementary information, Figure S1). We also examined the level of TGF-β1 in exosomes. The total amount of TGF-β1 in TGF-β1-EXO was about 15 pg/μg of exosomes and no TGF-β1 was expressed in exosomes derived from non-gene-modified BMDC (Control-EXO) or LacZ gene-modified BMDC (LacZ-EXO) (data not shown). When examined in mixed lymphocyte reaction system, Control-EXO and LacZ-EXO inhibited T cell proliferation at low-dose level. It was proposed that the inhibition was due to their low-level expression of costimulatory molecules. The T cell proliferation was promoted when dose was increased. Exosomes contain a large amount of allo-MHC molecules, which can be taken up by dendritic cells (DCs) and presented to T cells for allostimulation. This might be the reason why Control-EXO or LacZ-EXO did not inhibit, but promoted T-cell proliferation at a higher dose. On the other hand, TGF-β1-EXO showed a stronger inhibitory effect on T cell proliferation than on Control-EXO or LacZ-EXO via TGF-β1 and the effect was dose dependent (Supplementary information, Figure S2). We also analyzed the phenotype of exosomes by FACS (Supplementary information, Figure S3). In our results, there were no obvious difference in the expression level of costimulatory and adhesion molecules among Control-EXO, LacZ-EXO and except that TGF-β1-EXO express lower MHC class II molecules, which could explain that TGF-β1-EXO still showed stronger inhibitory effect than Control-EXO and LacZ-EXO after TGF-β1 was bloked. Moreover, TGF-β1-EXO plus anti-CD9 showed similar inhibitory effect to TGF-β1-EXO alone, excluding the possibility that anti-TGF-β1 can affect T cell modulatory properties of TGF-β1-EXO through enhancing exosome capture by BMDC after antibody binding. These results further confirm that the inhibitory effect of TGF-β1-EXO is TGF-β1 dependent (Supplementary information, Figure S2).
Then we explored the effect of TGF-β1-EXO during IBD development. We found that in vivo administration of Control-EXO or LacZ-EXO did not prevent DSS-induced weight loss, whereas administration of TGF-β1-EXO significantly prevented the weight loss (Figure 1A). The disease activity index (DAI) scores and intestinal bleeding were also significantly reduced by the administration of TGF-β1-EXO, but not Control-EXO or LacZ-EXO (Figure 1B and and1C).1C). Histological assessment of colonic damage revealed that the mice which were untreated or received Control-EXO or LacZ-EXO suffered severe diffuse inflammation involving the mucosa, submucosa, and in some cases extending through all intestinal layers (transmural inflammation). There was also pronounced disruption of the normal architecture and crypt loss. In contrast, DSS-mice treated with TGF-β1-EXO had much less damage in colon tissues, showing more conserved glandular structure and limited leukocyte infiltrations in mucosa and submucosa (Figure 1D). These results indicated that in vivo administration of TGF-β1-EXO could effectively inhibit the development of DSS-induced murine IBD. To further reveal the protective advantage of TGF-β1-EXO during the development of IBD, we compared the protective effect of TGF-β1-EXO and TGF-β1 cytokine. We found that 450 pg TGF-β1, the total amount in TGF-β1-EXO that we injected, showed no protective effect to IBD, and even if a higher dose of 4 500 pg TGF-β1 was injected, it showed only slight protective effect. The protective effect of TGF-β1-EXO was much better than TGF-β1 alone (Figure 1E). Although TGF-β1 is proved to play an important role in the protection of IBD, we could not detect the increase of TGF-β1 secretion at the inflammatory site (data not shown). The total TGF-β1 in exosomes that we injected into mice was about 450 pg; after diffusion in vivo, it was far from the detectable level. We supposed that the stability of TGF-β1 in TGF-β1-EXO would be favorable for the protective effect of TGF-β1-EXO, because we found that TGF-β1 presented in the form of exosomes showed stronger stability than TGF-β1 cytokine when digested by trypsin (Supplementary information, Figure S4). Moreover, the stability was related to the intact membrane structure of exosomes because disruption of the membrane structure of exosomes could decrease the ability of TGF-β1 and TSG101, another exosomal protein, in TGF-β1-EXO to resist the digestion of trypsin (Figure 1F). Inflammatory cells secret large amounts of proinflammatory cytokines such as TNF-α and IL-1, which increase the endotheliocytes to express ligands of adhesion molecule. Exosomes from BMDC are reported to express many kinds of adhesion molecules 8. It is possible that exosomes will be recruited to an inflammatory site and cause the enriched effect of TGF-β1, which may be another factor that TGF-β1-EXO possess better protective effect than TGF-β1 alone.
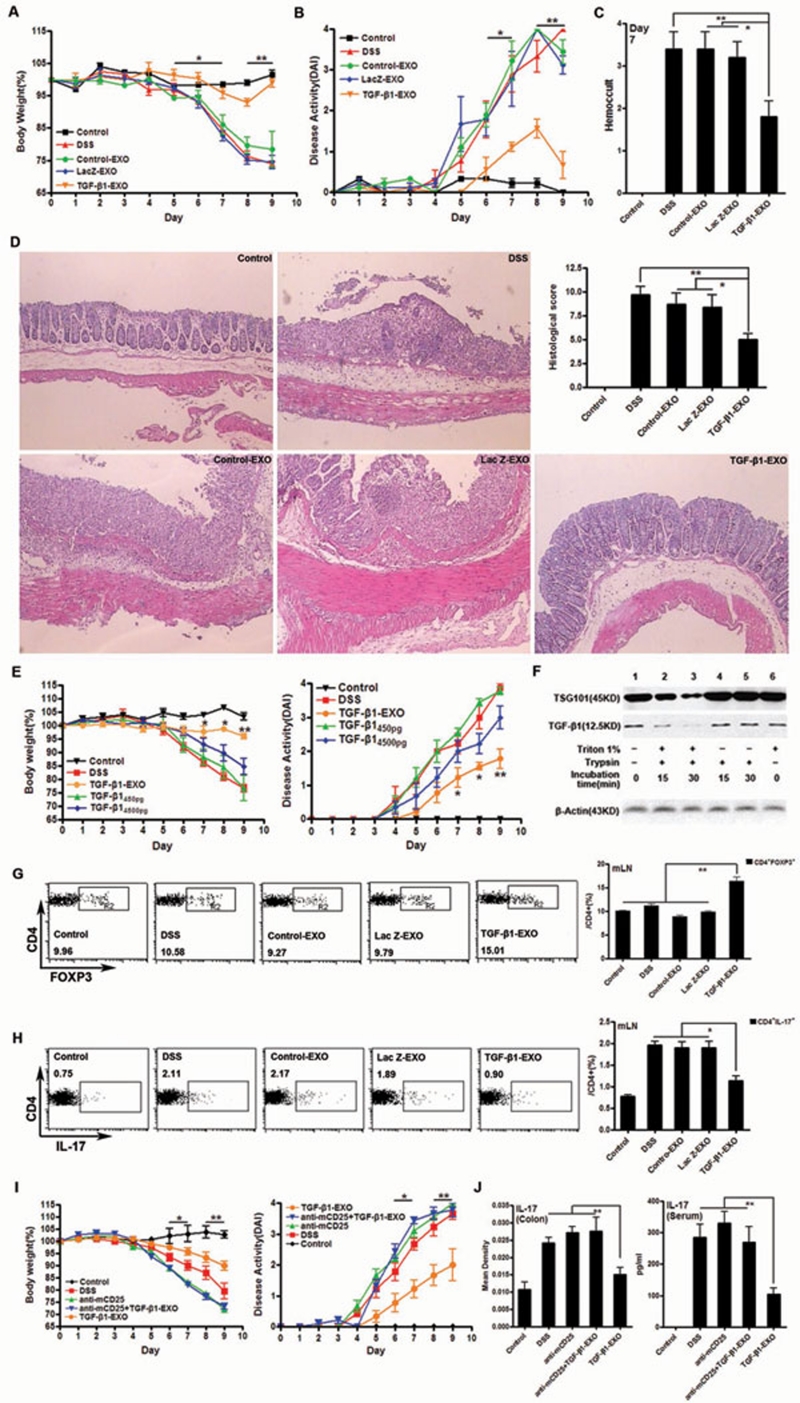
TGF-β1-EXO delayed the development of the DSS-induced murine IBD by increasing CD4+Foxp3+ Tregs and inhibiting Th17. IBD was induced by administering DSS in acidified drinking water, 1.5% (w/v) for 9 days. The day that mice started to drink DSS was regarded as day 0. For the treatment of IBD, mice were injected i.v. with exosomes (10 μg exosomes/mouse/injection) on day−2, −3, and −5. (A) Body weight loss. Each point represents average weight data pooled from eight mice ± SD. (B) DAI (combined score of body weight, bleeding and stool consistency). Each point represents average DAI data pooled from eight mice. (C) Rectal bleeding was detected on day 7 by one step fecal occult blood test. Each column represents average score data pooled from eight mice. (D) Histological appearance 9 days after DSS induction. Representative colonic sections stained with H&E. Magnifications: 40×. (E) The protective effect of TGF-β1-EXO versus TGF-β1 cytokine in IBD. To evaluate the protective effect of TGF-β1 in IBD, mice were injected i.v. with TGF-β1 (150 pg or 1 500 pg/mouse/injection) on day−2, −3, and −5 (n = 8). (F) TGF-β1 in exosomes resists the hydrolysis of proteases. Exosomes were disrupted by 1% Triton X-100 for 30 min at 4 °C. Equal amount of disrupted and intact exosomes (30 μg) were then subjected to trypsin digestion at 37 °C with a concentration of 25 μg/ml for 15 and 30 min. After trypsin digestion, the samples were subjected to western blot analysis. Lane 1 (no triton, no trypsin); Lanes 2, 3 (triton lysis followed by trypsin digestion); Lanes 4, 5 (mock treatment followed by trypsin digestion); Lane 6 (triton lysis without trypsin digestion). β-actin control showed that equal amount of exosomes were used in each experiment. (G) The percentage of CD4+Foxp3+ Tregs from mLNs of each group of mice (n = 3). (H) The percentage of Th17 from mLNs of each group mice (n = 3). (I) Severity of DSS-induced IBD after CD4+Foxp3+ Treg depletion. CD25+ T cells were depleted by i.p. injection of anti-mouse CD25 mAb (clone PC61.5.3). The anti-CD25 mAb (200 μg/mouse) was administered on day−8, −6 and −4. Control mice were injected with PBS alone without Ab (n = 5). (J) Level of IL-17 in inflammatory site and in murine sera after CD4+Foxp3+ Treg depletion (n = 5). All experiments were repeated three times. *P < 0.05, **P < 0.01, TGF-β1-EXO versus other groups except control.
Tregs were capable of suppressing colonic inflammation in IBD mice by downregulating Th17 responses via TGF-β 9. We speculated that TGF-β1-EXO might induce the Tregs to exert protective effect. As expected, we found that TGF-β1-EXO could effectively induce CD4+Foxp3+ Tregs via TGF-β1 in vitro (Supplementary information, Figure S5), and we also found that TGF-β1-EXO induced CD4+Foxp3+ Tregs in lymphocytes from mesentery lymph nodes (mLNs) of inflammatory site (Figure 1G), but not in splenocytes (data not shown). It is known that the TCR signaling is required for the generation of Tregs, but not for TGF-β1 signaling 10. Compared with lymphocytes of the inflammatory site, the possibility of splenocytes to contact intestinal antigen, which could provide the TCR signaling, was small. In addition, Tregs might transfer to spleen from mLNs and increase the Treg level in spleen. However, the increase of the transferred Tregs was too small to be detected in total CD4+ T cells in the spleen. Therefore, TGF-β1-EXO-induced CD4+Foxp3+ Tregs were mainly from mLNs of the inflammatory site, but not in the remote splenocytes.
There is a delicate balance between Th17 and Treg. A signature transcription factor, RORγT for Th17 is also induced by TGF-β1. In the absence of IL-6, Foxp3 can inhibit RORγT function and drive Treg differentiation. However, when the cells also receive a signal from IL-6, Foxp3 function is inhibited and the Th17 differentiation pathway is induced 11. Similar to CD4+Foxp3+ Tregs, we found that TGF-β1-EXO could decrease Th17 in lymphocytes from mLNs (Figure 1H), but not in splenocytes (data not shown). Furthermore, the levels of IL-17 and IL-6 in colon tissues were also inhibited by TGF-β1-EXO and results even showed that the serum level of IL-17 was decreased in TGF-β1-EXO-treated mice (Supplementary information, Figure S6), suggesting that TGF-β1-EXO could affect Th17 cells systemically.
To further confirm the protective effect of CD4+Foxp3+ Tregs, they were depleted by the pretreatment of mice with anti-CD25 monoclonal antibody (mAb). Then we found that the protective effect of TGF-β1-EXO against DSS-induced IBD was totally abrogated (Figure 1I) and the inhibitory effect of TGF-β1-EXO on IL-17 in colon tissues and serum also could not be observed (Figure 1J). These results further support the notion that CD4+Foxp3+ Tregs play a role in the prevention of DSS-induced IBD by treatment with TGF-β1-EXO.
This is the first report about utilizing exosomes that were derived from TGF-β1 gene-modified BMDCs to delay DSS-induced IBD. TGF-β1-EXO induced CD4+Foxp3+Tregs and decreased the proportion of Th17 in lymphocytes from mLNs of the inflammatory site. As acellular vesicles, exosomes can be preserved for a long time. Unlike DC, exosomes are derived from the DC and presumably reflect the function of the DC at the time of isolation. Moreover, the protective effect of TGF-β1-EXO in IBD was much better than TGF-β1 cytokine. All these specific characters make exosomes to be one of the attracting candidates for clinical application of autoimmune diseases. Experimental materials and methods aredescribed in the Supplementary information, Data S1.
Acknowledgments
We thank Ms Diya Yang for technical assistance. This work was supported by the National Key Basic Research Program of China (2007CB512403), the National Natural Science Foundation of China (30972725 and 31070795), the National Technology Key Projects of China (008ZX1002-008) and the Natural Science Foundation of Zhejiang Province (Z2090042).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Material
Supplementary information, Figure S1
Characterization of the exosomes from BMDCs.
Supplementary information, Figure S2
The serial concentrations of exosomes were added into culture of T cells isolated from BALB/c spleen and Control DCs from C57BL/6 at 10:1 ratio.
Supplementary information, Figure S3
FACS analysis of bead-coated exosomes.
Supplementary information, Figure S4
TGF-β1 in exosomes resists the hydrolysis of proteases.
Supplementary information, Figure S5
TGF-β1-EXO can increase the relative numbers of CD4+Foxp3+ Treg via TGF-β1 in vitro.
Supplementary information, Figure S6
TGF-β1-EXO inhibit Th17 in murine colon tissues and sera.
Supplementary information, Data S1
Materials and Methods
References
- Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. [Abstract] [Google Scholar]
- Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–1813. [Abstract] [Google Scholar]
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. [Abstract] [Google Scholar]
- Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol. 2007;42:16–25. [Europe PMC free article] [Abstract] [Google Scholar]
- Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–533. [Abstract] [Google Scholar]
- Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. [Abstract] [Google Scholar]
- Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J Immunol. 2007;179:2242–2249. [Abstract] [Google Scholar]
- Morelli AE, Larregina AT, Shufesky WJ, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. [Abstract] [Google Scholar]
- Ogino H, Nakamura K, Ihara E, Akiho H, Takayanagi R. CD4+CD25+ regulatory T cells suppress Th17-responses in an experimental colitis model. Dig Dis Sci. 2011;56:376–386. [Abstract] [Google Scholar]
- Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. [Europe PMC free article] [Abstract] [Google Scholar]
- Ziegler SF, Buckner JH. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009;11:594–598. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from Cell Research are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/cr.2011.196
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/cr2011196.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/cr.2011.196
Article citations
Extracellular vesicles for the treatment of ulcerative colitis: A systematic review and meta-analysis of animal studies.
Heliyon, 10(17):e36890, 24 Aug 2024
Cited by: 0 articles | PMID: 39281542 | PMCID: PMC11400994
The role of mesenchymal stem cells in attenuating inflammatory bowel disease through ubiquitination.
Front Immunol, 15:1423069, 09 Aug 2024
Cited by: 1 article | PMID: 39185411 | PMCID: PMC11341407
Review Free full text in Europe PMC
Exosomal mediators in sepsis and inflammatory organ injury: unraveling the role of exosomes in intercellular crosstalk and organ dysfunction.
Mil Med Res, 11(1):24, 22 Apr 2024
Cited by: 3 articles | PMID: 38644472 | PMCID: PMC11034107
Review Free full text in Europe PMC
Effects of Extracellular Vesicles Derived from Human Umbilical Cord Blood Mesenchymal Stem Cells on Cell Immunity in Nonobese Mice.
Stem Cells Int, 2024:4775285, 02 Feb 2024
Cited by: 1 article | PMID: 38343632 | PMCID: PMC10857880
Emerging role of extracellular vesicles in veterinary practice: novel opportunities and potential challenges.
Front Vet Sci, 11:1335107, 25 Jan 2024
Cited by: 2 articles | PMID: 38332755 | PMCID: PMC10850357
Review Free full text in Europe PMC
Go to all (89) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Exosomes with membrane-associated TGF-β1 from gene-modified dendritic cells inhibit murine EAE independently of MHC restriction.
Eur J Immunol, 43(9):2461-2472, 21 Jun 2013
Cited by: 59 articles | PMID: 23716181
The effects of TGF-βs on immune responses.
Nihon Rinsho Meneki Gakkai Kaishi, 39(1):51-58, 01 Jan 2016
Cited by: 13 articles | PMID: 27181235
Review
B cell-derived transforming growth factor-β1 expression limits the induction phase of autoimmune neuroinflammation.
Sci Rep, 6:34594, 06 Oct 2016
Cited by: 48 articles | PMID: 27708418 | PMCID: PMC5052622
TGF-beta1 gene-modified, immature dendritic cells delay the development of inflammatory bowel disease by inducing CD4(+)Foxp3(+) regulatory T cells.
Cell Mol Immunol, 7(1):35-43, 01 Jan 2010
Cited by: 24 articles | PMID: 20081874 | PMCID: PMC4003258




